Desymmetrization of cyclohexadienones viacinchonine derived thiourea-catalyzed enantioselective aza-Michael reaction and total synthesis of (-)-Mesembrine†
Qing
Gu
and
Shu-Li
You
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032, China. E-mail: slyou@sioc.ac.cn; Fax: (+86) 21-5492-5087
First published on 19th May 2011
Abstract
Desymmetrization of cyclohexadienones via aza-Michael reaction catalyzed by cinchonine derived thiourea has been realized to afford a series of highly enantioenriched pyrrolidine and morpholine derivatives in excellent yields and ees. With this newly established methodology, asymmetric total synthesis of (-)-Mesembrine in high enantiomeric excess (98% ee) was accomplished.
Oxidative dearomatization of arenes has attracted considerable attention because it offers a facile introduction of ring systems from cheap aromatics.1 On the other hand, the desymmetrization reaction is one of the most common and powerful methods for enantioselective synthesis of chiral molecules.2 When coupled with the dearomatization process, it provides a facile construction of optically active cyclic and polycyclic compounds from readily available starting materials. Recently, this strategy was demonstrated successfully in asymmetric desymmetrization of cyclohexadienones viaintramolecular Heck reaction,3 Stetter reaction,4 and Michael reaction,5 providing efficient carbon-carbon bond formation methods. Recent efforts from our group led to the desymmetrization of cyclohexadienonevia a chiral phosphoric acid6 catalyzed oxo-Michael reaction,7 providing highly enantioenriched 1,4-dioxane and tetrahydrofuran derivatives.
Development of efficient methods for the synthesis of chiral nitrogen-containing heterocyclic compounds is in great demand because of their frequent appearance in natural products and biologically interesting compounds (Scheme 1).8 Among the methods developed, the asymmetric catalytic aza-Michael reaction has become a simple and useful tool for the preparation of these compounds. Since the first example was introduced by Jørgensen in 1996,9 several asymmetric catalytic systems have been developed for this reaction.10 Despite the fact that highly enantioselective aza-Michael reactions have been known with either chiral amines11 or bifunctional thioureas12 as the catalysts, to our knowledge, the dearomatization/desymmetrization process via asymmetric aza-Michael reaction has not been reported yet (Scheme 2). As part of our ongoing program on asymmetric dearomatization reactions, herein we report the desymmetrization via asymmetric aza-Michael reaction catalyzed by cinchonine derived thiourea and their application in the total synthesis of (-)-Mesembrine.
 | ||
| Scheme 1 Natural products possessing a chiral pyrrolidine and piperidine moiety. | ||
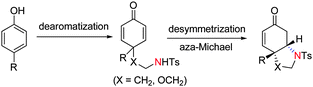 | ||
| Scheme 2 Dearomatization/desymmetrization process via asymmetric aza-Michael reaction. | ||
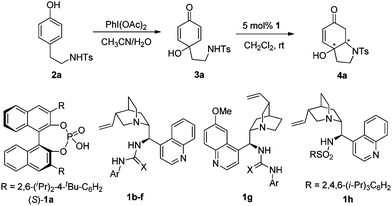
We began our investigation by testing the intramolecular aza-Michael reaction of 3a as the substrate with chiral phosphoric acid 1a as the catalyst. The aza-Michael addition proceeded in complete conversion but the cyclization product 4a was obtained with only moderate enantioselectivity (71% ee, Table 1, entry 1). We then screened cinchona alkaloid derived thioureas 1b–g, well-known as bifunctional catalysts,6b, 13 and sulfonamide 1h. With 5 mol% of thioureas (1b–g) in CH2Cl2 at room temperature, gratifyingly, all reactions proceeded smoothly to give the desired product 4a with yields from 89 to 95% and ee values from 92 to 97% (Table 1, entries 2–7). Thiourea 1b bearing 3,5-(CF3)2C6H3 derived from cinchonine proved to be the most efficient catalyst, affording 4a in 95% yield and 97% ee. Sulfonamide 1h bearing a monodentate hydrogen bond donor was found to be completely ineffective (Table 1, entry 8). Both cinchonine and quinine alone afforded product in moderate yields and ees (24% yield, 29% ee and 75% yield, 29% ee, Table 1, entries 9 and 10, respectively). The results indicate the importance of double hydrogen bonding interactions. Varying solvents disclosed that CH2Cl2 was the optimal one. Further screening of other reaction parameters including substrate concentration, reaction temperature and catalyst loading did not improve the results (for details, see the ESI†).
