Synthesis and properties of the ivyanes: the parent 1,1-oligocyclopropanes†
Gomotsang
Bojase
,
Thanh V.
Nguyen
,
Alan D.
Payne
,
Anthony C.
Willis
and
Michael S.
Sherburn
*
Research School of Chemistry, Australian National University, Canberra, ACT 0200, Australia. E-mail: sherburn@rsc.anu.edu.au; Fax: +61-2-6125-8114
First published on 12th November 2010
Abstract
Practical syntheses of the first six members of the ivyane (1,1-oligocyclopropane) family of fundamental hydrocarbons have been achieved from the cross-conjugated polyenes known as dendralenes. Evidence for helical conformations in both the solid state and in solution is presented. [6]Ivyane exhibits one of the highest experimental heats of combustion recorded for a hydrocarbon. Ring-opening reactions of ivyanes furnish new and interesting structures that are difficult to access by conventional means.
Hydrocarbons continue to provide science with important new theories, structures, reactions and concepts.1Cyclopropane-containing systems are particularly significant in this regard.2 Of the four fundamental hydrocarbon families comprising cyclopropanes (Fig. 1), the “acyclic” 1,1-linked systems are the least well studied.
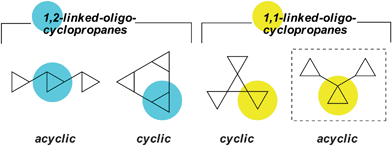 | ||
| Fig. 1 Selected fundamental hydrocarbon families based upon cyclopropanes. The sub-type under scrutiny is in a dashed box. | ||
In fact, of the parent (i.e. unsubstituted) 1,1-linked acyclic oligocyclopropanes, only the tricyclopropane is known. 1,1-Dicyclopropylcyclopropane has been made by several groups3 and substituted analogues are also known.4 Only one publication, however, describes higher “cyclopropanologues”. Thus, in an elegant application of the Matteson homologation, de Mejiere recently reported an iterative approach that produces mixtures of alcohol-substituted 1,1-oligocyclopropanes.5 Unfortunately, an attempt to prepare the unsubstituted systems from these substituted analogues was unsuccessful, with ring opened products being formed.5 Very recently, we reported short preparative syntheses of the first six members of the dendralene family.6 Herein we report the one-step synthesis of the first six members of the unsubstituted 1,1-oligocyclopropanes from the dendralenes. We disclose the physical and chemical properties of these new compounds, which we term the ivyanes, and provide evidence for their helical conformations in both the solid state and in solution.
The dendralenes are the ideal precursors to the ivyanes since, in principle, all that is required is the cyclopropanation of every alkene in the dendralene framework. Encouraged by the reported double cyclopropanation of 1,3-butadiene to bicyclopropyl7 and the single cyclopropanation of 1,1-dicyclopropylethylene to [3]ivyane,3a,b we embarked upon a Simmons–Smith approach. Whereas several different sets of conditions resulted in incomplete conversion and the generation of complex mixtures of products,8 Shi's trifluoroacetic acid-activated zinc carbenoid protocol9 induced a smooth and complete conversion of the complete series of [3]–[8]dendralenes (Scheme 1).
![Practical syntheses of [3]–[8]ivyanes through cyclopropanation of the dendralenes. Reagents and conditions: Et2Zn (1.2 mol equiv. per alkene), CF3CO2H (1.2 mol equiv. per alkene), hexanes–CH2Cl2, 0 °C, 20 min, then CH2I2 (1.2 mol equiv. per alkene), 20 min, then add dendralene (1 mol equiv.), 0 °C to rt, 2 h. a 1.5 mol equiv. of reagents per alkene used. b 18 h reaction time.](/image/article/2011/SC/c0sc00500b/c0sc00500b-s1.gif) | ||
Scheme 1 Practical syntheses of [3]–[8]ivyanes through cyclopropanation of the dendralenes. Reagents and conditions: Et2Zn (1.2 mol equiv. per alkene), CF3CO2H (1.2 mol equiv. per alkene), hexanes–CH2Cl2, 0 °C, 20 min, then CH2I2 (1.2 mol equiv. per alkene), 20 min, then add dendralene (1 mol equiv.), 0 °C to rt, 2 h. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 mol equiv. of reagents per alkene used. b 1.5 mol equiv. of reagents per alkene used. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 h reaction time. 18 h reaction time. | ||
As demonstrated in the Experimental section,10 the procedure lends itself to the gram-scale synthesis of the ivyanes. The efficiency of these reactions is best appreciated by considering the conversion of [8]dendralene into [8]ivyane; the transformation involves the formation of no less than 16 C–C bonds and affords the product in an isolated yield of 81%. The ivyanes are sensitive towards Brønsted acid (a reaction with an acid is discussed later). Interestingly, [3]ivyane appears to be the most acid-sensitive member of the family, and this is reflected in the abnormally low isolated yield of this hydrocarbon following flash column chromatography.
