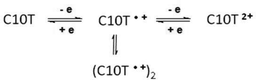Molecular and electronic structure of cyclo[10]thiophene in various oxidation states: polaron pair vs. bipolaron†
Fan
Zhang
a,
Günther
Götz
a,
Elena
Mena-Osteritz
a,
Matthias
Weil
b,
Biprajit
Sarkar
c,
Wolfgang
Kaim
c and
Peter
Bäuerle
*a
aInstitute of Organic Chemistry II and Advanced Materials, University of Ulm, Albert–Einstein–Allee 11, D-89081, Ulm, Germany. E-mail: peter.baeuerle@uni-ulm.de
bInstitute for Chemical Technologies und Analytics, Division of Structural Chemistry, Technical University of Vienna, Getreidemarkt 9/164-SC, A-1060, Vienna, Austria
cInstitute for Inorganic Chemistry, University Stuttgart, Pfaffenwaldring 55, D-70569, Stuttgart, Germany
First published on 15th February 2011
Abstract
The molecular structure of a cyclic oligothiophene, C10T, has been determined by single-crystal X-ray structure analysis. The exclusive syn-conformation of all thiophene units as confirmed in the solid state and the ring strain in this macrocycle result in its unusual and optoelectronic properties. This does not only apply to neutral C10T but also to its oxidized states, as demonstrated by absorption and ESR spectroscopy, supporting the formation of a polaron-pair structure upon oxidation of C10T to C10T2(·+) as has been discussed for linear oligothiophenes. To the best of our knowledge, C10T2(·+) represents an unambiguous example comprising a two-polaron structure (polaron-pair) of a thiophene-based conjugated macrocycle.
π-Conjugated cyclo[n]thiophenes (CnT)1 are of significance not only theoretically as model compounds,2 but also as a novel class of organic (semi)conductors with fascinating optical3 and self-assembling properties.4 However, investigation of this class of aesthetic molecules was limited due to their difficult availability.5 Very recently, we developed a novel effective one-pot synthesis of CnTs.6 A series of CnTs based on a linear quinquethiophene (L5T) building block was obtained in preparative scale, allowing to explore their structural and electronic properties in detail. In this respect, syn- or anti-conformations as well as β-substitution of repeating thiophene units have significant influence on the overall conjugation and consequently on the properties of the macrocycles.2a,7
In this communication, we report the X-ray structure analysis of cyclo[10]thiophene (C10T), the smallest member in the recent series obtained,6 unveiling its molecular structure and conformation in the solid state. In addition, unusual optoelectronic properties of C10T in various oxidation states are described and analyzed in detail by optical absorption and electron spin resonance. In the case of doubly oxidized C10T we were able to address a fundamental question in the physics of charge carriers in organic (semi)conducting systems: the relative stability of a radical cation (polaron) pair vs. a dication (bipolaron).8
Single crystals of C10T suitable for X-ray diffraction measurement were obtained by careful diffusion of n-heptane into a solution of C10T in a chloroform/tetrachloroethane mixture at low temperature, providing the first fully resolved crystallographic structure analysis of a cyclothiophene.† The top view on an individual molecule of C10T revealed a nearly circular shape with inner diameters of about 1.0 nm (Fig. 1, left). In contrast to the prevailing anti-conformation of linear oligothiophenes, in C10T all thiophenes in the centrosymmetric ring adopt a syn-conformation exhibiting interring bond lengths between 1.44(1) to 1.464(9) Å. Ring strain is reflected by the torsional angles of adjacent thiophene units, ranging from 25.9(8)° to 34.0(8)° (side view, Fig. 1, left), far above the values observed for linear oligothiophenes.4d Furthermore, short distances between sulfur atoms (d = 3.13–3.28 Å) in C10T, far below the sum of their van der Waals radii (3.6 Å), were found. Thus, the full syn-arrangement of the thiophene units in C10T and the ring strain lead to consequences in optoelectronic properties: a decrease in oxidation potential and thus a raised HOMO level (Fig. S1 in ESI†).2a,6b,7
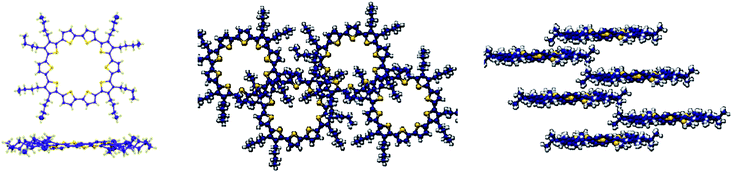 | ||
| Fig. 1 Molecular structure (front and side view, left) and packing view (middle, right) of C10T in the crystal lattice (solvent molecules were omitted for clarity). | ||
The macrocycles in the crystal lattice, stabilized by partly disordered solvent molecules in the cavities, pack in partially overlapping stacks (Fig. 1, right). The distance between macrocycles in one stack amounts to ∼7 Å, however, macrocycles from neighboring stacks significantly overlap with a global shortest distance of 3.41 Å resulting in typical π–π interactions (Fig. 1, middle, right).
