A breakthrough method for the accurate addition of reagents in multi-step segmented flow processing†
Heiko
Lange
a,
Catherine F.
Carter
a,
Mark D.
Hopkin
a,
Adrian
Burke
b,
Jon G.
Goode
c,
Ian R.
Baxendale
a and
Steven V.
Ley
*a
aInnovative Technology Centre, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, United Kingdom. E-mail: svl1000@cam.ac.uk; Fax: (+44) 1223-336-442
bMettler-Toledo AutoChem, Inc., 7075 Samuel Morse Drive, Columbia, MD 21046, USA
cMettler-Toledo AutoChem UK, 64 Boston Road, Beaumont Leys, Leicester, LE4 1AW, UK
First published on 28th January 2011
Abstract
In order to use segmented chemical flow processing in more complex reaction sequences, a method has been developed to precisely control the addition of reagent streams during multi-step operations. Using in-line infra-red monitoring with a new LabVIEW software application, it is possible to control additional pumps to dispense further reagents in real time based upon the concentration of reaction intermediates. This enables precise mixing with perfect timing thus greatly increasing product quality and enabling segmented chemical flow processing to be used in extended reaction sequences.
Introduction
Flow chemistry and other machine-assisted processing techniques are beginning to have a significant impact on the way molecules are made.1 Over the last decade there have been notable contributions to the field using both commercially available2 and self-made flow reactors,3 demonstrating their utility in conjunction with polymer-supported reagents4 and, more recently, in-line detection tools.5 However, the synthesis of more complex materials such as natural products in flow6via multi-step sequences has seen considerably less development when compared to single-step transformations. This is undoubtedly due to the currently unsolved problem of how to match the addition of sequential reagents, in terms of concentration, with a disperse product stream. Here, we report a breakthrough method to solve this problem using concentration profiles to control further pumps in the synthetic sequence.In-line IR monitoring has proved to be a powerful non-destructive in-line detection method5,7 and is consequently routinely used in our laboratories to determine product conversion,5,7 to measure the dispersion of product streams7a and to obtain mechanistic information.7b–d When performing multi-step sequences in flow, the unavoidable dispersion of the reaction “plug” is a significant issue, particularly when polymer-supported reagents are used either as a stoichiometric or excess component or for in-line purification. Dispersion is dependent on many variables (including diffusion, reactor set-up and interaction of intermediates with polymer-supported reagents) and therefore is difficult to predict without direct in-line measurement. The controlled addition of exact stoichiometries of further reagents to a product stream is therefore challenging, if not impossible, with the current commercially available flow equipment.7a,8,9
Technical solutions to this problem involve using IR or UV-vis detectors to measure the dispersion of a product stream from a single step. The dispersion curve obtained is then used as a visual signal to manually start a pump (C) delivering a third stream of reagent at a predetermined concentration (Scheme 1, top).7a,8
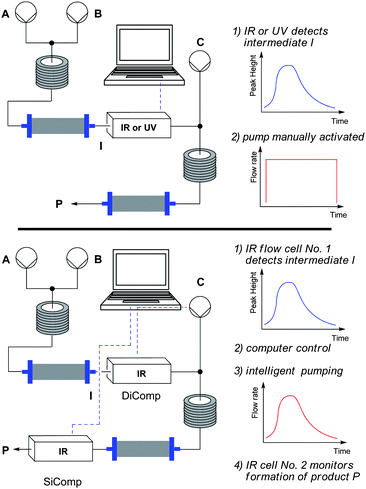 | ||
| Scheme 1 The previous solution: IR/UV detection with manual pump control (top) versus the solution presented here: IR-detector coupled to a computer to control a pump (bottom). | ||
However, this simple on/off method results in a large amount of the third component being wasted when the reaction is performed up to appreciable scales. This is particularly problematic if the component is precious (as is likely to be the case in the synthesis of complex molecules), toxic or hazardous. Additionally, this method is ineffective for reactions requiring an accurate and maintained stoichiometry ratio throughout the dispersion curve.
