N-heterocyclic carbene-catalyzed rearrangements of vinyl sulfones†
Roxanne L.
Atienza
,
Howard S.
Roth
and
Karl A.
Scheidt
*
Department of Chemistry, Center for Molecular Innovation and Drug Discovery, Chemistry of Life Processes Institute, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208, USA. E-mail: scheidt@northwestern.edu; Fax: (+1) 847-467-2184
First published on 10th June 2011
Abstract
N-heterocyclic carbenes catalyze the rearrangement of 1,1-bis(arylsulfonyl)ethylene to the corresponding trans-1,2-bis(phenylsulfonyl) under mild conditions. Tandem rearrangement/cycloadditions have been developed to capitalize on this new process and generate highly substituted isoxazolines and additional heterocyclic compounds. Preliminary mechanistic studies support a new conjugate addition/Umpolung process involving the ejection and subsequent unusual re-addition of a sulfinate ion.
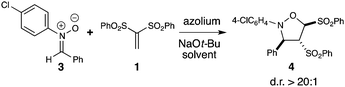
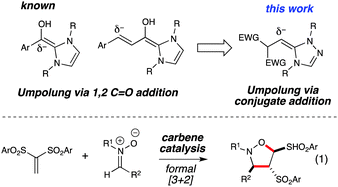
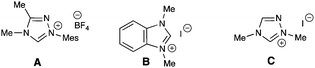
Metal free catalysis has undergone a renaissance over the last decade, leading to an explosion of efficient and stereoselective strategies for chemical synthesis.1 In the area of Lewis base-promoted reactions,2N-heterocyclic carbene (NHC) catalysis is a rapidly growing field that employs electron lone pair-bearing heterocycles to facilitate a wide breadth of chemical transformations.3Carbene catalysis is highly versatile and allows access to acyl anions,4 homoenolates,5 enolates,6 Cannizarro-type reductions,3c and oxidations.7 The majority of NHC catalysis currently involves the 1,2-addition of the carbene to a carbonyl-containing species which then proceeds to produce an acyl anion or homoenolate equivalent. A different mode for these nucleophilic catalysts is a conjugate addition manifold. By capitalizing on this 1,4-type reactivity, both Fu and Ye have separately reported the development of β-Umpolung-type and Morita-Baylis-Hilman reactions catalyzed by NHCs, respectively.8 This conjugate addition process provides a new direction beyond 1,2-additions. In this work, the combination of an NHC with a vinyl sulfone and nitrone results in the formation of an isoxazolidine product with an unusual transposition of a sulfone group (eqn 1). This process fundamentally differs from the previous 1,4 additions because it is neither an intramolecular alkylation8a nor a MBH reaction.8b
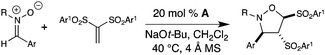
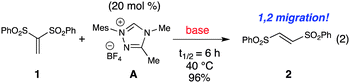
Vinyl sulfones are useful intermediates in organic synthesis that can act as 2π participants in cycloadditions9 and as electrophiles in organometallic10 and organocatalysis.11 When we began these specific studies, the use of vinyl sulfones as substrates in NHC-catalyzed transformations had not been previously reported. In our attempts to achieve homoenolate equivalent annulations with 1,1-bis(phenylsulfonyl)ethylene (1), we discovered that the carbene derived from triazolium A promoted an unanticipated rearrangement to the trans-1,2-bis(phenylsulfonyl)ethylene in 96% yield with a t1/2 of ∼6 h at 40 °C (2, eqn 2).