Despite their strain energy (a cyclopropane ring has strain energy of ca. 115 kJ mol−1),11 the ivyanes are kinetically stable to moderate temperatures. [8]Ivyane, for example, melts at 95 °C in air without significant decomposition and [6]ivyane is kinetically stable to ca. 200 °C, as demonstrated from DSC experiments.10 The experimental heat of combustion of [6]ivyane was found to be 50.8 ± 2.5 MJ kg−1 (12.3 ± 0.6 MJ mol−1),10 which is one of the highest reported for a hydrocarbon,12 and is significantly higher than that of cubane.13 The molar heat of combustion of [6]ivyane is roughly six times that of cyclopropane (2.1 MJ mol−1).12 Furthermore, the heats of combustion per kg for these two compounds are very similar (cyclopropane = 49.7 MJ kg−1).12 Thus, the thermodynamic strain energies in isolated and covalently connected cyclopropane rings are comparable, and the connected nature of the cyclopropane rings in the ivyane structure does not lead to a decrease in kinetic stability.
[3]–[6]Ivyanes are oils at ambient temperature but [7]- and [8]ivyanes are solids. Crystals suitable for single-crystal X-ray analysis were grown from n-pentane and the molecular structures of [7]ivyane and [8]ivyane are depicted in Fig. 2.
![Molecular structures of ivyanes based upon coordinates obtained from single-crystal X-ray analyses: (top) molecular structure of [8]ivyane; (bottom) molecular structure of [7]ivyane. Helical chains are highlighted green.14](/image/article/2011/SC/c0sc00500b/c0sc00500b-f2.gif) | ||
| Fig. 2 Molecular structures of ivyanes based upon coordinates obtained from single-crystal X-ray analyses: (top) molecular structure of [8]ivyane; (bottom) molecular structure of [7]ivyane. Helical chains are highlighted green.14 | ||
These first X-ray crystal structures of parent ivyanes (see ESI†) confirm the B3LYP/6-31G(d) predicted (gas phase) helical conformations and are consistent with X-ray structures of substituted [3]-, [4]- and [5]ivyanes reported by de Meijere.5 The gauche conformation between adjacent cyclopropanes means that one turn of the helix involves a five carbon chain. The composition of each crystal studied is racemic, with unit cells containing both enantiomeric structures.
Whereas bicyclopropyl (i.e. [2]ivyane) has long been known to exist as a roughly equimolar mixture of s-trans and gauche conformers in the liquid and gas phase,15 to our knowledge, no information relating to solution-phase conformations of ivyanes has been reported. 13C NMR spectra of [6]ivyane recorded at 25 and −80 °C are reproduced in Fig. 3. As might be expected from a symmetrical structure, the higher temperature spectrum contains six resonances (2 × C, 1 × CH, 3 × CH2). Nevertheless, the broad methylene signal at δ 8.1 ppm betrays exchange on the NMR timescale.
