Azomethine ylide annulations: facile access to polycyclic ring systems†
Chen
Zhang
,
Deepankar
Das
and
Daniel
Seidel
*
Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, Piscataway, New Jersey 08854. E-mail: seidel@rutchem.rutgers.edu
First published on 27th October 2010
Abstract
New annulation reactions of azomethine ylides are reported. Amino acids react with aldehydes that are linked to a pronucleophile (e.g. an indole subunit) to provide rapid access to polycyclic ring systems. Simple amines can also be used in place of amino acids.
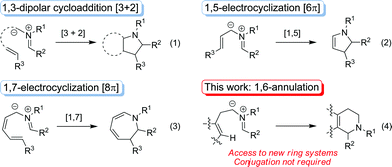
Azomethine ylides are used widely as reactive intermediates in inter- and intramolecular [3 + 2] cycloadditions (eqn (1)).1 While this reaction type is by far the most prevalent, azomethine ylides are known to also engage in 1,5- and 1,7-electrocyclizations (eqn (2) & (3)).2 In contrast, reports on the ability of these dipolar species to participate in non-pericyclic reactions are rare.3 Here we report a new type of azomethine ylide functionalization, namely a C–C bond forming annulation reaction, that results in the formation of six-membered rings (eqn (4)). This unprecedented process does not require a conjugated reaction pathway and significantly enhances the synthetic utility of azomethine ylides.
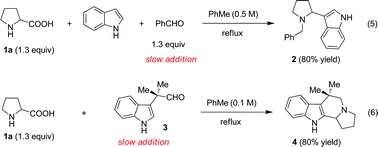
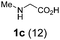
As part of a program aimed at developing redox neutral functionalizations of amines,4 we recently reported decarboxylative three-component coupling reactions of α-amino acids and aldehydes with indoles, naphthols, alkynes and nitroalkanes (eqn (5)).4e, 5–8 These reactions are thought to proceed through protonation of the intermediate azomethine ylide by the pronucleophile (e.g., indole), resulting in the formation of an iminium ion pair that ultimately gives rise to products such as 2.
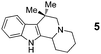
We envisioned that a new type of azomethine ylide annulation may be achieved by linking an aldehyde moiety to a pronucleophile and allowing this species to react with an α-amino acid. This approach would provide rapid access to products that would require several steps if they were to be synthesized by a classic Pictet–Spengler approach.9 Gratifyingly, slow addition (18 h) of indole-aldehyde 3 to proline (1a), heated under reflux in toluene, led to the formation of the desired annulation product 4 in 80% yield (eqn (6)). In this instance, slow addition of one reaction partner is not a strict requirement. The analogous reaction in which 1a and 3 were mixed directly resulted in an only slightly reduced yield of 4 (75%). The scope of this decarboxylative reaction was evaluated with regard to the aldehyde and the amino acid (Table 1).
| Entry | Aldehyde | Amino acid (equiv) | Product | Time/hb | Yield(%) |
|---|---|---|---|---|---|
| a Reactions were performed under reflux in PhMe (entries 1,4,8), in xylenes (entries 2,3,5,7) or in nBuOH (entry 6). b Slow addition time + additional reaction time (entries 1,4,6) or reaction time (all other entries). c under μW irradiation at 250 °C. d under μW irradiation at 200 °C. | |||||
| 1 | 3 |
|
|
18 + 24 | 90 |
| 2 | 3 |
|
|
20 | 61 |
| 3c | 3 |