As the intensity of IR radiation absorbed at a particular wavelength is defined by Beer's law to be proportional to concentration, a much better solution to this “third-stream problem” would be to control the flow rate of the third pump in real-time by using the concentration of a product-specific band measured in situ by an IR flow cell (Scheme 1, bottom). Here we report the development of such a pump control, using the commercially available iC IR software in combination with a LabVIEW application, that facilitates accurate multi-step synthesis in flow for the first time.
Results and discussion
The iC IR software (version 4.1) used to control the ReactIR device is able to export the values of the absorptions measured at selected wavelengths to a Microsoft Excel data sheet via a Mettler-Toledo prototype ActiveX interface in real-time. In order to provide a means of using this data to control a pump (or any other device that can receive commands from a computer), a simple application was developed using National Instruments LabVIEW10 that continuously reads the Excel data (see Supporting Information for details†). This data is then converted into a flow rate by multiplying by an empirical value, the “correlation factor”, that is pre-determined manually. | (1) |
| Flow rate for third pump vpump = fcorr.A | (2) |
Eqn (1) and 2 show the relationship between the peak height of the absorption measured with the IR flow cell and the flow rate of the third pump. A simple concentration screen of the desired intermediate compound can be performed to determine the relationship between peak height and concentration and hence the absorption coefficient, fA (Fig. 1). This was found to be linear within the region of concentrations that are normally used in mesoscale flow chemistry applications. To perform the concentration screen, solutions are injected into the newly developed IR flow cell head (10 μL) with a simple syringe adaptor.11
 | ||
| Fig. 1 Concentration screen for 4-chlorobenzophenone (1) in CH2Cl2. | ||
To investigate the reliability and accuracy of this technology as a proof of concept, a set-up was devised using two inert compounds (as shown in Scheme 2) to determine if a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry could be obtained along a flowing plug of material with varying concentration. Firstly, a concentration screen of 4-chlorobenzophenone (1) was performed as described above (Fig. 1), and the correlation factor used by the LabVIEW program was calculated to be 6630 μL min−1 in order to produce the desired stoichiometry. A solution of 1 was then pumped through a column of PS-Polyol (IRA-743) to disperse the solution. The C
1 stoichiometry could be obtained along a flowing plug of material with varying concentration. Firstly, a concentration screen of 4-chlorobenzophenone (1) was performed as described above (Fig. 1), and the correlation factor used by the LabVIEW program was calculated to be 6630 μL min−1 in order to produce the desired stoichiometry. A solution of 1 was then pumped through a column of PS-Polyol (IRA-743) to disperse the solution. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of 1 (ν = 1659 cm−1) was monitored in the first IR flow cell (DiComp), generating the dispersion curve shown in Scheme 2. The peak height recorded was automatically converted in real-time to a flow rate for a third pump, which dispensed a solution of 3-methyl-4-nitroanisole (2). The second IR flow cell (SiComp), positioned in-line at the outlet of the mixing chip, was used to measure the concentration profiles of both reagents (1 at ν = 1659 cm−1, 2 at ν = 1340 cm−1), which appear to be accurately matched. The absolute ratio of the two components was determined by collecting aliquots of the output of the reactor in fixed intervals (as indicated by the arrows on the SiComp graph in Scheme 2) and analysing them using 1H NMR spectroscopy. The results (Fig. 2) indicated that the desired 1
O stretch of 1 (ν = 1659 cm−1) was monitored in the first IR flow cell (DiComp), generating the dispersion curve shown in Scheme 2. The peak height recorded was automatically converted in real-time to a flow rate for a third pump, which dispensed a solution of 3-methyl-4-nitroanisole (2). The second IR flow cell (SiComp), positioned in-line at the outlet of the mixing chip, was used to measure the concentration profiles of both reagents (1 at ν = 1659 cm−1, 2 at ν = 1340 cm−1), which appear to be accurately matched. The absolute ratio of the two components was determined by collecting aliquots of the output of the reactor in fixed intervals (as indicated by the arrows on the SiComp graph in Scheme 2) and analysing them using 1H NMR spectroscopy. The results (Fig. 2) indicated that the desired 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was closely obtained for the majority of the eluted material. The calculated ratio from the integral of the IR traces (normalised to the 1H NMR ratio obtained from the central aliquot) is also displayed on the graph and shows a very good correlation to the ratios determined via1H NMR spectroscopy. At the leading and trailing edges of the dispersion curve where the concentration of the intermediate compound is much more dilute, the deviation from the desired stoichiometry is likely to be caused by the unavoidable inaccuracy of the piston pump at very low flow rates in conjunction with increased error in the calculated concentration. Nevertheless, the experiment clearly demonstrates that this protocol, designed to enable real-time communication between the IR detector and a third pump, is able to accurately provide the desired stoichiometry for the majority of the material in an automated fashion. To the best of our knowledge there is no other method for accurately matching concentration profiles in this manner and on these reaction scales. This method would also offer a distinct advantage in the event of a reaction failure, since the lowered concentration of intermediate would be accounted for in real-time thus saving potentially precious material (for example, within a natural product synthesis application).