![Broad-band decoupled 13C NMR spectra (125 MHz, CD2Cl2) of [6]ivyane at +25 and −80 °C. Assignments from DEPT experiments.](/image/article/2011/SC/c0sc00500b/c0sc00500b-f3.gif) | ||
| Fig. 3 Broad-band decoupled 13C NMR spectra (125 MHz, CD2Cl2) of [6]ivyane at +25 and −80 °C. Assignments from DEPT experiments. | ||
Indeed, at −80 °C, nine sharp carbon signals are resolved (2 × C, 1 × CH, 6 × CH2), a result consistent with the presence of a less symmetrical (i.e. helical) conformation.16 Interconversion between the two enantiomeric helical conformations (Scheme 2) results in an interchange of the environment of each cyclopropane's pair of methylenes. At the higher temperature, exchange is sufficiently rapid on the NMR timescale that a single averaged methylene resonance is witnessed for each cyclopropane.
![Enantioisomerisation in ivyanes. Interconversion between the two enantiomers is depicted for [6]ivyane, with the helical, six-carbon chain highlighted in green. H atoms are omitted for clarity. Note the interchange of environment for each cyclopropane's pair of methylenes (red tag).14](/image/article/2011/SC/c0sc00500b/c0sc00500b-s2.gif) | ||
| Scheme 2 Enantioisomerisation in ivyanes. Interconversion between the two enantiomers is depicted for [6]ivyane, with the helical, six-carbon chain highlighted in green. H atoms are omitted for clarity. Note the interchange of environment for each cyclopropane's pair of methylenes (red tag).14 | ||
The unique nature of the ivyane structure prompts the question of ring-opening transformations, and preliminary investigations towards this end are depicted in Scheme 3. Thus, regioselective hydrogenolysis of [6]ivyane gives the highly substituted acyclic hydrocarbon 1, bearing four contiguous quaternary centres. Effective routes to such compounds are lacking in the literature.17
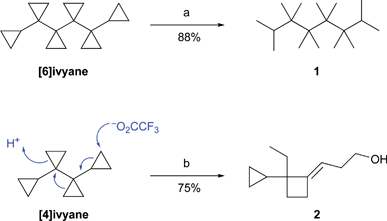 | ||
| Scheme 3 Ring-opening transformations of ivyanes. Reagents and conditions: (a) PtO2 (0.4 mol equiv.), CH3COOH, H2 (1.6 atm), 40 °C, 12 h. (b) (i) CF3COOH (6 mol equiv.), CH2Cl2, rt, 6 h; (ii) KOH, MeOH, rt, 2 h. | ||
On the other hand, treatment of [4]ivyane with trifluoroacetic acid furnishes alcohol 2 after hydrolysis of the corresponding trifluoroacetate.10 This last transformation demonstrates that adjacent cyclopropane rings in the ivyanes can react in a cooperative manner.18
Conclusions
In summary, the 1,1-linked acyclic oligocyclopropane family of fundamental hydrocarbons have been synthesised for the first time. Successful preparations of the first six members of the family involve efficient per-cyclopropanation reactions of the corresponding dendralenes. The ivyanes adopt chiral helical conformations in both solid and solution phases. The simplicity of this synthetic approach should lend itself to the construction of related systems, perhaps including compounds with a barrier towards interconversion that is sufficiently high to allow the isolation of single enantiomeric helices at ambient temperature. Given the importance of helical molecules in nature, ivyanes are clearly worthy of further investigation.Experimental section
Representative procedure: synthesis of [6]ivyane
