DOI:
10.1039/C1SC00176K
(Edge Article)
Chem. Sci., 2011,
2, 1470-1473
The intramolecular asymmetric allylation of aldehydes via organo-SOMO catalysis: A novel approach to ring construction†
Received
22nd March 2011
, Accepted 19th April 2011
First published on 19th May 2011
Abstract
The intramolecular asymmetric cyclization of aldehydes has been accomplished using singly occupied molecular orbital (SOMO) catalysis. Selective oxidation of chiral enamines (formed by the condensation of an aldehyde and a secondary amine catalyst) leads to the formation of a 3π-electron radical species. These chiral SOMO-activated radical cations undergo enantioselective cyclization with an array of pendent allylsilanes thus efficiently providing a new approach to the construction of five-, six- and seven-membered carbocycles and heterocycles.
Introduction
The enantioselective formation of stereochemically complex carbocyclic and heterocyclic ring systems remains a longstanding goal within the field of chemical synthesis.1 The Diels–Alder reaction remains perhaps the archetypal example (eq 1), a powerful technology that builds molecular complexity from simple dienes and dienophiles via a routine and predictable intermolecular or intramolecular pathway.1a Likewise, ring-closing metathesis has emerged as a robust approach for the intramolecular cyclization of simple diene systems, a transformation that has now found widespread utility for the construction of natural products and medicinal agents, broadly defined.1b Recently, we hypothesized that SOMO-catalysis (singly occupied molecular orbital) might provide an additional approach to the enantioselective construction of complex ring systems via the asymmetric intramolecular α-allylation of aldehydes using formyl-tethered allylsilanes (eq 2).2–4 Enantioselective SOMO-catalysis is a unique and versatile mode of organocatalytic activation that features the transient generation of a 3π-radical cation species, which can participate in asymmetric bond construction with a variety of π-rich nucleophiles or electron-neutral SOMO-philes. Since its introduction in 2007, we have successfully utilized this activation mode to overcome a series of elusive challenges in asymmetric catalysis, including the α-allylic alkylation, α-enolation, α-vinylation, α-chlorination, and α-arylation of aldehydes and ketones.5 On this basis, we envisioned that various formyl tethered allylsilanes should readily undergo asymmetric cyclization in the presence of an oxidant and a chiral amine catalyst to stereoselectively generate five-, six- and seven-membered rings. Herein, we describe the successful execution of these ideals and outline a facile, intramolecular aldehyde α-allylation protocol, which, to our knowledge, is not known in any previous format (racemic or asymmetric).6 This new approach to enantioenriched carbocycles and heterocycles of various size and substitution pattern should be of utility to practitioners of both natural product and medicinal agent synthesis.
Design plan
In accord with our previous SOMO-catalysis studies,5 we reasoned that exposure of an allylsilane-tethered aldehyde7,8 to amine catalyst 1 in the presence of a suitable oxidant would render the radical-cation 2 (Scheme 1). Rapid and enantioselective cyclization of the pendent olefin onto the 3π-electron species would then provide a β-silyl radical 3 that, upon further oxidation, would generate a stabilized cation (not shown). At this stage, we presumed that β-silyl elimination would be facile prior to hydrolysis of the iminium species to reconstitute catalyst 1 and deliver the desired 1,2-disubstituted ring system in enantioenriched form. In alignment with previous studies, we anticipated that the activated radical 2 would position the 3π-electron system away from the bulky tert-butyl moiety, while the benzyl group would effectively shield the Re-face,5 such that internal cyclization of the pendent allylsilane moiety would occur from the less shielded Si-face.