1 stoichiometry was closely obtained for the majority of the eluted material. The calculated ratio from the integral of the IR traces (normalised to the 1H NMR ratio obtained from the central aliquot) is also displayed on the graph and shows a very good correlation to the ratios determined via1H NMR spectroscopy. At the leading and trailing edges of the dispersion curve where the concentration of the intermediate compound is much more dilute, the deviation from the desired stoichiometry is likely to be caused by the unavoidable inaccuracy of the piston pump at very low flow rates in conjunction with increased error in the calculated concentration. Nevertheless, the experiment clearly demonstrates that this protocol, designed to enable real-time communication between the IR detector and a third pump, is able to accurately provide the desired stoichiometry for the majority of the material in an automated fashion. To the best of our knowledge there is no other method for accurately matching concentration profiles in this manner and on these reaction scales. This method would also offer a distinct advantage in the event of a reaction failure, since the lowered concentration of intermediate would be accounted for in real-time thus saving potentially precious material (for example, within a natural product synthesis application).
 | ||
Scheme 2 Proof of principle experiment illustrating precise addition of a third stream with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. 1 stoichiometry. | ||
Pyrazole formation
Equipped with this new level of control, we reinvestigated a previously reported procedure for the segmented flow12 synthesis of pyrazole-based scaffolds. In this synthesis an ynone is first formed from an acid chloride and a terminal acetylene. A further stream of a hydrazine derivative in ethanol is then introduced to meet the output containing the product ynone. The cyclisation event occurs in the second coil, and the product stream is passed through a series of polymer-supported scavengers for in-line purification. In the initial work, the strongly coloured ynone mixture was used as a visual trigger to manually start the third pump. Once all the coloured material had passed through the T-piece, the pump was manually switched off. This led to the unavoidable use of 5–10 equivalents of toxic hydrazine, creating the need for large-scale downstream purification using acidic scavenger resins. Considering that the corresponding batch reaction only requires 1.1 equivalents of hydrazine, our new method would greatly improve this useful process in terms of both safety and economy if the amount of hydrazine could be reduced. Trifluoromethylbenzoyl chloride (3a) was chosen as a previously unused starting material for pyrazole formation. A concentration screen was performed using the intermediate ynone (see Supporting Information†) and the correlation factor for this system was found to be 1790 μL min−1. The advanced set-up (Fig. 3, Scheme 3) consisted of the first IR flow cell (DiComp) inserted after the polyol in-line scavenger and the second IR flow cell (SiComp) positioned after the second series of scavengers. The first run proceeded smoothly on a technical level, with the intended amount of hydrazine reagent (1.5 mmol, 3 equivalents) dispensed via the third stream pump. However, a polymeric impurity, presumably formed from uncontrolled polymerisation of the terminal acetylene at the elevated temperatures applied in the first step, was observed. Reducing the temperature of the first coil to 80 °C in order to suppress polymerisation and the introduction of a column of silica gel at the end of the flow set-up significantly improved the in-line work-up procedure. Product formation was now observed in the second IR flow cell (SiComp) before any coloured material began to exit the reactor. Further increasing the amount of silica gel allowed us to perform an in-line chromatographic separation, resulting in the simple isolation of nearly colourless crystalline pyrazole 5a in 69% yield after the output solution was left to stand. | ||