| Entry | Catalyst 1 (Ar, X) | Time (h) | Yield (%)b | ee (%)c |
|---|---|---|---|---|
| a Reaction conditions: 5 mol% of catalyst, 0.1 mol L−13a in CH2Cl2 at room temperature. b Isolated yields. c Determined by HPLC analysis (chiralpak AD-H). d Conversion determined by 1H NMR. | ||||
| 1 | 1a | 20 | 99 d | 71 |
| 2 | 1b (3,5-(CF3)2C6H3, S) | 12 | 94 | 97 |
| 3 | 1c (C6H5, S) | 60 | 89 | 93 |
| 4 | 1d (4-FC6H4, S) | 36 | 95 | 95 |
| 5 | 1e (4-CF3C6H4, S) | 24 | 95 | 95 |
| 6 | 1f (3,5-(CF3)2C6H3, O) | 20 | 94 | 94 |
| 7 | 1g (3,5-(CF3)2C6H3, S) | 12 | 95 | 92 |
| 8 | 1h | 6 days | NR | ND |
| 9 | cinchonine | 24 | 24d | 29 |
| 10 | quinine | 24 | 75 | 29 |
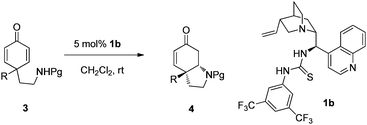
With the optimized conditions in hand, the reaction scope for the synthesis of pyrrolidine derivatives was investigated. The results are summarized in Table 2. As seen from the results, the nitrogen protecting groups have great effect on both reactivity and enantioselectivity (Table 2, entries 1–4). Acidic N–H14 was required for the reactivity since it could be easily deprotonated by the tertiary amine of the bifunctional catalyst 1b. When Ts was used as a protecting group, the best yield and enantioselectivity were obtained (94% yield and 97% ee, Table 2, entry 1). To our great delight, regardless of the substituent R in the 4-position of cyclohexadienones, all the asymmetric aza-Michael reaction proceeded smoothly to afford pyrrolidine derivatives with excellent enantioselectivities (89–99% ees, Table 2, entries 5–11). The substrate 3j, bearing an acetamide substituent, gave the best ee (99% ee, Table 2, entry 10). In addition, substrate 3k, with a bulky aromatic group, also gave the cyclization product in 91% yield and 97% ee (Table 2, entry 11). The ee values of product 4a were invariable during the reaction by examining the ee at different conversions. When racemic 4a was subjected to the optimal conditions with 5 mol% of 1b, the product 4a was recovered as a racemic compound. These two experiments suggest that the aza-Michael addition reaction is not reversible under our conditions. The one-pot dearomatization/desymmetrization process was also explored for the synthesis of 4e. After the dearomatization reaction (N-(4-hydroxyphenethyl)-4-methylbenzenesulfonamide with PhI(OAc)2 in MeOH), the solvent was removed and then the residue was treated with 5 mol% of 1b in CH2Cl2. The product 4e was obtained in 28% overall yield and 95% ee.