|
|
20 min | 52 |
| 4 |

|
1a (1.5) |
|
18 + 2 | 79 |
| 5 |

|
1a (2) |
|
1.75 | 52 |
| 6 |

|
1a (3) |

|
18 + 2 | 63 |
| 7d |
|
1a (2) |
|
30 min | 61 |
| 8 |
|
1a (1.5) |
|
30 min | 91 |
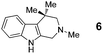
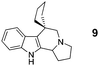
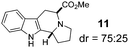
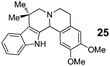
In addition to proline, indole-aldehyde 3 readily underwent reactions with other amino acids (entries 1–3). In the case of amino acid 1d, in addition to the expected product 7, the corresponding regioisomeric product 23 (vide infra) was obtained in 25% yield. This indicates the possibility that the initially formed azomethine ylide might undergo additional isomerisation processes prior to ring closure. The enolizable α-ketoester 10 underwent the annulation with proline to yield product 11 as a mixture of diastereomers.
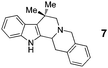
While conjugation is evidently not a requirement in this new annulation process, aldehydes directly linked to conjugated π-systems participated readily in this reaction (entries 6–8). However, the last three examples in Table 1 represent processes that are distinctly different from more common 1,5- or 1,7-electrocyclization reactions. Whereas the intermediates shown in eqn (2) and (3) react through fully conjugated pericyclic reaction pathways, the same is not possible for azomethine ylides derived from 12, 14 or 16.
As an alternative to decarboxylation, the deprotonation of intermediate iminium ions has previously been reported as an entry to azomethine ylides.10 This suggested the use of simple amines and amino acid esters as reaction partners for indole and β-naphthol derived aldehydes. As summarized in Table 2, various combinations of these starting materials gave rise to a diverse set of products that were obtained in generally good yields.
| Entry | Aldehyde | Amine (equiv) | Product | Time/hb | Yield(%) |
|---|---|---|---|---|---|
| a Reactions were performed under reflux in nBuOH (entries 1,2,3,6), in xylenes (entries 4,5,7,8) or PhMe (entry 9). b Slow addition time + additional reaction time (entries 1–3) or reaction time (all other entries). c under μW irradiation at 250 °C. d under μW irradiation at 200 °C. | |||||
| 1 | 14 |

|
|
18 + 1 | 81 |
| 2 | 12 |
|
|
18 + 2 | 78 |
| 3 | 12 |
|

|
18 + 2 | 54 |
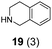
| 4c | 3 | 19 (3) |

|
20 min | 64 |
| 5d | 3 |

|
|
20 min | 61 |
| 6c | 3 |
|
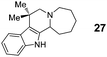
|
5 | 43 |
| 7c | 3 |

|

|
1 | 54 |
| 8c | 3 |
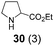
|
|
20 min | 73 |
| 9 | 16 | 19 (3) |