| Fig. 3 Top: Set-up for pyrazole formation incorporating IR control for third stream addition. Bottom: Screen shot showing the correlation between peak-height variation and the flow rate of the third pump. | ||
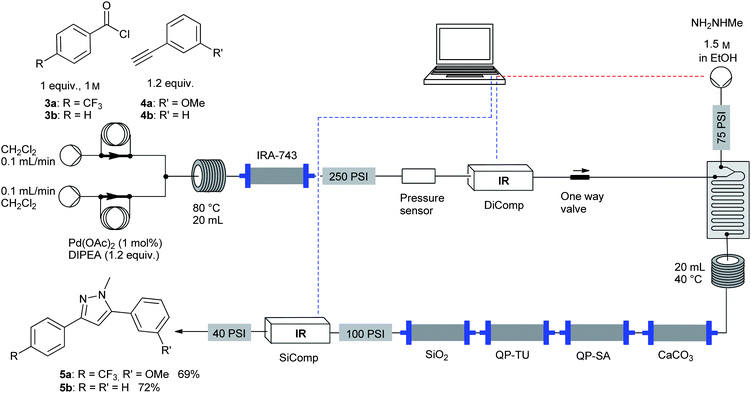 | ||
| Scheme 3 Optimised set-up for the machine-assisted synthesis of pyrazoles. | ||
Pleasingly, the set-up worked well for an alternative pyrazole, giving 5b in 72% yield. Whilst this is less than previously reported (88%12), the product was isolated in much higher purity using only 3 equivalents of hydrazine.
The output from the IR cell could theoretically be used to control other devices that can be attached to a computer via an appropriate interface. The data from the second IR cell could then potentially be used to control a fraction collector after the chromatography step, resulting in a fully automated process for the synthesis of pure pyrazole products.
Admittedly, having to perform a concentration screen for each intermediate is not ideal. However, it is a reliable way of having absolute control and accuracy within the flow process. Additionally, when performing multi-step sequences in flow the intermediates are often isolated as a matter of course, providing ready access to the material.
Reduction crotylation sequence
We next decided to apply the new method to an ongoing field of research in our group; the application of flow chemistry to complex organic transformations requiring low temperatures and inert conditions.13 One such example is the Roush crotylation, whereby an aldehyde is mixed with a stream of boronate reagent 7 to yield propionate relationships. Closely connected to this transformation is the reduction of an ester to the aldehyde oxidation level, as it delivers the starting materials that can be sensitive and inappropriate for storage. Using the precise control over stoichiometry and temperature within a flow process, we can now combine the two reactions such that the aldehyde is immediately consumed and requires no manual handling.Consequently, a process was designed in which the aldehyde signal (ν = 1737 cm−1, fcorr. = 8379 μL min−1) was monitored such that a stream of crotylation reagent could be precisely added, thus avoiding wasting the precious chiral reagent. The set-up (Scheme 4) uses two newly developed Vapourtec low temperature reactors, and two columns of IRA-743 resin to chelate and scavenge both the aluminium and boron residues. The entire flow process is completed in approximately three hours, and yields olefin 8 in 78% from ester 6. This should be contrasted to a full day's work that is required for performing the two individual transformations using conventional batch methods and purifications. The throughput of the flow process could be improved either by increasing the concentration of the reagent streams, or by scaling out with a larger reactor. While both of these reactions are individually useful flow processes,13 telescoping them to a single sequence is very powerful and represents a significant advance over the batch procedures.
 | ||
| Scheme 4 Telescoped reduction–Roush crotylation sequence using IR-controlled addition of the boronate reagent (7). | ||
Conclusions
A software based application has been developed that enables the flow rate of a pump to be controlled in real-time as a function of the concentration of a component measured by in-line infra-red spectroscopy. We have demonstrated the accuracy of the technology at providing matched 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry along a flowing stream of varying concentration, together with its application in various syntheses. This higher level of control via in-line monitoring greatly improves our ability to effect multi-step sequences using segmented flow processing.