 | ||
| Scheme 1 Known NHC methodology and this work. | ||
The isomerization of 1,1-bis(phenylsulfonyl)ethylene to date involves harsh, non-catalytic reaction conditions such as autoclave with high pressure (no isolated yield) or ammonium salts at 140 °C.12 Our survey of standard Lewis bases for this process, such as 1,4-diazabicyclo[2.2.2]octane (DABCO) and triphenylphosphine, revealed there was poor and incomplete conversion when compared to the NHC catalyst generated from azolium catalyst A and sodium tert-butoxide.13Trans-1,2-bis(arylsulfonyl)ethylene has been used in organic synthesis,14 but its preparation typically involves the use of vinyl chloride gas.15 Alternatively, the straightforward preparation of 1,1-bis(phenylsulfonyl)ethylene involves paraformaldehyde, piperidine, and acid.12b
 | ||
| Scheme 2 Isomerization of 1,1-bis(phenylsulfonyl)ethylene. | ||
2. Results and discussion
We viewed this unusual NHC-catalyzed generation of trans-1,2-bis(phenylsulfonyl)ethylene in situ under mild reaction conditions as an interesting opportunity to develop a two-step single flash process involving a cycloaddition step (vide infra). The initial test bed reaction we chose was the formation of the isomerization product in a one-pot process with a nitrone in a Huisgen [3 + 2] cyclization to form the isoxazolidine product.16 Our optimization studies for this NHC-catalyzed reaction began by treating the 1,1-bis(phenylsulfonyl)ethylene with N-aryl nitrone and surveying various azolium salts and reaction conditions (Table 1). Of the azolium salts, triazolium A was the only catalyst which led to appreciable amounts of desired cycloadduct 4. Notably, the cycloaddition yielded a single diastereomer (as observed by 1H NMR spectroscopy).17 The reduction of catalyst loading from 20 to 10 mol% of A resulted in significant depression of yield for the two-step process (rearrangement/cycloaddition). Further experimentation revealed dichloromethane as the best solvent and sodium tert-butoxide was the optimal base, presumably since its minimal nucleophilicity did not compete with the NHC for addition to the vinyl sulfone.18| Entry | Conditionsa | Yield [%]b |
|---|---|---|
| a General conditions: vinyl sulfone (1 equiv), nitrone (3 equiv), NaOt-Bu (20 mol%), solvent (0.10 M), 4 Å MS. b Yield of isolated products. | ||
| 1 | 20 mol% B, 40 °C, CH2Cl2 | Trace |
| 2 | 20 mol% C, 40 °C, CH2Cl2 | 4 |
| 3 | 20 mol% A, 40 °C, CH2Cl2 | 94 |
| 4 | 10 mol% A, 40 °C, CH2Cl2 | 37 |
| 5 | 20 mol% A, 40 °C, CH3CN | 0 |
| 6 | 20 mol% A, 40 °C, THF | 50 |
|
||
With the optimized reaction conditions, we examined the scope of the reaction with regard to the nitrone component (Table 2). A chloride substituent in the 4 position of the N-aryl ring delivered the products in good yields, presumably due to the increased electrophilicity of the nitrone being complimentary to the cyclization step (entries 1–7). Increasing the temperature to 40 °C and adding 4 Å molecular sieves generally improved conversion and yields. Reactions with N-aryl nitrones with non-electron withdrawing groups provided moderate yields and an N-alkyl nitrone provided 86% of the desired cycloadduct (entry 13). Similar to the initial optimization reaction in Scheme 1, the isoxazolidine products were observed as a single diastereomer (1H NMR, 500 MHz). Substituted aryl sulfones are also compatible with the system (entries 14–16), but currently both sulfones are required. With these NHC conditions, mixed sulfone/carbonyl group compounds are not substrates and neither are β-substituted bis-sulfones.