| Entry | 3: R, Pg | Time (h) | Yield (%)b | ee (%)c |
|---|---|---|---|---|
| a Reaction conditions: 5 mol% of 1b, 0.1 mol L−13 in CH2Cl2. b Isolated yields. c Determined by HPLC analysis. d 10 mol% of 1b. | ||||
| 1 | 3a: OH, p-Ts | 12 | 94 | 97 |
| 2 | 3b: OH, Ms | 72 | 26 | 80 |
| 3 | 3c: OH, p-Ns | 60 | 75 | 87 |
| 4 | 3d: OH, Boc | 72 | NR | ND |
| 5 | 3e: OMe, Ts | 10 | 83 | 94 |
| 6 | 3f: OEt, Ts | 24 | 89 | 90 |
| 7d | 3g: O(CH2)2OH, Ts | 15 | 88 | 89 |
| 8 | 3h: O(CH2)3OH, Ts | 36 | 80 | 97 |
| 9 | 3i: OAc, Ts | 48 | 75 | 89 |
| 10 | 3j: NHAc, Ts | 24 | 70 | 99 |
| 11 | 3k: 3,4-(MeO)2C6H3, Ts | 10 | 91 | 97 |
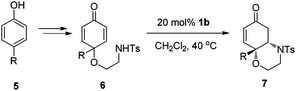
For substrate 6a, readily prepared from 4-phenyl phenol, the desired product was obtained in a low yield (20%) but with high ee (97%) under the above reaction conditions. After further optimizing the reaction conditions (for details, see the ESI†), the aza-Michael adduct 7a was obtained in excellent yield and ee when the reaction was carried with 20 mol% of 1b in CH2Cl2 at 40 °C. As summarized in Table 3, the reaction was general for substrates 6 bearing different aromatic groups at the 4-position. The substrates 6a–g, containing either an electron-donating group or an electron-withdrawing group on the phenyl ring, all led to their desired cyclization products in 76–97% yields and 93–98% ees (Table 3, entries 1–7). The alkyl group in the 4-position of cyclohexadienones can also be well tolerated and the bulkiness of the group has great influence on the reactivity (Table 3, entries 8–11). The reactivity was significantly decreased when sterically more hindered group was introduced (Me, 96% yield; Et, 97% yield; i-Pr, 73% yield; t-Bu, NR). In the case of substrates 6h–j, excellent ees were obtained for the cyclization products.
| Entry | 6: R | Time (h) | Yield (%)b | ee (%)c |
|---|---|---|---|---|
| a Reaction conditions: 20 mol% of 1b, 0.5 mol L−16 in CH2Cl2 at 40 °C. b Isolated yields. c Determined by HPLC analysis. | ||||
| 1 | 6a: C6H5 | 72 | 82 | 97 |
| 2 | 6b: 2-MeC6H4 | 84 | 76 | 95 |
| 3 | 6c: 3-MeC6H4 | 84 | 79 | 98 |
| 4 | 6d: 4-MeC6H4 | 72 | 96 | 96 |
| 5 | 6e: 4-FC6H4 | 72 | 97 | 98 |
| 6 | 6f: 4-ClC6H4 | 72 | 96 | 96 |
| 7 | 6g: 4-BrC6H4 | 72 | 84 | 93 |
| 8 | 6h: Me | 60 | 96 | 97 |
| 9 | 6i: Et | 72 | 97 | 97 |
| 10 | 6j: i-Pr | 96 | 73 | 96 |
| 11 | 6k: t-Bu | 96 | NR | ND |
The multifunctionalized products obtained here could undergo versatile transformations. As shown in Scheme 3, product 4g bearing another nucleophilic moiety can undergo oxo-Michael addition in the presence of catalytic amount of p-TsOH affording a spiro-tricyclic compound 8. Hydrogenation of 4a afforded ketone 10 in 95% yield. In both cases, the products were obtained without loss of the optical purity. To determine the absolute configuration of the products, crystals of enantiopure 9 (>99% ee after recrystallization, Scheme 3) derived from 4a were obtained and single crystal X-ray analysis revealed the configuration to be (3aS, 7aS) (see the ESI†).
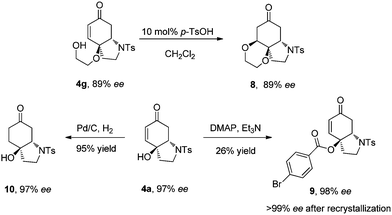 | ||
| Scheme 3 Derivatization of the aza-Michael adducts. | ||
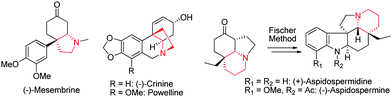
(-)-Mesembrine,15 isolated as the major alkaloid component of Sceletium tortuosum, has been found to have potent serotonin re-uptake inhibitor activity.16 Due to its interesting biological activity, (-)-Mesembrine has received extensive synthetic studies during the past several decades.17 The main challenge in the synthesis of (-)-Mesembrine is the installation of sterically congested chiral arylated quaternary carbon center. Although remarkable progress has been made for its enantioselective total syntheses, most of them employed chiral auxiliaries strategy17b–n rather than asymmetric catalysis.17o–t As a further demonstration of the utility of our newly developed desymmetrization process via intramolecular aza-Michael reaction, a concise total synthesis of (-)-Mesembrine was developed as shown in Scheme 4. The product 4k was hydrogenated under 1 atm H2 at room temperature to afford saturated ketone 14 in 91% yield. Subjecting ketone 14 to NaBH4 in CH3OH, followed by removal of the tosyl group using sodium naphthalenide, N-methylation,18 and Jones oxidation, led to (-)-Mesembrine in 35% yield over four steps with 98% ee. The synthetic (-)-Mesembrine has spectral characteristics identical to those reported in the literatures.