 |
| | Scheme 1 Proposed mechanism of organo-SOMO allylic cyclization. | |
Results and discussion
The feasibility of this proposed intramolecular cyclization was first examined using aldehyde 4, catalyst 1·TFA and CAN (ceric ammonium nitrate) in DME at −20 °C. As shown in Table 1, the desired cyclization could be accomplished using these initial conditions, albeit with modest yield and enantiocontrol along with poor diastereoinduction (entry 1, 53% yield, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 82% ee). Changing the reaction medium from DME to acetone served to increase both reaction efficiency and stereoselectivity (entry 2, 71% yield, 88% ee), and further improvements could be realized through the addition of water (entry 3, 80% yield, 91% ee). We presume that the addition of water allows for rapid capture of the putative silyl-cation that is generated at the final step in the catalytic cycle (see Scheme 1), a species that is often found to be detrimental to organocatalytic systems.5h As might be expected, lowering the reaction temperature to −30 °C provided a modest enhancement in diastereoselectivity, however, the accompanying decrease in reaction efficiency rendered these conditions less favorable (entry 4). The superior levels of induction and efficiency exhibited by amine 1 in acetone at −20 °C to afford α-formyl cyclopentane 5 in 91% ee, 15
1 dr, 82% ee). Changing the reaction medium from DME to acetone served to increase both reaction efficiency and stereoselectivity (entry 2, 71% yield, 88% ee), and further improvements could be realized through the addition of water (entry 3, 80% yield, 91% ee). We presume that the addition of water allows for rapid capture of the putative silyl-cation that is generated at the final step in the catalytic cycle (see Scheme 1), a species that is often found to be detrimental to organocatalytic systems.5h As might be expected, lowering the reaction temperature to −30 °C provided a modest enhancement in diastereoselectivity, however, the accompanying decrease in reaction efficiency rendered these conditions less favorable (entry 4). The superior levels of induction and efficiency exhibited by amine 1 in acetone at −20 °C to afford α-formyl cyclopentane 5 in 91% ee, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 80% yield, prompted us to select these catalytic conditions for further exploration.
1 dr and 80% yield, prompted us to select these catalytic conditions for further exploration.
Table 1 Enantioselective organo-SOMO cyclization: Initial studies.
As revealed in Table 2, this new enantioselective α-formyl cyclization reaction can be employed to build a diverse range of carbo- and heterocyclic ring systems. With respect to five-membered rings, this amine-catalyzed protocol is tolerant to electronically diverse allylsilane terminators (entries 1–3). Moreover, enantiopure β-chiral aldehydes can also be used for the diastereoselective construction of 1,2-anti, 2,3-anti as well as 1,2-syn, 2,3-anti cyclopentane adducts (entries 2 and 3, 64–73%, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 6
1 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, respectively). It is interesting to note that the induced stereochemistry in both examples is dominated by the catalyst architecture rather than a matched and mismatched pairing with the specific catalyst antipode employed. Cyclohexyl systems can also be accessed with high levels of efficiency and enantiocontrol (entries 4–6, 70–83% yield, ≥90% ee). In this series, dramatic changes in the inherent π-nucleophilicity of the allylsilane unit can also be accommodated (cf. α,β-unsaturated esters and styrenyl olefins, entries 5 and 6, respectively). Moreover, heteroatoms can be readily incorporated into the formyl-allylic silane linker thereby allowing direct access to heterocycles such as tetrahydropyranyl rings (entry 7, 74% yield, >20
1 dr, respectively). It is interesting to note that the induced stereochemistry in both examples is dominated by the catalyst architecture rather than a matched and mismatched pairing with the specific catalyst antipode employed. Cyclohexyl systems can also be accessed with high levels of efficiency and enantiocontrol (entries 4–6, 70–83% yield, ≥90% ee). In this series, dramatic changes in the inherent π-nucleophilicity of the allylsilane unit can also be accommodated (cf. α,β-unsaturated esters and styrenyl olefins, entries 5 and 6, respectively). Moreover, heteroatoms can be readily incorporated into the formyl-allylic silane linker thereby allowing direct access to heterocycles such as tetrahydropyranyl rings (entry 7, 74% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 91% ee). Finally, synthetically challenging enantioenriched seven-membered systems may be accessed using this new organocatalytic cyclization protocol. Efforts to accomplish 7-exo-cyclization resulted in formation of the desired adduct with high levels of enantiocontrol (entry 8, 95% ee), while the corresponding 7-endo cyclization is also possible, albeit with modest levels of enantiocontrol (entry 9, 60% ee). Importantly, for all cases examined in this manuscript, the potentially competitive intramolecular Sakurai-allylation pathway is almost completely suppressed.8
1 dr, 91% ee). Finally, synthetically challenging enantioenriched seven-membered systems may be accessed using this new organocatalytic cyclization protocol. Efforts to accomplish 7-exo-cyclization resulted in formation of the desired adduct with high levels of enantiocontrol (entry 8, 95% ee), while the corresponding 7-endo cyclization is also possible, albeit with modest levels of enantiocontrol (entry 9, 60% ee). Importantly, for all cases examined in this manuscript, the potentially competitive intramolecular Sakurai-allylation pathway is almost completely suppressed.8
Table 2 Enantioselective organo-SOMO cyclization: Reaction scope
Finally, to further demonstrate the value of this new α-allylation cyclization strategy, we focused upon the development of a unique and expeditious route to enantioenriched 3,4-disubstituted piperidine rings, a structural motif that is prevalent in drug design and commercial medicines (e.g. Paxil). As shown in detail in Fig. 1, implementation of our standard cyclization conditions with N-tosyl-linked formyl-allylsilanes enabled the rapid production of the desired trans-substituted 3-vinyl, 4-formyl piperidine rings with excellent levels of enantiocontrol (78–86% yield, 89–91% ee, 11–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Intriguingly, an examination of the impact of olefin geometry on product diastereoselectivity suggests that E-allylsilanes cyclize to form products with slightly higher levels of diastereocontrol than those observed with the corresponding Z-allylsilane substrates, while the reverse trend is observed in enantioselectivity.