| Entry | R | Ar | Ar1 | d.r. | Yield [%] |
|---|---|---|---|---|---|
| a General conditions: vinyl sulfone (1 equiv), nitrone (1.05 equiv), azolium salt A (20 mol%), NaOt-Bu (20 mol%), CH2Cl2 (0.10 M). b Yield of isolated products. c Vinyl sulfone (2 equiv), nitrone (1 equiv). d 1,2-dichloroethane (0.10 M), 40 °C, no 4 Å MS. | |||||
| 1 | 4-ClPh | Ph | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 (4) |
| 2 | 4-ClPh | 4-BrPh | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 (5) |
| 3 | 4-ClPh | 4-MePh | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
61 (6) |
| 4 | 4-ClPh | 4-ClPh | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
76 (7) |
| 5 | 4-ClPh | 2-Napth | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
74 (8) |
| 6 | 4-ClPh | 2-ClPh | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 (9) |
| 7 | 4-ClPh | 4-MeOPh | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
71 (10)c |
| 8 | Ph | 2-ClPh | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 (11)c |
| 9 | Ph | 4-MeOPh | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
63 (12)c |
| 10 | Ph | 4-MePh | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 (13)c |
| 11 | Ph | 2-Napth | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
77 (14)c |
| 12 | 4-MePh | 4-ClPh | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 (15)c |
| 13 | PhCH2 | Ph | Ph | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86 (16)c |
| 14 | 4-ClPh | Ph | 4-MePh | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
72 (17)d |
| 15 | 4-ClPh | Ph | 4-BrPh | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 (18)d |
| 16 | Ph | Ph | 4-MePh | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
77 (19)d |
Since N-alkyl nitrones are productive substrates, this tandem 1,1-1,2 rearrangement/cycloaddition with a D-ribose-based chiral auxiliary is highly selective and provides the optically active isoxazoline in 65% yield as a single diastereomer (Scheme 3, eqn 3).19 Importantly, this streamlined process is not limited to nitrones20 as the dipole coupling partner. For example, azomethine imines (such as 22) and dienes (including furan) undergo efficient reactions in [3 + 2] and [4 + 2] cycloaddition21 reactions (respectively, eqn 4 and 5).
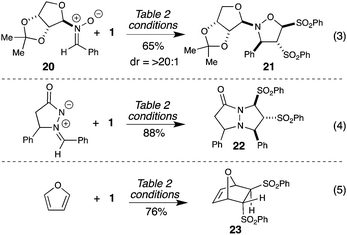 | ||
| Scheme 3 Compatibility with other cycloaddition reactions. | ||
We performed the studies outlined below to a) determine if the NHC was involved in the Huisgen cycloaddition,22 and b) provide insight into the mechanism of the sulfone rearrangement process. Our initial control experiments showed the triazolium salt and base were not needed for the cycloaddition with trans-1,2-bis(phenylsulfonyl)ethylene, thereby supporting an uncatalyzed thermal [3 + 2] process.23
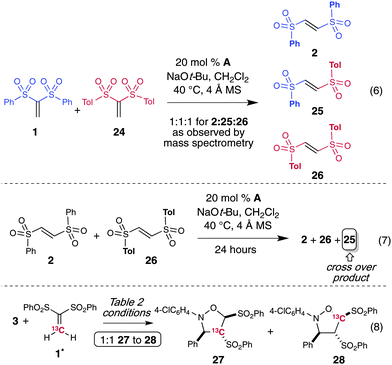
To further probe the proposed NHC-catalyzed isomerization mechanism, a cross-over experiment with 1,1-bis(tosyl)ethylene (24) and 1,1-bis(phenylsulfonyl)ethylene (1) was performed (Scheme 4, eqn 6). The two different 1,1-sulfones were placed in the same reaction mixture and subjected to the NHC reaction conditions from Table 2. Based on the high resolution mass spectrometry (EI), the cross-over product (25) was present in the reaction mixture in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with the trans-1,2-bis(phenylsulfonyl)ethylene (2) and trans-1,2-bis(tosyl)ethylene (26).24 Additionally, we have exposed a 1
1 ratio with the trans-1,2-bis(phenylsulfonyl)ethylene (2) and trans-1,2-bis(tosyl)ethylene (26).24 Additionally, we have exposed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2 and 26 to the NHC conditions and observed compound 25, thereby supporting that the NHC-addition/sulfinate ejection/addition pathway is operative with 1,2-bis(arylsulfonyl)ethylenes and underscoring the complexity and dynamic aspects of this process.