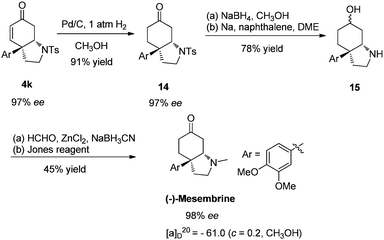 | ||
| Scheme 4 Total synthesis of (-)-Mesembrine. | ||
In summary, we have developed cinchonine-derived thiourea catalyzed desymmetrization of cyclohexadienones via asymmetric aza-Michael reaction, affording a series of highly enantioenriched pyrrolidine and morpholine derivatives in excellent yields and ees. In addition, the utility of this method has been demonstrated in the total synthesis of (-)-Mesembrine. The application of the current methods in the total syntheses of other natural products is currently underway in our lab.
Acknowledgements
We thank the NSFC (20732006, 20821002, 21025209, 21002111), MOST (973 Program 2010CB833300) for financial support and CNIBR for a postdoctoral fellowship to Q. Gu.Notes and references
- For reviews: (a) S. Quideau, L. Pouységu and D. Deffieux, Synlett, 2008, 467 CrossRef CAS; (b) L. Pouységu, D. Deffieux and S. Quideau, Tetrahedron, 2010, 66, 2235 CrossRef CAS; Selected recent examples: (c) T. A. Wenderski, S. Huang and T. R. R. Pettus, J. Org. Chem., 2009, 74, 4104 CrossRef CAS; (d) L. Pouységu, T. Sylla, T. Garnier, L. B. Rojas, J. Charris, D. Deffieux and S. Quideau, Tetrahedron, 2010, 66, 5908 Search PubMed; (e) T. A. Wenderski, C. Hoarau, L. Mejorado and T. R. R. Pettus, Tetrahedron, 2010, 66, 5873 CrossRef CAS.
- For reviews: (a) E. García-Urdiales, I. Alfonso and V. Gotor, Chem. Rev., 2005, 105, 313 CrossRef; (b) A. Studer and F. Schleth, Synlett, 2005, 3033 CrossRef CAS.
- (a) Y. Sato, M. Sodeoka and M. Shibasaki, J. Org. Chem., 1989, 54, 4738 CrossRef CAS; (b) R. Imbos, A. J. Minnaard and B. L. Feringa, J. Am. Chem. Soc., 2002, 124, 184 CrossRef CAS.
- Q. Liu and T. Rovis, J. Am. Chem. Soc., 2006, 128, 2552 CrossRef CAS.
- (a) Y. Hayashi, H. Gotoh, T. Tamura, H. Yamaguchi, R. Masui and M. Shoji, J. Am. Chem. Soc., 2005, 127, 16028 CrossRef CAS; (b) N. T. Vo, R. D. M. Pace, F. O'Hara and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 404 CrossRef CAS.
- For reviews: (a) T. Akiyama, Chem. Rev., 2007, 107, 5744 CrossRef CAS; (b) X. Yu and W. Wang, Chem.–Asian J., 2008, 3, 516 CrossRef CAS; (c) G. Adair, S. Mukherjee and B. List, Aldrichim. Acta, 2008, 41, 31 CAS; (d) M. Terada, Synthesis, 2010, 1929 CrossRef CAS.
- Q. Gu, Z.-Q. Rong, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2010, 132, 4056 CrossRef CAS.
- For reviews: (a) G. A. Cordell, The alkaloid; Academic Press: New York, 1998. Vol. 51 Search PubMed; (b) P. Pelmutter, Conjugate Addition Reactions in Organic Synthesis; Pergamon: Oxford, 1992 Search PubMed; (c) C. Kibayashi, Chem. Pharm. Bull., 2005, 53, 1375 CrossRef CAS; (d) T. Naito, Chem. Pharm. Bull., 2008, 56, 1367 CrossRef CAS.