|
30 min | 81 |
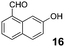
The examples shown in Tables 1 and 2 represent a broad range of different substrate reactivities. In general, reaction times can be significantly shortened by the application of microwave irradiation. In some cases, the use of microwave irradiation enabled the efficient preparation of products whose formation was found to be very sluggish under reflux conditions (e.g., products 23, 27 and 29). For some substrate combinations, slow addition of the aldehyde under reflux conditions resulted in higher yields of product. The superior reactivity of 7-hydroxy-1-naphthaldehyde (16) may be related to the higher acidities of naphthols compared to indoles. This higher acidity would be expected to facilitate the azomethine ylide protonation step.
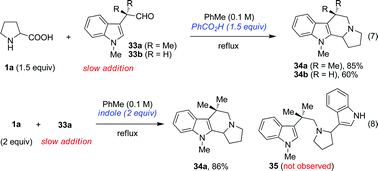
In order for the annulation to proceed under the standard reaction conditions, the nucleophile must bear a moderately acidic proton. For instance, no reaction was observed between proline and either 33a or 33b. However, these reactions proceeded smoothly in the presence of benzoic acid to provide compounds 34 (eqn (7)). The acid serves to protonate the azomethine ylide to form an iminium ion that subsequently cyclizes to product.4e In an experiment designed to test potentially competing inter- and intramolecular reaction pathways, 33a was allowed to react with proline in the presence of excess indole (eqn (8)). Interestingly, no product 35 was obtained that would have been the result of an intermolecular reaction. Apparently, added indole assumes the role of an external acid, enabling the formation of 34a.
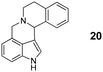
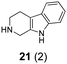
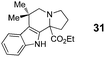
In summary, we have developed a new 1,6-annulation reaction for azomethine ylides. The utility of this process was demonstrated by the rapid generation of various polycyclic ring systems, including analogues of the alkaloid harmicine (products 4, 9, 11, 31 and 34).11Azomethine ylide annulations are expected to find widespread use in the synthesis of alkaloids and related biologically active compounds.
Acknowledgements
We thank the National Science Foundation for support of this research (Grant CHE-0911192). We thank Dr. Tom Emge for crystallographic analysis.Notes and references
- For selected reviews, see: (a) A. Padwa, ed., 1,3-Dipolar Cycloaddition Chemistry, Wiley: New York, N.Y., 1984, vol. 1 & 2, pp. 1521 Search PubMed; (b) A. Padwa and W. H. Pearson, Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, Wiley: Chichester, U.K., 2002, vol. 59, pp. 940 Search PubMed; (c) I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765 CrossRef CAS; (d) G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484 CrossRef CAS; (e) C. Najera and J. M. Sansano, Top. Heterocycl. Chem., 2008, 12, 117 Search PubMed.
- For examples, see: (a) A. Arany, D. Bendell, P. W. Groundwater, I. Garnett and M. Nyerges, J. Chem. Soc., Perkin Trans. 1, 1999, 2605 RSC; (b) M. Nyerges, A. Pinter, A. Viranyi, G. Blasko and L. Toke, Tetrahedron, 2005, 61, 8199 CrossRef CAS; (c) T. M. V. D. Pinho e Melo, Eur. J. Org. Chem., 2006, 2873 CrossRef; (d) M. Nyerges, J. Toth and P. W. Groundwater, Synlett, 2008, 1269 CrossRef CAS.
- For examples, see: (a) N. Cohen, J. F. Blount, R. J. Lopresti and D. P. Trullinger, J. Org. Chem., 1979, 44, 4005 CrossRef CAS; (b) L. Zheng, F. Yang, Q. Dang and X. Bai, Org. Lett., 2008, 10, 889 CrossRef CAS.