In order to gain insight into how the geometric structure influences the optoelectronic properties of C10T in various redox states, absorption spectra in 1,1,2,2-tetrachloroethane were monitored upon stepwise chemical oxidation and compared to the behavior of linear quinquethiophene L5T and decithiophene L10T (Fig. 2, Table 1).
| CnT/LnT | nT | nT+. | (nT+.)2 | nT2+ |
|---|---|---|---|---|
| C10T | 424 (490) | 2052, ∼500 | 1576 | 1334 (1212), 683 (620) |
| L10T | 439 | 2050, 840, 780 | 1705 (1410), 680 (822) | |
| L5T | 385 | 1284, 722 | 1110 (630) | 855 (722, 700), 470 |
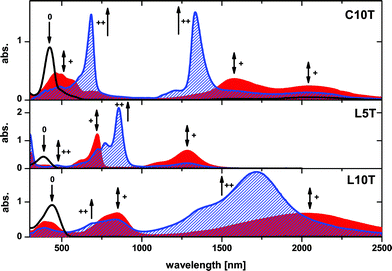 | ||
| Fig. 2 Electronic absorption of C10T (top), linear L5T (middle) and linear L10T (bottom) in 1,1,2,2-tetrachloroethane at room temperature under stepwise oxidation with ThiSbCl6: 0.0 eq. (black), 1.0 eq. (red plain) and 2.0 eq. (blue patterned). “0”, “+”, and “++” denote bands for neutral, radical cationic, and dication species, respectively. | ||
Thianthrenium hexachloridoantimonate(V) (ThiSbCl6) was used as the oxidizing agent because its first oxidation potential (E°1 = 0.83 V vs. Fc/Fc+)9 allows the controlled oxidation of C10T up to the second oxidation level. C10T in its neutral state is characterized by one sharp absorption band peaking at 424 nm and an unresolved shoulder at around 490 nm which we assign to the S2 ← S0 and S1 ← S0 transitions, respectively (Fig. 2 top, black curve).6b Upon addition of 0.5 (not shown) and 1 eq. of ThiSbCl6, the main band of neutral C10T is gradually replaced by three new bands, one broad at 500 nm and two others in the near-infrared with maxima at 2052 nm and 1576 nm, which we assign to radical cation species (red filled curve).10,11 Temperature-dependent measurements revealed that with increasing temperature the intensity of the absorption band at 1576 nm decreases by a simultaneous increase of the absorption intensity at 2052 nm, which is indicative for reversible aggregation of the radical species C10T·+ (Fig. S2 in ESI†). Thus, we assign the longer wavelength band at 2052 nm to the monomeric radical cation C10T·+ and the band at 1576 nm to the dimer (C10·+)2, which previously was also characterized by cyclic voltammetry (CV) studies on C10T.6b The broad absorption band centered at 500 nm for C10T·+ (Fig. 2, middle), which has no analogy in L5T and other linear oligothiophenes,12 can be ascribed to a high energy electronic transition (bg ← au) specific for cyclic compounds due to their higher symmetry related to the linear ones.6b,13 Conversion of neutral C10T into C10T·+ generates an isosbestic point at λ = 457 nm indicating a clean transformation.