- L. Falborg and K. A. Jørgensen, J. Chem. Soc., Perkin Trans. 1, 1996, 1, 2823 Search PubMed.
- For reviews: (a) J. L. Vicario, D. Badía, L. Carrillo, J. Etxebarria, E. Reyes and N. Ruiz, Org. Prep. Proced. Int., 2005, 37, 513 CrossRef CAS; (b) L.-W. Xu and C.-G. Xia, Eur. J. Org. Chem., 2005, 633 CrossRef CAS; (c) J. L. Vicario, D. Badía and L. Carrillo, Synthesis, 2007, 2065 CrossRef CAS; (d) D. Enders, C. Wang and J. X. Liebich, Chem.–Eur. J., 2009, 15, 11058 CrossRef CAS; (e) P. R. Krishna, A. Sreeshailam and R. Srinivas, Tetrahedron, 2009, 65, 9657 CrossRef CAS; Selected recent examples: (f) Z. Feng, Q.-L. Xu, L.-X. Dai and S.-L. You, Heterocycles, 2010, 80, 765 Search PubMed; (g) Q. Cai, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2010, 49, 8666 CrossRef CAS.
- Selected examples catalyzed by chiral amines: (a) Y. K. Chen, M. Yoshida and D. W. C. MacMillan, J. Am. Chem. Soc., 2006, 128, 9328 CrossRef CAS; (b) J. Vesely, I. Ibrahem, G.-L. Zhao, R. Rios and A. Córdova, Angew. Chem., Int. Ed., 2007, 46, 778 CrossRef CAS; (c) P. Dinér, M. Nielsen, M. Marigo and K. A. Jørgensen, Angew. Chem., Int. Ed., 2007, 46, 1983 CrossRef; (d) H. Jiang, J. B. Nielsen, M. Nielsen and K. A. Jørgensen, Chem.–Eur. J., 2007, 13, 9068 CrossRef CAS; (e) X. Lu and L. Deng, Angew. Chem., Int. Ed., 2008, 47, 7710 CrossRef CAS; (f) F. Pesciaioli, F. D. Vincentiis, P. Galzerano, G. Bencivenni, G. Bartoli, A. Mazzanti and P. Melchiorre, Angew. Chem., Int. Ed., 2008, 47, 8703 CrossRef CAS; (g) D. Enders, C. Wang and G. Raabe, Synthesis, 2009, 4119 CAS; (h) S. Gogoi, C.-G. Zhao and D. Ding, Org. Lett., 2009, 11, 2249 CrossRef CAS; (i) G. Luo, S. Zhang, W. Duan and W. Wang, Synthesis, 2009, 1564 Search PubMed; (j) L. Hong, W. Sun, C. Liu, L. Wang and R. Wang, Chem.–Eur. J., 2010, 16, 440 CrossRef CAS.
- Selected examples catalyzed by bifunctional thioureas: (a) J. Wang, L. Zu, H. Li, H. Xie and W. Wang, Synthesis, 2007, 2576 CrossRef CAS; (b) M. P. Sibi and K. Itoh, J. Am. Chem. Soc., 2007, 129, 8064 CrossRef CAS; (c) D. Pettersen, F. Piana, L. Bernardi, F. Fini, M. Fochi, V. Sgarzani and A. Ricci, Tetrahedron Lett., 2007, 48, 7805 CrossRef CAS; (d) D. Enders, D. P. Göddertz, C. Beceño and G. Raabe, Adv. Synth. Catal., 2010, 352, 2863 Search PubMed; (e) L. Lykke, D. Monge, M. Nielsen and K. A. Jørgensen, Chem.–Eur. J., 2010, 16, 13330 Search PubMed; (f) X. Liu and Y. Lu, Org. Biomol. Chem., 2010, 8, 4063 RSC; (g) X. Liu and Y. Lu, Org. Lett., 2010, 12, 5592 Search PubMed.
- For reviews: (a) Y. Takemoto, Org. Biomol. Chem., 2005, 3, 4299 RSC; (b) M. S. Taylor and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 1520 CrossRef CAS; (c) T. Akiyama, J. Itoh and K. Fuchibe, Adv. Synth. Catal., 2006, 348, 999 CrossRef CAS; (d) S. J. Connon, Chem.–Eur. J., 2006, 12, 5418 CrossRef; (e) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713 CrossRef CAS; (f) S. J. Connon, Chem. Commun., 2008, 2499 RSC; (g) Y. Takemoto, Chem. Pharm. Bull., 2010, 58, 593 CrossRef CAS.