1 mixture of 2 and 26 to the NHC conditions and observed compound 25, thereby supporting that the NHC-addition/sulfinate ejection/addition pathway is operative with 1,2-bis(arylsulfonyl)ethylenes and underscoring the complexity and dynamic aspects of this process.
 | ||
| Scheme 4 Mechanistic studies. | ||
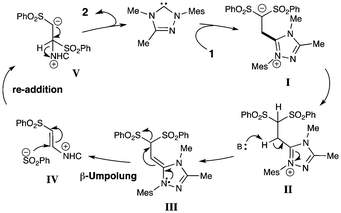
In addition to the cross over experiments, the reaction with a 13C-labeled isomer of bis-vinyl sulfone 1 provided evidence for the isomerization of the sulfonyl groups occurring prior to cyclization (Scheme 4, eqn 8). Based on the proposed reaction pathway in Scheme 5, there should be a mixture of 13C-labeled products (27 and 28) if the isomerization occurs prior to Huisgen cycloaddition with the nitrone. Alternatively, if there is only 13C-labeled vinyl sulfone isomer present at the cycloaddition step, then this label would be in a single position in the product. A cycloadduct with a singularly labeled position would necessitate a difficult migration of a sulfone moiety after formation of an initial isoxazoline product. The use of 1* provided a mixture of isotope-labeled isoxazolidine products as observed by NMR spectroscopy.23
 | ||
| Scheme 5 Proposed reaction pathway. | ||
Based on the data above, our proposed pathway of the NHC-induced sulfone rearrangement involves the carbene addition to the beta position of 1,1-bis(phenylsulfonyl)ethylene (Scheme 5). The nucleophilic addition of the NHC to the vinyl sulfone drives the process. After protonation of the intermediate anion (I), base can remove a β-proton of II due to the stabilization of the anion by the triazolium catalyst, thus promoting the ejection of a sulfinate moiety from III.25 The sulfinate group then undergoes intermolecular addition at the beta position of IV to produce another nucleophilic anion (V).26 This sulfinate rebound promotes the regeneration of the NHC catalyst through elimination and the formation of the trans-1,2-bis(phenylsulfonyl)ethylene. The mixed arylsulfone product (25) from the cross over experiment in eqn 6 supports this unusual ejection/rebound of a sulfinate group.
Conclusions
We have discovered that triazolium-derived carbenes promote the facile rearrangement of 1,1-bis(arylsulfonyl)ethylenes to trans-1,2-bis(arlylsulfonyl)ethylenes. We have combined this new sulfone transposition reaction with subsequent cycloadditions to provide a single flask, tandem rearrangement/cycloaddition process. The combination of the 1,1-bis(arylsulfonyl)ethylene, a nitrone and an NHC provides highly substituted isoxazolines through a stereoselective [3 + 2] Huisgen cylcoaddition. Mechanistic studies support an unusual carbene-promoted ejection and then re-addition of a sulfinate group. This NHC-catalyzed reaction is unusual in that the substrates are vinyl sulfones and not the standard reaction partner: α,β-unsaturated carbonyl compound, ketone, or aldehyde. Further methodology development with this promising NHC reactivity pattern and its application in chemical synthesis is under investigation.Acknowledgements
Support for this work was provided by the NIH (GM73072-01) and an NIGMS Research Supplement to Promote Diversity in Health-Related Research (to R.L.A.). We thank the Integrated Molecular Structure Education and Research Center (IMSERC) at NU for assistance with mass spectrometry and John M. Roberts (NU) for assistance with X-ray crystallography.Notes and references
- (a) P. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138–5175 CrossRef CAS; (b) B. List, Chem. Commun., 2006, 819–824 RSC; (c) D. W. C. MacMillan, Nature, 2008, 455, 304–308 CrossRef CAS; (d) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713–5743 CrossRef CAS; (e) S. Bertelsen and K. A. Jorgensen, Chem. Soc. Rev., 2009, 38, 2178–2189 RSC; (f) D. Kampen, C. M. Reisinger and B. List, Top. Curr. Chem., 2010, 291, 395–456 CAS.