1 stoichiometry along a flowing stream of varying concentration, together with its application in various syntheses. This higher level of control via in-line monitoring greatly improves our ability to effect multi-step sequences using segmented flow processing.
Acknowledgements
The authors gratefully acknowledge financial support from the Alexander von Humboldt Foundation (HL), the EPSRC (HL, MDH), Pfizer (MDH), AstraZeneca (CFC), the Royal Society (IRB), and the BP 1702 Endowment (SVL).References
- Recent reviews on the concepts, benefits, applications and the tools of flow chemistry: (a) M. Baumann, I. R. Baxendale and S. V. Ley, Mol. Divers., 2010 DOI:10.1007/s11030-010-9282-1; (b) D. Webb and T. F. Jamison, Chem. Sci., 2010, 1, 675 RSC; (c) S. Marrea and K. F. Jensen, Chem. Soc. Rev., 2010, 39, 1183 RSC; (d) T. Illg, P. Löb and V. Hessel, Bioorg. Med. Chem., 2010, 18, 3707 CrossRef CAS; (e) X. Y. Mak, P. Laurino and P. H. Seeberger, Beilstein J. Org. Chem., 2009, 5 (No. 19) Search PubMed; (f) A. Kirschning, Beilstein J. Org. Chem., 2009, 5 (No. 15) Search PubMed; (g) V. Hessel, Chem. Eng. Technol., 2009, 32, 1655 CrossRef CAS; (h) K. Geyer, T. Gustafsson and P. H. Seeberger, Synlett, 2009, 2382 CAS; (i) W. Lin, Y. Wang, S. Wang and H. Tseng, Nano Today, 2009, 4, 470 CrossRef; (j) R. L. Hartman and K. F. Jensen, Lab Chip, 2009, 9, 2495 RSC; (k) I. R. Baxendale, J. J. Hayward, S. Lanners, S. V. Ley and C. D. Smith, in Microreactors in Organic Synthesis and Catalysis (Ed: T. Wirt(h), Wiley-VCH, Weinheim, 2008, Chapter 4.2 Heterogeneous Reactions, pp. 84 Search PubMed; (l) S. Y. Teh, R. Lin, L. H. Hung and A. P. Lee, Lab Chip, 2008, 8, 198 RSC; (m) D. Wilms, J. Klos and H. Frey, Macromol. Chem. Phys., 2008, 209, 343 CrossRef; (n) B. K. Singh, N. Kaval, S. Tomar, E. Van der Eycken and V. S. Parmar, Org. Process Res. Dev., 2008, 12, 468 Search PubMed; (o) C. Wiles and P. Watts, Eur. J. Org. Chem., 2008, 1655 CrossRef CAS; (p) T. Fukuyama, M. T. Rahman, M. Sato and I. Ryu, Synlett, 2008, 151 CAS; (q) J. Yoshida, A. Nagaki and T. Yamada, Chem.–Eur. J., 2008, 14, 7450 CrossRef CAS; (r) P. B. Mason, K. E. Proce, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300 CrossRef; (s) I. R. Baxendale, J. J. Hayward, S. V. Ley and G. K. Tranmer, Chem. Med. Chem., 2007, 2, 768–788 CrossRef CAS.
- Examples: (a) The H-Cube® is available from ThalesNano. http://www.thalesnano.com; (b) The FlowSyn is available from Uniqsis Ltd. http://www.uniqsis.com; (c) Vapourtec R2+ and R4 units are available from Vapourtec Ltd. http://www.vapourtec.co.uk; (d) The ReactIR 45m and the flow cells are available from Mettler-Toledo AutoChem. http://www.mt.com.
- Recent examples from our laboratory: (a) C. H. Hornung, B. Hallmark, M. R. Mackley, I. R. Baxendale and S. V. Ley, Adv. Synth. Catal., 2010, 352, 1736 CrossRef CAS; (b) C. H. Hornung, B. Hallmark, M. Baumann, I. R. Baxendale, S. V. Ley, P. Hester, P. Clayton and M. R. Mackley, Ind. Eng. Chem. Res., 2010, 49, 4576 CrossRef CAS; (c) M. O'Brien, I. R. Baxendale and S. V. Ley, Org. Lett., 2010, 12, 1596 CrossRef CAS; (d) C. H. Hornung, M. R. Mackley, I. R. Baxendale and S. V. Ley, Org. Process Res. Dev., 2007, 11, 399 Search PubMed.