Upon further oxidation with 1.5 eq. (not shown) and 2 eq. of ThiSbCl6 (Fig. 2 top, blue curve) to form the dication, the intensities of the three bands of the radical cation species C10T·+/(C10T·+)2 progressively decreased and two very intensive sharp bands appeared at λmax = 683 nm and 1334 nm. The direct and clean conversion of C10T·+/(C10T·+)2 into C10T2+ is indicated by two isosbestic points at λ = 587 nm and 1469 nm. Treatment of monocations C10T·+/(C10T·+)2 or the dication C10T2+ with an excess of hydrazine monohydrate easily allowed the recovery of neutral C10T, indicating the stability of the various charged species and the reversibility of the oxidation processes. The redox behavior of C10T investigated by cyclic voltammetry6b is consistent with the observed changes in the absorption spectra by stepwise oxidation and clearly supports the formation of two distinct reversible electron transfer equilibria.
For the dicationic species, C10T2+, two fundamentally different electronic structures have to be considered:12 a polaron pair configuration, consisting of two locally separated radical cations with minimized charge interaction (diradical alternative), and a bipolaron structure, described as a spinless dication with minimized structural distortion (Fig. 3, right). The polaron pair can exist as singlet or triplet species.12b The optical spectrum of C10T2+ (Fig. 2 top, blue curve), characterized by two equally intense and narrow absorption bands at 1334 and 683 nm, differs substantially from that expected for a bipolaron system, for which typically only one strong absorption band is observed, like in the case of L5T2+ (855 nm) (Fig. 2 middle, blue curve). In stark contrast to C10T2+, the absorption spectrum of the linear analogue L10T2+ shows two broad bands of different intensity at 1705 nm and 680 nm (Fig. 2 bottom, blue curve). In the bipolaron configuration, the interaction of the electrons in the single geometrical conformation leads to a negligible value for the probability coefficient of the second absorption band.12a Thus, the observed single absorption band in the electronic spectrum of L5T2+ indicates a bipolaron structure and is in accordance with previous reports on oligothiophenes.12 In contrast, in the case of a polaron-pair (non-interacting single polarons) distributed over the π-system, similar coefficients for both transitions are expected from theoretical analysis.12a Our experimental findings on C10T2+ completely support these theoretical assumptions, and it can be denoted thus as C10T2(·+). Furthermore, similar absorption energies of the corresponding bands for polaronic species L5T·+ and those of C10T2+, 722 vs. 683 nm and 1284 vs. 1334 nm, respectively (Fig. 2 middle red curve vs. top blue curve), indicate a similarity between their electronic configuration and, as a first hint, the formation of two single polarons in C10T2(·+) confined in two separated structural distortions on the macrocycle. The electronic spectrum of the longer linear oligomer L10T in its doubly oxidized state also reveals as well two absorption bands albeit of different intensities; we attribute these two subgap absorptions to two polarons in separated geometrical distortions (vide infra), which, due to the limited delocalization length, are most probably not independent. This interaction is responsible for the broadness of the bands and the inhomogeneity in their intensity (Fig. 2 bottom, blue curve).13