- (a) F. G. Bordwell and D. Algrim, J. Org. Chem., 1976, 41, 2507 CrossRef CAS; (b) C. Gimbert, M. Moreno-Mañas, E. Pérez and A. Vallribera, Tetrahedron, 2007, 63, 8305 CrossRef CAS; (c) R. A. Scherrer and S. F. Donovan, Anal. Chem., 2009, 81, 2768 CrossRef CAS.
- (a) A. Popelak, E. Haack, G. Lettenbauer and H. Spingler, Naturwissenschaften, 1960, 47, 156 Search PubMed; (b) E. Smith, N. Hosansky, M. Shamma and J. B. Moss, Chem. Ind., 1961, 402 Search PubMed.
- N. P. Gericke and B.-E. VanWyk, PCT Int. Appl., WO 9746234 CAN 128
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80030, 1997 Search PubMed.
80030, 1997 Search PubMed. - For a review: (a) Y. Zhao, Y. Zhou, F. Du, L. Liang and H. Zhang, Chin. J. Org. Chem., 2010, 30, 47 Search PubMed; For chiral auxiliary strategy: (b) S.-I. Yamada and G. Otani, Tetrahedron Lett., 1971, 12, 1133 Search PubMed; (c) H. F. Strauss and A. Wiechers, Tetrahedron Lett., 1979, 20, 4495 Search PubMed; (d) A. I. Meyers, R. Hanreich and K. T. Wanner, J. Am. Chem. Soc., 1985, 107, 7776 CrossRef CAS; (e) S. Takano, K. Samizu and K. Ogasawara, Chem. Lett., 1990, 1239 CAS; (f) T. Yokomatsu, H. Iwasawa and S. Shibuya, Tetrahedron Lett., 1992, 33, 6999 CrossRef CAS; (g) H. Kosugi, Y. Miura, H. Kanna and H. Uda, Tetrahedron: Asymmetry, 1993, 4, 1409 CrossRef CAS; (h) S. E. Denmark and L. R. Marcin, J. Org. Chem., 1997, 62, 1675 CrossRef CAS; (i) P. I. Dalko, V. Brun and Y. Langlois, Tetrahedron Lett., 1998, 39, 8979 CrossRef CAS; (j) D. F. Taber and T. D. Neubert, J. Org. Chem., 2001, 66, 143 CrossRef CAS; (k) T. Paul, W. P. Malachowski and J. Lee, Org. Lett., 2006, 8, 4007 CrossRef CAS; (l) M. Saito, J.-i. Matsuo and H. Ishibashi, Tetrahedron, 2007, 63, 4865 CrossRef CAS; (m) E. A. Ilardi, M. J. Isaacman, Y.-c. Qin, S. A. Shelly and A. Zakarian, Tetrahedron, 2009, 65, 3261 CrossRef CAS; (n) L. A. Tuan and G. Kim, Tetrahedron Lett., 2010, 51, 2354 CrossRef CAS; For asymmetric catalysis strategy: (o) H. Nemoto, T. Tanabe and K. Fukumoto, Tetrahedron Lett., 1994, 35, 6499 CrossRef CAS; (p) H. Nemoto, T. Tanabe and K. Fukumoto, J. Org. Chem., 1995, 60, 6785 Search PubMed; (q) T. Yoshimitsu and K. Ogasawara, Heterocycles, 1996, 42, 135 CrossRef CAS; (r) M. Mori, S. Kuroda, C.-S. Zhang and Y. Sato, J. Org. Chem., 1997, 62, 3263 CrossRef CAS; (s) O. Yamada and K. Ogasawara, Tetrahedron Lett., 1998, 39, 7747 CrossRef CAS; (t) D. F. Taber and Y. He, J. Org. Chem., 2005, 70, 7711 CrossRef CAS.
- Z. L. Song, B. M. Wang, Y. Q. Tu, C. A. Fan and S. Y. Zhang, Org. Lett., 2003, 5, 2319 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and analysis data for new compounds, CIF file of 9. CCDC reference number 805906. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1sc00083g |
| This journal is © The Royal Society of Chemistry 2011 |