To a two-neck round bottom flask containing dry dichloromethane (40 mL) at 0 °C under a nitrogen atmosphere was added a 1 M solution of diethyl zinc in hexanes (47.6 mL, 47.6 mmol 7.2 mol equiv.). A solution of trifluoroacetic acid (3.67 mL, 47.6 mmol, 7.2 mol equiv.) in dry dichloromethane (20 mL) was added dropwise and the resulting mixture was vigorously stirred for 20 min. A solution of diiodomethane (3.83 mL, 47.6 mmol, 7.2 mol equiv.) in dichloromethane (20 mL) was added dropwise and the resulting mixture was vigorously stirred for another 20 min. The reaction mixture was maintained at 0 °C during this time. A solution of [6]dendralene (1.04 g, 6.6 mmol, 1.0 mol equiv.) in dichloromethane (20 mL) was then added and the reaction mixture was slowly warmed to rt and stirred for 5 h. The reaction mixture was then quenched with a solution of saturated aqueous NH4Cl (50 mL) and the organic and aqueous layers were separated. The aqueous layer was extracted with more dichloromethane (2 × 20 mL) and the combined organic extracts were washed with water, saturated aqueous NaCl then dried over anhydrous MgSO4 and concentrated in vacuo (20 mbar, 0 °C) to give an oil. The oil was purified by flash chromatography (40 g SiO2, pentane) to give [6]ivyane (1.42 g, 89%) as a colourless oil: Rf 0.60 (pentane); δH (500 MHz, CDCl3): 1.56 (2H, tt, J 8.0, 6.0), 0.33 (4H, m), 0.24 (8H, m), 0.19 (4H, br s), 0.02 (4H, br s), −0.09 (4H, m) ppm; δC (125 MHz, CDCl3): 25.4 (2 × C), 23.5 (2 × C), 15.3 (2 × CH), 8.9 (4 × CH2), 7.4 (4 × CH2), 2.1 (4 × CH2) ppm; νmax (thin film)/cm−1 3079, 3008, 1469, 1018; m/z (40 eV, EI) 242 (M+, 1%), 214 (M+ − C2H4, 72), 185 (70), 91 (100), 79 (92); HRMS: calc. for C18H26 (M+): 242.2035; found 242.2021.Acknowledgements
Funding from the Australian Research Council (ARC) is gratefully acknowledged. We thank A/Prof. Hans Riesen and Dr Barry Gray of the University of New South Wales at the Australian Defence Force Academy, Canberra for assistance in conducting calorimetric analyses.Notes and references
- (a) H. Hopf, in Classics in Hydrocarbon Chemistry: Syntheses, Concepts, Perspectives, Wiley-VCH, Weinheim, 2000 Search PubMed; (b) Carbon-Rich Compounds: From Molecules to Materials, ed. M. M. Haley, R. R. Tykwinski, Wiley-VCH, Weinheim, 2006 Search PubMed.
- (a) A. de Meijere, S. I. Kozhushkov and H. Schill, Chem. Rev., 2006, 106, 4926–4996 CrossRef CAS; (b) A. de Meijere, S. I. Kozhushkov, A. A. Folkin, I. Emme, S. Redlich and P. R. Schreiner, Pure Appl. Chem., 2003, 75, 549–562 CrossRef CAS.
- (a) F. Effenberger and W. Podszun, Angew. Chem., Int. Ed. Engl., 1969, 8, 976 CrossRef CAS; (b) O. M. Nefedov, I. E. Dolgii, I. B. Shvedova and R. N. Shafran, Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1972, 21, 1834–1836 Search PubMed; Izv. Akad. Nauk SSSR Ser. Khim., 1972, 21, 1885–1887 Search PubMed; (c) W. Weber, U. Behrens and A. de Meijere, Chem. Ber., 1981, 114, 1196–1199 CrossRef CAS; (d) T. K. Klindukhova, G. N. Suvorova, L. B. Koroleva and M. I. Komendantov, Zh. Org. Khim., 1984, 20, 529–532 CAS; J. Org. Chem. USSR (Engl. Transl.), 1984, 20, 477–479 Search PubMed.
- (a) S. Nishida, M. Murakami, T. Mizuno and T. Tsuji, J. Org. Chem., 1989, 54, 3868–3872 CrossRef CAS; (b) N. A. Donskaya, A. G. Bessmertnikh and Y. S. Shabarov, Zh. Org. Khim., 1989, 25, 332–339 CAS; J. Org. Chem. USSR (Engl. Transl.), 1989, 25, 295–301 Search PubMed; (c) N. S. Zefirov, K. A. Lukin, S. I. Kozhushkov, T. S. Kuznetsova, A. M. Domarev and I. M. Sosonkin, Zh. Org. Khim., 1989, 25, 312–319 CAS; J. Org. Chem. USSR (Engl. Transl.), 1989, 25, 278–284 Search PubMed; (d) T. Imamoto, Y. Kamiya, T. Hatajima and H. Takahashi, Tetrahedron Lett., 1989, 30, 5149–5152 CrossRef CAS; (e) T. Imamoto, T. Hatajima, N. Takiyama, T. Takeyama, Y. Kamiya and T. Yoshizawa, J. Chem. Soc., Perkin Trans. 1, 1991, 3127–3135 RSC; (f) N. A. Donskaya and A. G. Bessmertnykh, Zh. Org. Khim., 1991, 27, 1681–1685 CAS; J. Org. Chem. USSR (Engl. Transl.), 1991, 27, 1474–1478 Search PubMed; (g) S. Nishida, N. Asanuma, M. Murakami, T. Tsuji and T. Imai, J. Org. Chem., 1992, 57, 4658–4663 CrossRef CAS.