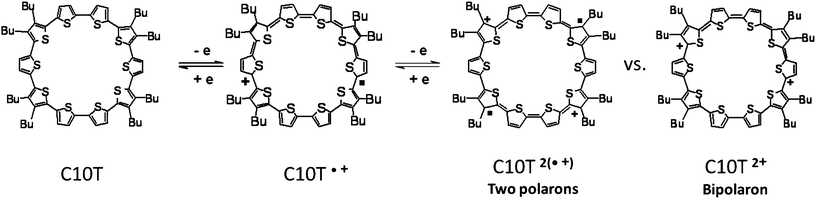 | ||
| Fig. 3 Electronic structure description of neutral C10T, of radical cationic C10T·+ and of the two dicationic species: radical cation pair C10T2(·+) (polaron-pair) and dication C10T2+ (bipolaron). | ||
The bipolaron/polaron pair alternative with singlet or triplet configuration of the latter implies that the absence of an ESR signal is not necessarily a signature of a bipolaron, it could also be caused by a strongly stabilized singlet ground state in a polaron-pair arrangement.12b The ESR spectra of polaron-pairs and diradicals in general can vary considerably, depending on the strength of the exchange interaction between the two electrons.12a For very small exchange interaction, the ESR spectrum of the diradical is equivalent to that of two independent monoradicals. When the exchange interaction increases beyond an observable electron-nuclear hyperfine coupling constant, that hyperfine coupling parameter is halved compared with that of the corresponding monoradical. Strong exchange interaction can produce a singlet diradical ground state14,15 with a close lying triplet excited state.12a
The ESR results for the stepwise oxidation of C10T in 1,1,2,2-tetrachloroethane showed signal intensity at all ratios of oxidant/C10T between 0.5 and 3.5 (Fig. 4). The unresolved ESR line is similar to that of L10T·+ (Fig. 5) and the maximum signal intensity was observed for the ratios 1.0 and 1.5, i.e. for predominantly one-electron oxidized C10T·+ (monoradical). The partial but never complete decrease of ESR intensity for two-electron oxidized C10T and beyond is attributed to formation of C10T2(·+) in both singlet and triplet states. This hypothesis is supported by the temperature-dependence of that signal (Fig. 6) which shows a significant increase, e.g., on going from 293 to 343 K, suggesting a thermally populated triplet state above a singlet ground state. The absence of a half field ESR feature in the frozen state (110 K) confirms this interpretation. An interesting observation is the small but notable monotonous increase of the g factor on addition of oxidant, ranging from 2.0011 (after adding 0.5 eq. ThiSbCl6) to 2.0022 (after addition of 3.5 eq., Fig. 4). We tentatively attribute this effect to an association of the spin-carrying π system with the SbCl6− counter anions which contain heavy elements with high spin–orbit coupling constants.
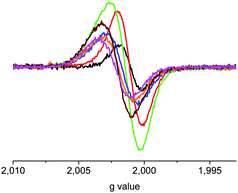 | ||
| Fig. 4 ESR spectra of C10T in 1,1,2,2-tetrachloroethane under stepwise oxidation with ThiSbCl6 at room temperature: 0.5 eq. (black), 1.0 eq. (red), 1.5 eq. (green), 2.0 eq. (blue), 2.5 eq. (orange), 3.0 eq. (brown), 3.5 eq. (pink). | ||
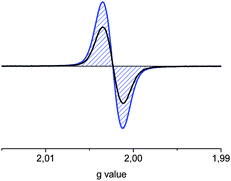 | ||
| Fig. 5 ESR spectra of the mono radical cation (black) and dication (blue) of L10T in 1,1,2,2-tetrachlorethane. | ||
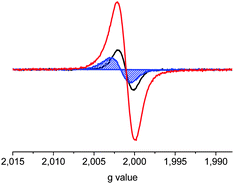 | ||
| Fig. 6 ESR spectra of the radical cation C10T·+ (black) and radical cation pair C10T2(·+) (blue at 295 K and red at 343 K). | ||