- Examples: (a) I. R. Baxendale, S. V. Ley, M. Nessi and C. Piutti, Tetrahedron, 2002, 58, 6285 CrossRef CAS; (b) I. R. Baxendale, S. V. Ley and C. Piutti, Angew. Chem., 2002, 114, 2298 CrossRef ; Angew. Chem., Int. Ed., 2002. 41, 2194; (c) I. R. Baxendale, S. V. Ley and H. F. Sneddon, Synlett, 2002, 5, 775 CrossRef; (d) S. V. Ley, A. G. Leach and R. I. Storer, J. Chem. Soc., Perkin Trans. 1, 2001, 24, 358 Search PubMed; (e) S. V. Ley, I. R. Baxendale, R. N. Bream, P. S. Jackson, A. G. Leach, D. A. Longbottom, M. Nesi, J. S. Scott, R. I. Storer and S. J. Taylor, J. Chem. Soc., Perkin Trans. 1, 2000, 23, 3815 RSC.
- C. F. Carter, H. Lange, I. R. Baxendale, S. V. Ley, J. Goode, N. Gaunt and B. Wittkamp, Org. Process Res. Dev., 2010, 14, 393 Search PubMed.
- Flow syntheses of natural products including in-line purification protocols: (a) M. Brasholz, J. M. MacDonald, S. Saubern, J. H. Ryan and A. Holmes, Chem.–Eur. J., 2010, 16, 11471 CrossRef CAS; (b) I. R. Baxendale, J. Deeley, C. M. Griffiths-Jones, S. V. Ley, S. Saaby and G. Tranmer, J. Chem. Soc., Chem. Commun., 2006, 2566 RSC; (c) I. R. Baxendale, C. M. Griffiths-Jones, S. V. Ley and G. K. Tranmer, Synlett, 2006, 427.
- (a) C. J. Smith, N. Nikbin, S. V. Ley, H. Lange and I. R. Baxendale, Org. Biomol. Chem., 2010 10.1039/c0ob00815j; (b) Z. Qian, I. R. Baxendale and S. V. Ley, Synlett, 2010, 505; (c) L. Malet-Sanz, J. Madrzak, S. V. Ley and I. R. Baxendale, Org. Biomol. Chem., 2010, 8, 5324–5332 RSC; (d) C. F. Carter, I. R. Baxendale, J. B. J. Pavey and S. V. Ley, Org. Biomol. Chem., 2010, 8, 1588 RSC; (e) C. F. Carter, I. R. Baxendale, M. O'Brien, J. B. J. Pavey and S. V. Ley, Org. Biomol. Chem., 2009, 7, 4594 RSC; (f) S. Suga, M. Okalima, K. Fujiwara and J. Yoshida, J. Am. Chem. Soc., 2001, 123, 7941 CrossRef CAS.
- M. D. Hopkin, I. R. Baxendale and S. V. Ley, Chem. Commun., 2010, 46, 2450 RSC.
- Vapourtec offers a software application which, based on theoretical models, predicts dispersion curves. See: http://www.vapourtec.co.uk.
- LabVIEW is commercially available from National Instruments. http://www.ni.com/labview/.
- We found that it does not make a difference whether the DiComp IR flow cell or the SiComp IR flow cell are used for the pump control feature.
- I. R. Baxendale, S. C. Schou, J. Sedelmeier and S. V. Ley, Chem.–Eur. J., 2010, 16, 89 CrossRef CAS.
- C. F. Carter, H. Lange, D. S. Dakai, I. R. Baxendale and S. V. Ley, Chem.–Eur. J., 2010, 16 Search PubMed , accepted.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, LabVIEW scripts, and detailed analytical data. See DOI: 10.1039/c0sc00603c |
| This journal is © The Royal Society of Chemistry 2011 |