- S. E. Denmark and G. L. Beutner, Angew. Chem., Int. Ed., 2008, 47, 1560–1638 CrossRef CAS.
- (a) D. Enders and T. Balensiefer, Acc. Chem. Res., 2004, 37, 534–541 CrossRef CAS; (b) N-heterocyclic Carbenes in Synthesis, ed. S. P. Nolan, Wiley-VCH, 2006 Search PubMed; (c) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS; (d) V. Nair, S. Vellalath and B. Babu, Chem. Soc. Rev., 2008, 37, 2691–2698 RSC; (e) E. M. Phillips, A. Chan and K. A. Scheidt, Aldrichimica Acta, 2009, 42, 55–66 CAS; (f) J. L. More and T. Rovis, Top. Curr. Chem., 2010, 291, 77–144 CAS.
- (a) T. Ugai, S. Tanaka and S. Dokawa, J. Pharm. Soc. Jpn., 1943, 63, 269; (b) R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719–3726 CrossRef CAS; (c) Y. Hachisu, J. W. Bode and K. Suzuki, J. Am. Chem. Soc., 2003, 125, 8432–8433 CrossRef CAS; (d) A. Chan and K. Scheidt, J. Am. Chem. Soc., 2006, 128, 4558–4559 CrossRef CAS ; For asymmetric variants, see; (e) D. Enders, K. Breuer, J. Runsink and J. H. Teles, Helv. Chim. Acta, 1996, 79, 1899–1902 CAS; (f) D. Enders, K. Breuer and J. H. Teles, Helv. Chim. Acta, 1996, 79, 1217–1221 CAS; (g) D. Enders and U. Kallfass, Angew. Chem., Int. Ed., 2002, 41, 1743–1745 CrossRef CAS; (h) M. S. Kerr, J. R. de Alaniz and T. Rovis, J. Am. Chem. Soc., 2002, 124, 10298–10299 CrossRef CAS.
- (a) C. Burstein and F. Glorius, Angew. Chem., Int. Ed., 2004, 43, 6205–6208 CrossRef CAS; (b) S. S. Sohn, E. L. Rosen and J. W. Bode, J. Am. Chem. Soc., 2004, 126, 14370–14371 CrossRef CAS; (c) A. Chan and K. Scheidt, Org. Lett., 2005, 7, 905–908 CrossRef CAS; (d) V. Nair, S. Vellalath, M. Poonoth and E. Suresh, J. Am. Chem. Soc., 2006, 128, 8736–8737 CrossRef CAS; (e) A. Chan and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 5334–5335 CrossRef CAS; (f) E. M. Phillips, M. Wadamoto, A. Chan and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 3107–3110 CrossRef CAS; (g) V. Nair, S. C. Mathew, A. T. Biju and E. Suresh, Angew. Chem., 2007, 119, 2116–2119 CrossRef; (h) A. Chan and K. A. Scheidt, J. Am. Chem. Soc., 2008, 130, 2740–2741 CrossRef CAS; (i) E. M. Phillips, T. E. Reynolds and K. A. Scheidt, J. Am. Chem. Soc., 2008, 130, 2416–2417 CrossRef CAS ; For NHC catalysis with Lewis acids, see; (j) B. Cardinal-David, D. E. A. Raup and K. A. Scheidt, J. Am. Chem. Soc., 2010, 132, 5345–5347 CrossRef CAS; (k) D. E. A. Raup, B. Cardinal-David, D. Holte and K. A. Scheidt, Nat. Chem., 2010, 2, 766–771 CrossRef CAS.