The spectral evidence for C10T2(·+) supports a polaron-pair configuration, as in the cases of stabilized dicationic oligothiophenes16 and of the linear analogue L10T2(·+) (Fig. 5), which was also predicted theoretically.8b,c To assure the correct assignment of the species responsible for the various ESR signals, absorption spectra of the solutions were taken before and after the acquisition of the ESR spectra. While the resonance intensities and g-values in the spectra change with stepwise oxidation (vide supra), no annihilation of the ESR signal has been observed. The increase of spin intensity for C10T2(·+) with increasing temperature (Fig. 6) can be attributed either to dimer formation at room temperature, which is neither observed in temperature-dependent absorption spectroscopy of the dication nor supported by theory (vide supra), or to a singlet to triplet state transition of the polaron-pair.12b
In summary, the molecular structure of a cyclic oligothiophene, C10T, has been determined by single-crystal X-ray structure analysis. The exclusive syn-conformation of all thiophene units as confirmed in the solid state and the ring strain in this macrocycle result in its unusual and optoelectronic properties. This does not only apply to neutral C10T6b but also to its oxidized states, as demonstrated by absorption and ESR spectroscopy, supporting the formation of a polaron-pair structure upon oxidation of C10T to C10T2(·+) as has been discussed for linear oligothiophenes. To the best of our knowledge, C10T2(·+) represents an unambiguous example comprising a two-polaron structure (polaron-pair) of a thiophene-based conjugated macrocycle.
This work was supported by the German Research Foundation (DFG) in the frame of Collaborative Research Center (SFB) 569.
Notes and references
- A. Mishra, C.-Q. Ma and P. Bäuerle, Chem. Rev., 2009, 109, 1141 CrossRef CAS.
- (a) S. S. Zade and M. Bendikov, J. Org. Chem., 2006, 71, 2972 CrossRef CAS; (b) S. Fomine and P. Guadarrama, J. Phys. Chem. A, 2006, 110, 10098 CrossRef CAS; (c) J. Fabian and H. Hartmann, J. Phys. Org. Chem., 2007, 20, 554 CrossRef CAS; (d) S. Fomine, P. Guadarrama and P. Flores, J. Phys. Chem. A, 2007, 111, 3124 CrossRef CAS; (e) P. Flores, P. Guadarrama, E. Ramos and S. Fomine, J. Phys. Chem. A, 2008, 112, 3996 CrossRef CAS.
- (a) J. Casado, V. Hernandez, M. C. Ruiz Delgado, J. T. Lopez Navarrete, G. Fuhrmann and P. Bäuerle, J. Raman Spectrosc., 2004, 35, 592 CrossRef CAS; (b) A. Bhaskar, G. Ramakrishna, K. Hagedorn, O. Varnavski, E. Mena-Osteritz, P. Bäuerle and T. Goodson III, J. Phys. Chem. B, 2007, 111, 946 CrossRef CAS; (c) O. Varnavski, P. Bäuerle and T. Goodson III, Opt. Lett., 2007, 32, 3083 Search PubMed.
- (a) E. Mena-Osteritz and P. Bäuerle, Adv. Mater., 2001, 13, 243 CrossRef CAS; (b) E. Mena-Osteritz, Adv. Mater., 2002, 14, 609 CrossRef CAS; (c) E. Mena-Osteritz and P. Bäuerle, Adv. Mater., 2006, 18, 447 CrossRef CAS; (d) R. Azumi, E. Mena-Osteritz, R. Boese, J. Benet-Buchholz and P. Bäuerle, J. Mater. Chem., 2006, 16, 728 RSC.
- (a) J. Krömer, I. Rios-Carreras, G. Fuhrmann, C. Musch, M. Wunderlin, T. Debaerdemaeker, E. Mena-Osteritz and P. Bäuerle, Angew. Chem. Int. Ed., 2000, 39, 3481 CrossRef CAS; (b) G. Fuhrmann, T. Debaerdemaeker and P. Bäuerle, Chem. Commun., 2003, 948 RSC.
- (a) F. Zhang and P. Bäuerle, J. Am. Chem. Soc., 2007, 129, 3090 CrossRef CAS; (b) F. Zhang, G. Götz, H. D. F. Winkler, C. A. Schalley and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 6632 CrossRef CAS.