1 dr). Intriguingly, an examination of the impact of olefin geometry on product diastereoselectivity suggests that E-allylsilanes cyclize to form products with slightly higher levels of diastereocontrol than those observed with the corresponding Z-allylsilane substrates, while the reverse trend is observed in enantioselectivity.
 |
| | Fig. 1 Catalyst-controlled stereoselective piperidine formation. Isolated yields. Diastereomeric ratio determined by 1H NMR analysis. Enantiomeric excess determined by GC analysis. Reactions performed using Method B. | |
Conclusions
In summary, we have applied the mechanistic paradigm of organo-SOMO catalysis to the successful development of a novel cyclization reaction based upon the enantioselective intramolecular α-allylation of aldehydes. This protocol can be successfully employed to construct five-, six-, and seven-membered carbocycles as well as delivering a new route to tetrahydropyran and piperidine heterocyclic ring systems. We expect this new bond forming technology will find application in the construction of both natural products and medicinal agents. Further studies into the scope of this new transformation are ongoing in our laboratory.
Acknowledgements
Financial support was provided by NIHGMS (R01 GM078201-05) and kind gifts from Merck, Amgen and Abbott. P. V. P. thanks the Vietnamese government for a predoctoral fellowship, and K. A. thanks Merck for a postdoctoral fellowship.
Notes and references
-
(a) H. B. Kagan and O. Riant, Chem. Rev., 1992, 92, 1007–1019 CrossRef CAS;
(b) S. Monfette and D. E. Fogg, Chem. Rev., 2009, 109, 3783–3816 CrossRef CAS.
- For a review of transition metal-catalyzed, asymmetric allylic alkylations, see:
(a) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395–422 CrossRef CAS, and references therein; and for a recent example of a π-allyl palladium approach which employs an achiral organocatalyst, see:
(b) F. Bihelovic, R. Matovic, B. Vulovic and R. N. Saicic, Org. Lett., 2007, 9, 5063–5066 CrossRef CAS.