- (a) K. Chow and J. Bode, J. Am. Chem. Soc., 2004, 126, 8126–8127 CrossRef CAS; (b) N. Reynolds and T. Rovis, J. Am. Chem. Soc., 2005, 127, 16406–16407 CrossRef; (c) E. M. Phillips, M. Wadamoto, A. Chan and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 3107–3110 CrossRef CAS; (d) J. J. Song, Z. Tan, J. T. Reeves, D. R. Fandrick, N. K. Yee and C. H. Senanayake, Org. Lett., 2008, 10, 877–880 Search PubMed; (e) X.-L. Huang, X.-Y. Chen and S. Ye, J. Org. Chem., 2009, 74, 7585–7587 Search PubMed; (f) E. M. Phillips, M. Wadamoto, H. S. Roth and K. A. Scheidt, Org. Lett., 2009, 11, 105–108 CrossRef CAS; (g) Y. Kawanaka, E. M. Phillips and K. A. Scheidt, J. Am. Chem. Soc., 2009, 131, 18028–18029 CrossRef CAS; (h) E. M. Phillips, M. Wadamoto, H. S. Roth, A. W. Ott and K. A. Scheidt, Org. Lett., 2009, 11, 105–108 CrossRef CAS.
- (a) J. Castells, H. Llitjos and M. Morenomanas, Tetrahedron Lett., 1977, 2, 205–206 Search PubMed; (b) H. Inoue and K. Higashiura, J. Chem. Soc., Chem. Commun., 1980, 549–550 RSC; (c) A. Miyashita, Y. Suzuki, I. Nagasaki, C. Ishiguro, K. Iwamoto and T. Higashino, Chem. Pharm. Bull., 1997, 45, 1254–1258 CAS; (d) B. E. Maki, A. Chan, E. M. Phillips and K. A. Scheidt, Org. Lett., 2007, 9, 371–374 CrossRef CAS; (e) B. E. Maki and K. A. Scheidt, Org. Lett., 2008, 10, 4331–4334 CrossRef CAS; (f) B. E. Maki, A. Chan, E. M. Phillips and K. A. Scheidt, Tetrahedron, 2009, 65, 3102–3109 CrossRef CAS; (g) S. De Sarkar, S. Grimme and A. Studer, J. Am. Chem. Soc., 2010, 132, 1190–1191 CrossRef CAS.
- (a) C. Fischer, S. W. Smith, D. A. Powell and G. C. Fu, J. Am. Chem. Soc., 2006, 128, 1472–1473 CrossRef CAS; (b) L. He, T.-Y. Jian and S. Ye, J. Org. Chem., 2007, 72, 7466–7468 CrossRef CAS.
- (a) P. L. Fuchs and T. F. Braish, Chem. Rev., 1986, 86, 903–917 CrossRef CAS; (b) N. Simpkins, Tetrahedron, 1990, 46, 6951–6984 CrossRef CAS ; For vinyl sulfones in organocatalysis, see; (c) M. Nielsen, C. B. Jacobsen, N. Holub, M. W. Paixão and K. A. Jørgensen, Angew. Chem. Int. Ed., 2010, 49, 2668–2679 CAS.
- K. Grela and M. Bieniek, Tetrahedron Lett., 2001, 42, 6425–6428 CrossRef CAS.
- (a) S. Mossé and A. Alexakis, Org. Lett., 2005, 7, 4361–4364 CrossRef CAS; (b) S. Mossé, M. Laars, K. Kriis, T. Kanger and A. Alexakis, Org. Lett., 2006, 8, 2559–2562 CrossRef CAS; (c) Q. Zhu, L. Cheng and Y. Lu, Chem. Commun., 2008, 6315–6317 RSC; (d) Q. Zhu and Y. Lu, Org. Lett., 2008, 10, 4803–4806 CrossRef CAS; (e) A. Landa, M. Maestro, C. Masdeu, Á. Puente, S. Vera, M. Oiarbide and C. Palomo, Chem.–Eur. J., 2009, 15, 1562–1565 CrossRef CAS; (f) Q. Zhu and Y. Lu, Org. Lett., 2009, 11, 1721–1724 CrossRef CAS; (g) S. A. Moteki, S. Xu, S. Arimitsu and K. Maruoka, J. Am. Chem. Soc., 2010, 132, 17074–17076 CrossRef CAS; (h) A. Quintard, A. Alexakis and C. Mazet, Angew. Chem., Int. Ed., 2011, 50, 1393–1396 Search PubMed; (i) J. Xiao, Y.-P. Lu, Y.-L. Liu, P.-S. Wong and T.-P. Loh, Org. Lett., 2011, 13, 876–879 CrossRef CAS.