- T. Kurahashi, S. I. Kozhushkov, H. Schill, K. Meindl, S. Rühl and A. de Meijere, Angew. Chem., Int. Ed., 2007, 46, 6545–6548 CrossRef CAS.
- A. D. Payne, G. Bojase, M. N. Paddon-Row and M. S. Sherburn, Angew. Chem., Int. Ed., 2009, 48, 4836–4839 CrossRef CAS.
- (a) M. C. Guerreiro and U. Schuchardt, Synth. Commun., 1996, 26, 1793–1800 CrossRef CAS; (b) G. Wittig and F. Wingler, Chem. Ber., 1964, 97, 2146–2164 CAS; (c) C. G. Overberger and G. W. Halek, J. Org. Chem., 1963, 28, 867–868 CrossRef CAS.
- The original Simmons–Smith procedure (Zn/Cu couple, CH2I2, Et2O, reflux: H. E. Simmons and R. D. Smith, J. Am. Chem. Soc., 1959, 81, 4256–4264 Search PubMed ) and variations led to incomplete conversions, as did attempts involving dihalocarbene additions under PTC conditions (CHX3, Et3NBnCl, NaOH, CH2Cl2: C. M. Starks, J. Am. Chem. Soc., 1971, 93, 195–199 CrossRef CAS ).
- Z. Yang, J. C. Lorenz and Y. Shi, Tetrahedron Lett., 1998, 39, 8621–8624 CrossRef CAS.
- See ESI† for details.
- R. D. Bach and O. Dmitrenko, J. Am. Chem. Soc., 2006, 128, 4598–4611 CrossRef CAS.
- E. S. Domalski, J. Phys. Chem. Ref. Data, 1972, 1, 221–278 CAS ; see ESI† for details.
- The value for cubane is 46.5 MJ kg−1: P. Eaton, N. Nodari, C. Neri, L. Cassar, F. Monti and F. Alberici, Fuel Composition with a High Energy Content, Eur. Pat., 036 4051, 1990 Search PubMed; values for related strained structures are provided in ESI†.
- Molecule visualisations were created using CYLview, v1.0b; C. Y. Legault, Université de Sherbrooke, 2009. http://www.cylview.org (accessed Sept. 22, 2010).
- (a) W. Lüttke, A. de Meijere, H. Wolff, H. Ludwig and H. W. Schrötter, Angew. Chem., Int. Ed. Engl., 1966, 5, 123–124 CrossRef; (b) O. Bastiansen and A. de Meijere, Angew. Chem., Int. Ed. Engl., 1966, 5, 124–125 CrossRef CAS.
- While the proposed helical conformation in solution is consistent with these VTNMR data, we cannot rule out other conformations at this stage.
- For a recent method involving a Hg-mediated radical photodimerisation: S. P. Verevkin, M. Nölke, H.-D. Beckhaus and C. Rüchardt, J. Org. Chem., 1997, 62, 4683–4686 Search PubMed.
- This sequence differs from that witnessed in the rotane series, see: L. Fitjer and D. Wehle, Angew. Chem., Int. Ed. Engl., 1979, 18, 868–869 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data of all new compounds, X-ray crystal structures of [7]- and [8]ivyanes and thermal stability/calorimetry studies on [6]ivyane. CCDC reference numbers 766962 ([7]ivyane) and 766963 ([8]ivyane). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00500b |
| This journal is © The Royal Society of Chemistry 2011 |