- For lead references concerning radical cyclizations, see:
(a)
B. B. Snider, “Manganese(III)-Mediated Radical Reactions” in Radicals in Organic Synthesis, Vol. 2, P. Renaud and M. P. Sibi (ed.), Wiley-VCH: 2000, and references therein Search PubMed;
(b)
A.-L. Dhimane, L. Fensterbank, M. Malacria, “Polycyclic Compounds via Radical Cascade Reactions” in Radicals in Organic Synthesis, Vol. 2, P. Renaud and M. P. Sibi (ed.), Wiley-VCH: 2000, and references therein Search PubMed;
(c)
H. Togo, Advanced Free Radical Reactions for Organic Synthesis, Springer: New York, 1999, Vol. 3 Search PubMed, and references therein. For recent examples of radical cyclizations, see:
(d) I. V. Alabugin, V. I. Timokhin, J. N. Abrams, M. Manoharan, R. Abrams and I. Ghiviriga, J. Am. Chem. Soc., 2008, 130, 10984–10995 CrossRef CAS;
(e) A. Servais, M. Azzouz, D. Lopes, C. Courillon and M. Malacria, Angew. Chem., Int. Ed., 2007, 46, 576–579 CrossRef CAS;
(f) F. G.-L. de Turiso and D. P. Curran, Org. Lett., 2005, 7, 151–154 CrossRef;
(g) B. M. Trost, H. C. Shen and J.-P. Surviet, J. Am. Chem. Soc., 2004, 126, 12565–12579 CrossRef CAS;
(h) M. Toyota, M. Yokota and M. Ihara, J. Am. Chem. Soc., 2001, 123, 1856–1861 CrossRef CAS;
(i) H. Tokuyama, T. Yamashita, M. T. Reding, Y. Kaburagi and T. Fukuyama, J. Am. Chem. Soc., 1999, 121, 3791–3792 CrossRef CAS. For reviews of enantioselective radical processes, see:
(j) E. Yoshioka, S. Kohtani and H. Miyabe, Heterocycles, 2009, 79, 229–242 Search PubMed , and references therein;
(k) M. P. Sibi, S. Manyem and J. Zimmerman, Chem. Rev., 2003, 103, 3263–3295 CrossRef CAS, and references therein; for recent examples of enantioselective radical processes, see:
(l) A. Bauer, F. Westkämper, S. Grimme and T. Bach, Nature, 2005, 436, 1139–1140 CrossRef CAS , and references therein;
(m) D. Yang, B.-F. Zheng, Q. Gao, S. Gu and N.-Y. Zhu, Angew. Chem., Int. Ed., 2006, 45, 255–258 CrossRef CAS;
(n) D. Yang, S. Gu, Y.-L. Yan, N.-Y. Zhu and K.-K. Cheung, J. Am. Chem. Soc., 2001, 123, 8612–8613 CrossRef CAS; for reviews of oxidative cyclizations, see:
(o) B. B. Snider, Chem. Rev., 1996, 96, 339–363 CrossRef CAS , and references therein;
(p) for examples of oxidative cyclizations, see: B. B. Snider, J. Y. Kiselgof and B. M. Foxman, J. Org. Chem., 1998, 63, 7945–7952 Search PubMed;
(q) D. Yang, X.-Y. Ye, S. Gu and M. Xu, J. Am. Chem. Soc., 1999, 121, 5579–5580 CrossRef CAS;
(r) D. Yang, X.-Y. Ye, M. Xu, K.-W. Pang and K.-K. Cheung, J. Am. Chem. Soc., 2000, 122, 1658–1663 CrossRef CAS;
(s) D. Yang, M. Xu and M.-Y. Bian, Org. Lett., 2001, 3, 111–114 CrossRef CAS;
(t) Y. Huang and K. D. Moeller, Org. Lett., 2004, 6, 4199–4202 Search PubMed.
- For recent examples of SOMO-catalyzed cyclizations, see:
(a) K. C. Nicolaou, R. Reingruber, D. Sarlah and S. Bräse, J. Am. Chem. Soc., 2009, 131, 2086–2087 CrossRef CAS;
(b) J. C. Conrad, J. Kong, B. N. Laforteza and D. W. C. MacMillan, J. Am. Chem. Soc., 2009, 131, 11640–11641 CrossRef CAS;
(c) J. M. Um, O. Gutierrez, F. Schoenebeck, K. N. Houk and D. W. C. MacMillan, J. Am. Chem. Soc., 2010, 132, 6001–6005 CrossRef CAS.
- For examples of our group's development of SOMO catalysis, see:
(a) T. D. Beeson, A. Mastracchio, J.-B. Hong, K. Ashton and D. W. C. MacMillan, Science, 2007, 316, 582–585 CrossRef CAS;
(b) H.-Y. Jang, J.-B. Hong and D. W. C. MacMillan, J. Am. Chem. Soc., 2007, 129, 7004–7005 CrossRef CAS;
(c) H. Kim and D. W. C. MacMillan, J. Am. Chem. Soc., 2008, 130, 398–399 CrossRef CAS;
(d) M. Amatore, T. D. Beeson, S. P. Brown and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2009, 48, 5121–5124 CrossRef CAS;
(e) J. E. Wilson, A. D. Casarez and D. W. C. MacMillan, J. Am. Chem. Soc., 2009, 131, 11332–11334 CrossRef CAS;
(f) S. Rendler and D. W. C. MacMillan, J. Am. Chem. Soc., 2010, 132, 5027–5029 CrossRef CAS;
(g) N. T. Jui, E. C. Y. Lee and D. W. C. MacMillan, J. Am. Chem. Soc., 2010, 132, 10015–10017 CrossRef CAS;
(h) J. J. Devery III, J. C. Conrad, D. W. C. MacMillan and R. A. Flowers II, Angew. Chem., Int. Ed., 2010, 49, 6106–6110 CrossRef;
(i) A. Mastracchio, A. A. Warkentin, A. M. Walji and D. W. C. MacMillan, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20648–20651 Search PubMed.