- (a) C. Zhang, C. K. De, R. Mal and D. Seidel, J. Am. Chem. Soc., 2008, 130, 416 CrossRef CAS; (b) S. Murarka, C. Zhang, M. D. Konieczynska and D. Seidel, Org. Lett., 2009, 11, 129 CrossRef CAS; (c) C. Zhang, S. Murarka and D. Seidel, J. Org. Chem., 2009, 74, 419 CrossRef CAS; (d) S. Murarka, I. Deb, C. Zhang and D. Seidel, J. Am. Chem. Soc., 2009, 131, 13226 CrossRef CAS; (e) C. Zhang and D. Seidel, J. Am. Chem. Soc., 2010, 132, 1798 CrossRef CAS; (f) I. Deb and D. Seidel, Tetrahedron Lett., 2010, 51, 2945 CrossRef CAS.
- For an excellent review on the α-functionalization of amines, see: K. R. Campos, Chem. Soc. Rev., 2007, 36, 1069 Search PubMed.
- For the alkynylation of azomethine ylides, see also: H.-P. Bi, Q. Teng, M. Guan, W.-W. Chen, Y.-M. Liang, X. Yao and C.-J. Li, J. Org. Chem., 2010, 75, 783 Search PubMed.
- For oxidative variants of these reactions, see: (a) H.-P. Bi, L. Zhao, Y.-M. Liang and C.-J. Li, Angew. Chem., Int. Ed., 2009, 48, 792 CrossRef CAS; (b) H.-P. Bi, W.-W. Chen, Y.-M. Liang and C.-J. Li, Org. Lett., 2009, 11, 3246 CrossRef CAS.
- For other examples of redox-neutral amine functionalizations, see: (a) J. Ten Broeke, A. W. Douglas and E. J. J. Grabowski, J. Org. Chem., 1976, 41, 3159 CrossRef CAS; (b) W. Verboom, D. N. Reinhoudt, R. Visser and S. Harkema, J. Org. Chem., 1984, 49, 269 CrossRef CAS; (c) B. De Boeck, S. Jiang, Z. Janousek and H. G. Viehe, Tetrahedron, 1994, 50, 7075 CrossRef CAS; (d) O. Meth-Cohn, Adv. Heterocycl. Chem., 1996, 65, 1 CAS; (e) S. J. Pastine, K. M. McQuaid and D. Sames, J. Am. Chem. Soc., 2005, 127, 12180 CrossRef CAS; (f) P. Matyus, O. Elias, P. Tapolcsanyi, A. Polonka-Balint and B. Halasz-Dajka, Synthesis, 2006, 2625 CrossRef CAS; (g) S. V. Ryabukhin, A. S. Plaskon, D. M. Volochnyuk, A. N. Shivanyuk and A. A. Tolmachev, J. Org. Chem., 2007, 72, 7417 CrossRef CAS; (h) J. Barluenga, M. Fananas-Mastral, F. Aznar and C. Valdes, Angew. Chem., Int. Ed., 2008, 47, 6594 CrossRef CAS; (i) A. Polonka-Balint, C. Saraceno, K. Ludányi, A. Bényei and P. Matyus, Synlett, 2008, 2846 CAS; (j) K. Mori, Y. Ohshima, K. Ehara and T. Akiyama, Chem. Lett., 2009, 38, 524 CrossRef CAS; (k) J. C. Ruble, A. R. Hurd, T. A. Johnson, D. A. Sherry, M. R. Barbachyn, P. L. Toogood, G. L. Bundy, D. R. Graber and G. M. Kamilar, J. Am. Chem. Soc., 2009, 131, 3991 CrossRef CAS; (l) L. Cui, Y. Peng and L. Zhang, J. Am. Chem. Soc., 2009, 131, 8394 CrossRef CAS; (m) P. A. Vadola and D. Sames, J. Am. Chem. Soc., 2009, 131, 16525 CrossRef CAS; (n) N. K. Pahadi, M. Paley, R. Jana, S. R. Waetzig and J. A. Tunge, J. Am. Chem. Soc., 2009, 131, 16626 CrossRef CAS; (o) V. K.-Y. Lo, C.-Y. Zhou, M.-K. Wong and C.-M. Che, Chem. Commun., 2010, 46, 213 RSC; (p) J. Kuang and S. Ma, J. Am. Chem. Soc., 2010, 132, 1786 CrossRef CAS; (q) L. Cui, L. Ye and L. Zhang, Chem. Commun., 2010, 46, 3351 RSC; (r) G. Zhou and J. Zhang, Chem. Commun., 2010, 46, 6593 RSC; (s) Y. K. Kang, S. M. Kim and D. Y. Kim, J. Am. Chem. Soc., 2010, 132, 11847 CrossRef CAS.
- For selected reviews, see: (a) E. D. Cox and J. M. Cook, Chem. Rev., 1995, 95, 1797 CrossRef CAS; (b) J. Royer, M. Bonin and L. Micouin, Chem. Rev., 2004, 104, 2311 CrossRef CAS.
- H. Ardill, R. Grigg, V. Sridharan, S. Surendrakumar, S. Thianpatanagul and S. Kanajun, J. Chem. Soc., Chem. Commun., 1986, 602 RSC.
- (a) T.-S. Kam and K.-M. Sim, Phytochemistry, 1998, 47, 145 CrossRef CAS For recent syntheses of harmicine, see:; (b) W.-H. Chiou, G.-H. Lin, C.-C. Hsu, S. J. Chaterpaul and I. Ojima, Org. Lett., 2009, 11, 2659 CrossRef CAS; (c) W. A. da Silva, M. T. Rodrigues, N. Shankaraiah, R. B. Ferreira, C. K. Z. Andrade, R. A. Pilli and L. S. Santos, Org. Lett., 2009, 11, 3238 CrossRef CAS; (d) L. Evanno, J. Ormala and P. M. Pihko, Chem.–Eur. J., 2009, 15, 12963 CrossRef CAS and references cited therein.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization. CCDC reference numbers 796597 and 796598. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00432d |
| This journal is © The Royal Society of Chemistry 2011 |