- (a) H. Stetter and K. Steinbeck, Liebigs Ann. Chem., 1974, 1315–1321 Search PubMed; (b) V. M. Naplyuev and I. M. Bazavova, Zh. Org. Khim., 1981, 17, 2231–2232 Search PubMed.
- The reaction conditions were Lewis base (20 mol%), dichloromethane (0.1(M)), and 1,1-bis(phenylsulfonyl)ethylene.
- (a) S. Mirsadeghi and B. Rickborn, J. Org. Chem., 1985, 50, 4340–4345 CrossRef; (b) S. W. McCombie, B. B. Shankar and A. K. Ganguly, Tetrahedron Lett., 1985, 26, 6301–6304 CrossRef CAS; (c) L. A. Pacquette, H. Kuenzer, K. E. Green, O. De Lucchi, G. Licini, L. Pasquato and G. Valle, J. Am. Chem. Soc., 1986, 108, 3453–3460 CrossRef CAS; (d) A. Padwa, W. H. Bullock, A. D. Dyszlewski, S. W. McCombie, B. B. Shankar and A. K. Ganguly, J. Org. Chem., 1991, 56, 3556–3564 CrossRef CAS; (e) K. S. Feldman, C. K. Weinreb, W. J. Youngs and J. D. Bradshaw, J. Am. Chem. Soc., 1994, 116, 9019–9026 CrossRef CAS; (f) P. A. Evans and T. Manangan, Tetrahedron Lett., 1997, 38, 8165–8168 CrossRef CAS; (g) K. C. Nicolau, J. A. Pfefferkorn, S. Kim and H. X. Weo., J. Am. Chem. Soc., 1999, 121, 4724–4725 CrossRef CAS; (h) P. A. Evans and T. Manangan, J. Org. Chem., 2000, 65, 4523–4528 CrossRef CAS; (i) F. Chery, P. Rollin, O. De Lucchi and S. Cossu, Tetrahedron Lett., 2000, 41, 2357–2360 CrossRef CAS; (j) F. Chery, P. Rollin, O. De Lucchi and S. Cossu, Synthesis, 2001, 2, 286–292 Search PubMed; (k) S. Berlin, C. Ericsson and L. Engman, J. Org. Chem., 2003, 68, 8386–8396 Search PubMed; (l) A.-P. Schaffner, V. Darmency and P. Renaud, Angew. Chem., Int. Ed., 2006, 45, 5847–5849 CrossRef CAS; (m) J. L. G. Ruano, J. Aleman and C. G. Paredes, Org. Lett., 2006, 8, 2683–2686 Search PubMed; (n) A. Lopez-Perez, R. Robles-Machin, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2007, 46, 9261–9264 Search PubMed; (o) M. Luethy, V. Darmency and P. Renaud, Eur. J. Org. Chem., 2011, 3, 547–552 Search PubMed.
- D. Reddy, N. Babu, V. Padmavathi and R. Sumathi, Synthesis, 1999, 491–494 CrossRef CAS.
- (a) R. Huisgen, Angew. Chem., Int. Ed. Engl., 1963, 2, 565–632 CrossRef; (b) R. Huisgen, Angew. Chem., Int. Ed. Engl., 1968, 7, 321–406 CrossRef CAS.
- Stereochemistry of 4 determined by X-ray crystallography and other assignments made by analogy. See Supporting Information (a) P. N. Confalone and E. M. Huie, Org. React., 1988, 36, 1 CAS; (b) M. Burdisso, R. Gandolfi, P. Grunanger and A. Rastelli, J. Org. Chem., 1990, 55, 3427–3429 CrossRef CAS and references cited therein; (c) H. M. I. Osborn, N. Gemmell and L. M. Harwood, J. Chem. Soc., Perkin Trans. 1, 2002,(22), 2419–2438 RSC; (d) I. A. Grigorev, in Nitrile Oxides, Nitrones and Nitronates in Organic Synthesis: Novel Strategies in Synthesis, ed. H. Feuer, John Wiley & Sons, UK, 2nd edn, 2008, pp. 129–434 Search PubMed.