- An enamine catalysis cyclization strategy was applied by the List group to construct 3- to 5-membered rings: N. Vignola and B. List, J. Am. Chem. Soc., 2004, 126, 450–451 Search PubMed.
- For the synthesis of allylsilanes, see:
(a) T. K. Sarkar, Synthesis, 1990, 969–983 CrossRef CAS;
(b) T. K. Sarkar, Synthesis, 1990, 1011–1111 Search PubMed;
(c) W. E. Crowe, D. R. Goldberg and Z. J. Zhang, Tetrahedron Lett., 1996, 37, 2117–2120 CrossRef CAS;
(d) Y. Tsuji, M. Funato, M. Ozawa, H. Ogiyama, S. Kajita and T. Kawamura, J. Org. Chem., 1996, 61, 5779–5787 CrossRef CAS.
- For examples of intramolecular Sakurai reactions with similar starting materials, see:
(a) P. J. Jervis, B. M. Kariuki and R. L. Cox, Org. Lett., 2006, 8, 4649–4652 CrossRef CAS;
(b) M. Schlosser, L. Franzini, C. Bauer and F. Leroux, Chem.–Eur. J., 2001, 7, 1909–1914 Search PubMed;
(c) Identical substrates to those in Table 2, entries 2 and 3 are employed in ref. 8b. In our reaction, less than 20% of the Sakurai by-product is observed in either case. We presume this arises from an acid co-catalyst activation pathway.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, structural proofs, and spectral data for all new compounds are provided. See DOI: 10.1039/c1sc00176k |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 82% ee). Changing the reaction medium from DME to acetone served to increase both reaction efficiency and stereoselectivity (entry 2, 71% yield, 88% ee), and further improvements could be realized through the addition of water (entry 3, 80% yield, 91% ee). We presume that the addition of water allows for rapid capture of the putative silyl-cation that is generated at the final step in the catalytic cycle (see Scheme 1), a species that is often found to be detrimental to organocatalytic systems.5h As might be expected, lowering the reaction temperature to −30 °C provided a modest enhancement in diastereoselectivity, however, the accompanying decrease in reaction efficiency rendered these conditions less favorable (entry 4). The superior levels of induction and efficiency exhibited by amine 1 in acetone at −20 °C to afford α-formyl cyclopentane 5 in 91% ee, 15
1 dr, 82% ee). Changing the reaction medium from DME to acetone served to increase both reaction efficiency and stereoselectivity (entry 2, 71% yield, 88% ee), and further improvements could be realized through the addition of water (entry 3, 80% yield, 91% ee). We presume that the addition of water allows for rapid capture of the putative silyl-cation that is generated at the final step in the catalytic cycle (see Scheme 1), a species that is often found to be detrimental to organocatalytic systems.5h As might be expected, lowering the reaction temperature to −30 °C provided a modest enhancement in diastereoselectivity, however, the accompanying decrease in reaction efficiency rendered these conditions less favorable (entry 4). The superior levels of induction and efficiency exhibited by amine 1 in acetone at −20 °C to afford α-formyl cyclopentane 5 in 91% ee, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 80% yield, prompted us to select these catalytic conditions for further exploration.
1 dr and 80% yield, prompted us to select these catalytic conditions for further exploration.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Isolated yield. b
Isolated yield. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Diastereomeric ratio determined by 1H NMR analysis. c
Diastereomeric ratio determined by 1H NMR analysis. c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Enantiomeric excess determined by GC analysis. 2,6-DTBP: 2,6-di-tert-butylpyridine; DME: 1,2-dimethoxyethane.