- B. Jousselme, P. Blanchard, E. Levillain, J. Delaunay, M. Allain, P. Richomme, D. Rondeau, N. Gallego-Planas and J. Roncali, J. Am. Chem. Soc., 2003, 125, 1363 CrossRef CAS.
- (a) A. J. W. Tol, Synth. Met., 1995, 74, 95 CrossRef CAS; (b) A. J. W. Tol, Chem. Phys., 1996, 208, 73 CrossRef CAS; (c) Y. Gao, C.-G. Liu and Y.-S. Jia, J. Phys. Chem. A, 2002, 106, 5380 CrossRef CAS.
- (a) C.-C. You and F. Würthner, J. Am. Chem. Soc., 2003, 125, 9716 CrossRef CAS; (b) A. L. Balch, C. R. Cornman, L. Latos-Grażyński and M. M. Olmstead, J. Am. Chem. Soc., 1990, 112, 7552 CrossRef CAS.
- The reduction product, thianthrene, mainly absorbs lower than 300 nm.
- A. Knorr and J. Daub, Angew. Chem., Int. Ed. Engl., 1997, 36, 2817 CAS.
- (a) J. A. E. H. van Haare, E. E. Havinga, J. L. J. van Dongen, R. A. J. Janssen, J. Cornil and J.-L. Brédas, Chem. Eur. J., 1998, 4, 1509 CrossRef; (b) V. M. Geskin and J.-L. Brédas, ChemPhysChem, 2003, 4, 498 CrossRef CAS.
- M. Bednarz, P. Reineker, E. Mena-Osteritz and P. Bäuerle, J. Lumin., 2004, 110, 225 CrossRef CAS.
- V. Bachler, G. Olbrich, F. Neese and K. Wieghardt, Inorg. Chem., 2002, 41, 4179 CrossRef CAS.
- S. Samanta, P. Singh, J. Fiedler, S. Záliš, W. Kaim and S. Goswami, Inorg. Chem., 2008, 47, 1625 CrossRef CAS.
- T. Nishinaga, A. Wakamiya, D. Yamazaki and K. Komatsu, J. Am. Chem. Soc., 2004, 126, 3163 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Data for sample preparation and measurement, CV and absorption spectra. CCDC reference numbers 779279. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00560f |
‡ Crystal data for compound C10T C77.5H86Cl10.2S10, Mr = 1699.65, red fragment, 0.18 × 0.18 × 0.08 mm3, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 11.0629(4), b = 13.6610(5), c = 15.0564(6) Å, α = 93.282(2), β = 107.469(2), γ = 106.870(2)°, V = 2050.98(13) Å3, Z = 1, ρc = 1.376 kg m−3, μ = 0.643 mm−1, Mo radiation (λ = 0.71073 Å), 100(2) K, 2θmax = 46°, 19882 measured reflections, 5618 independent reflections, Rint = 0.057, R = 0.078 (for 3702 reflections I > 2σ(I)), wR = 0.219 (for all 5618 unique reflections), ρmin = −0.90 e−·Å −3, ρmax = −1.69 e−·Å−3. , a = 11.0629(4), b = 13.6610(5), c = 15.0564(6) Å, α = 93.282(2), β = 107.469(2), γ = 106.870(2)°, V = 2050.98(13) Å3, Z = 1, ρc = 1.376 kg m−3, μ = 0.643 mm−1, Mo radiation (λ = 0.71073 Å), 100(2) K, 2θmax = 46°, 19882 measured reflections, 5618 independent reflections, Rint = 0.057, R = 0.078 (for 3702 reflections I > 2σ(I)), wR = 0.219 (for all 5618 unique reflections), ρmin = −0.90 e−·Å −3, ρmax = −1.69 e−·Å−3. |
| This journal is © The Royal Society of Chemistry 2011 |