- Additional bases (Et3N, DBU, i-Pr2EtN, TBD, 2,6-lutidine, sodium amylate, KO t-Bu, K2CO3) were also examined and gave inferior results.
- (a) M. Kiso and A. Hasegawa, Carbohydr. Res., 1976, 52, 95–101 CrossRef CAS; (b) A. Vasella, Helv. Chim. Acta, 1977, 60, 426–446 CrossRef CAS; (c) A. Vasella, Helv. Chim. Acta, 1977, 60, 1273–1295 CrossRef CAS; (d) D. K. Thompson, C. N. Hubert and R. H. Wightman, Tetrahedron, 1993, 49, 3827–3840 CrossRef CAS; (e) S. Mzengeza and R. A. Whitney, J. Org. Chem., 1988, 53, 4074–4081 CrossRef CAS; (f) K. Kasahara, H. Iida and C. Kibayashi, J. Org. Chem., 1989, 54, 2225–2233 CrossRef CAS; (g) F. Machetti, F. M. Cordero, F. De Sarlo, A. Guarna and A. Brandi, Tetrahedron Lett., 1996, 37, 4205–4208 CrossRef CAS; (h) U. Chiacchio, A. Corsaro, G. Gumina, A. Rescifina, D. Iannazzo, A. Piperno, G. Romeo and R. Romeo, J. Org. Chem., 1999, 64, 9321–9327 CrossRef CAS.
- M. Burdisso, A. Gamba and R. Gandolgi, Tetrahedron, 1988, 44, 3735–3748 CrossRef CAS.
- O. De Lucchi and G. Modena, Tetrahedron Lett., 1983, 24, 1653–1656 CrossRef CAS.
- A possible NHC-catalyzed Huisgen cycloaddition pathway would involve the addition of the Lewis base to trans-1,2-bis(phenylsulfonyl)ethylene (2) produced in situ, thereby generating a stabilized carbanion. The addition of this plausible intermediate to the nitrone followed by an intramolecular SN2 reaction would generate the free NHC catalyst and isoxazoline product.
- The possible role of the NHC in the [3 + 2] cycloaddition was investigated through the comparison of the overall rates with a) trans-1,2-bis(phenylsulfonyl)ethylene and nitrone 3, b) trans-1,2-bis(phenylsulfonyl)ethylene with nitrone 3, azolium A, and sodium tert-butoxide, and c) trans-1,2-bis(phenylsulfonyl)ethylene with nitrone 3 and sodium tert-butoxide. Based on the observed rates of these three reactions (as determined by HPLC), the NHC does not accelerate the [3 + 2] cycloaddition step relative to the thermal process.
- See supporting information† for details.
- Alexakis has reported an unusual 1,2 to 1,1 rearrangement under a proline-mediated organocatalytic addition of aldehydes to cis-1,2-bis(phenylsulfonyl)ethylene. This process is fundamentally different than carbene catalysed reaction here (vs.proline catalysis) and proceeds in the opposite sense of migration relative to the results reported herein. They propose an intramolecular migration of the sulfinate ion: A. Quintard and A. Alexakis, Chem.–Eur. J., 2009, 15, 11109–11113 Search PubMed.
- Balenkova observed the unexpected phenylsulfonyl group migration to the beta position with their work with β-trifluoroacetylketene diphenyldithioacetal S, S-tetroxide, see: A. L. Krazovsky, S. V. Druzhinin, V. G. Nenajdenko and E. S. Balenkova, Tetrahedron Lett., 2004, 45, 1129–1132 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data for all new compounds. See DOI: 10.1039/c1sc00194a |
| This journal is © The Royal Society of Chemistry 2011 |