Enantiomeric excess determined by GC analysis. 2,6-DTBP: 2,6-di-tert-butylpyridine; DME: 1,2-dimethoxyethane.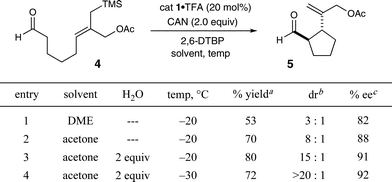
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 6
1 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, respectively). It is interesting to note that the induced stereochemistry in both examples is dominated by the catalyst architecture rather than a matched and mismatched pairing with the specific catalyst antipode employed. Cyclohexyl systems can also be accessed with high levels of efficiency and enantiocontrol (entries 4–6, 70–83% yield, ≥90% ee). In this series, dramatic changes in the inherent π-nucleophilicity of the allylsilane unit can also be accommodated (cf. α,β-unsaturated esters and styrenyl olefins, entries 5 and 6, respectively). Moreover, heteroatoms can be readily incorporated into the formyl-allylic silane linker thereby allowing direct access to heterocycles such as tetrahydropyranyl rings (entry 7, 74% yield, >20
1 dr, respectively). It is interesting to note that the induced stereochemistry in both examples is dominated by the catalyst architecture rather than a matched and mismatched pairing with the specific catalyst antipode employed. Cyclohexyl systems can also be accessed with high levels of efficiency and enantiocontrol (entries 4–6, 70–83% yield, ≥90% ee). In this series, dramatic changes in the inherent π-nucleophilicity of the allylsilane unit can also be accommodated (cf. α,β-unsaturated esters and styrenyl olefins, entries 5 and 6, respectively). Moreover, heteroatoms can be readily incorporated into the formyl-allylic silane linker thereby allowing direct access to heterocycles such as tetrahydropyranyl rings (entry 7, 74% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, 91% ee). Finally, synthetically challenging enantioenriched seven-membered systems may be accessed using this new organocatalytic cyclization protocol. Efforts to accomplish 7-exo-cyclization resulted in formation of the desired adduct with high levels of enantiocontrol (entry 8, 95% ee), while the corresponding 7-endo cyclization is also possible, albeit with modest levels of enantiocontrol (entry 9, 60% ee). Importantly, for all cases examined in this manuscript, the potentially competitive intramolecular Sakurai-allylation pathway is almost completely suppressed.8
1 dr, 91% ee). Finally, synthetically challenging enantioenriched seven-membered systems may be accessed using this new organocatalytic cyclization protocol. Efforts to accomplish 7-exo-cyclization resulted in formation of the desired adduct with high levels of enantiocontrol (entry 8, 95% ee), while the corresponding 7-endo cyclization is also possible, albeit with modest levels of enantiocontrol (entry 9, 60% ee). Importantly, for all cases examined in this manuscript, the potentially competitive intramolecular Sakurai-allylation pathway is almost completely suppressed.8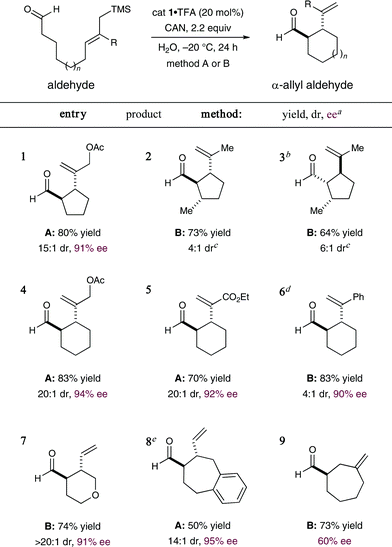
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Intriguingly, an examination of the impact of olefin geometry on product diastereoselectivity suggests that E-allylsilanes cyclize to form products with slightly higher levels of diastereocontrol than those observed with the corresponding Z-allylsilane substrates, while the reverse trend is observed in enantioselectivity.
1 dr). Intriguingly, an examination of the impact of olefin geometry on product diastereoselectivity suggests that E-allylsilanes cyclize to form products with slightly higher levels of diastereocontrol than those observed with the corresponding Z-allylsilane substrates, while the reverse trend is observed in enantioselectivity.

