Conceptual integration of hybridization by Algerian students intending to teach physical sciences
Hazzi
Salah
a and
Alain
Dumon
b
aEcole Normale Superieure de Kouba, Algers, Algeria. E-mail: hazzi_salah@yahoo.fr
bInstitut Universitaire de Formation des Maîtres d'Aquitaine - Bordeaux 4 University, Laboratory LACES, site de Pau, 44 bd du recteur J. Sarrailh, 64000, Pau, France. E-mail: alain.dumon@aquitaine.iufm.fr; Tel: 0559626581
First published on 4th October 2011
Abstract
This work aims to assess the difficulties encountered by students of the Ecole Normale Superieure of Kouba (Algeria) intending to teach physical science in the integration of the hybridization of atomic orbitals. It is a concept that they should use in describing the formation of molecular orbitals (σ and π) in organic chemistry and gaps in the mastery of these concepts may represent an obstacle for the interpretation of the reactivity of organic compounds. Several studies have noted that the concept of hybridization is among one of the most difficult to understand for students at all levels of learning chemistry. In this work we try to analyze the alternative conceptions that students have constructed and how they have brought together in a conceptual structure the various concepts related to hybridization. The analysis of responses to a written questionnaire and exchanges between students in group activities sequences shows that for most students, the hybridization is not assimilated correctly. It seems that many students can speak about hybridization only once the bonds are formed and there is confusion between the formation of hybrid orbitals and the formation of molecular orbitals. Moreover, various alternative conceptions for the hybridization and the meaning of the designation of hybrid orbitals (sp, sp2, sp3) appear. Finally, from the reasoning used by students in achieving the proposed tasks, we inferred a knowledge structure of their possible integration of the concept of hybridization.
1. Introduction
The interpretation of the reactivities of organic compounds requires the use of the concepts related to the modelling of the covalent bond. To describe this bond, Lewis or quantum models are possible. In the case of Lewis model, all electron pairs of the outer atomic shells in the form of ‘dot-pairs’ are represented in the formula by dashes. These electron pairs are shared by two atoms (bonding pairs) or free (non-bonding pairs), and electronic vacancies can also exist. With the quantum model, the bonds result from an axial (called σ bond) or lateral (called π bond) overlap of atomic orbitals. In this model, two parallel ways for the description of the molecule appear (Dumon and Sauvaitre, 1995). The first adopted the delocalized way based on the concept of molecular orbital obtained by linear combination of pure AO of atoms and leading to the molecular geometry by minimizing the energy of the atomic system. The second is the localized way (called the valence bond method) based on the formation of hybridized orbitals obtained by linear combination from pure AO of the same atom followed by combination with pure or hybrid AO of bonded atoms to a maximum electron density of binding along favoured directions.The concepts involved in the quantum model of the atom and molecule are complex and abstract and therefore, they are considered among the more difficult subjects to understand for students at all levels of learning chemistry (Gold, 1988; Tsaparlis, 1997; Taber, 2001, 2002a; Tsaparlis and Papaphotis, 2002; Papaphotis and Tsaparlis, 2008a; Nakiboglu, 2003; Dumon and Sauvaitre, 1995). These concepts of molecular orbital (MO) and hybridization, introduced when teaching physical chemistry, are mainly used for their operational character in organic or inorganic chemistry and it is during their handling in these areas that students will be challenged. In organic chemistry, the difficulties encountered in the mastery of these concepts constitute the major impediment towards the right interpretation of the chemical reactivity of the chemical species (Rushton et al., 2008). For example, difficulties of the microscopic modeling based on the representation of electronic densities associated with bonds have been highlighted by various studies (Barlet and Plouin, 1997; Rivera-Huizar, 1997; Agrebi, 2004; Hassan et al., 2004; Treagust, 2004). In this work, we seek to analyze how the hybridization concept, taught in Algeria in the first and second year of bachelor's degree and widely used in third year when teaching organic chemistry, has been assimilated by students.
The theoretical framework
Learning the concept of hybridization requires the linking of different abstract concepts of the quantum model (electrons, atomic orbital s and p, linear combination of atomic orbitals, orbital symmetry, orbital overlap respecting the symmetry rules, molecular orbital, electronic density, etc.). To make sense of such complex knowledge, students will, from information received during the teaching, perform their “conceptual integration” (Taber, 2005a) by linking the various concepts involved and thus build up a personal “knowledge structure” (Champagne et al., 1981; Tsai, 1998; Nakiboglu, 2008; Taber, 2005a). If using a model of “information processing”, as proposed by Johnstone (1991), to interpret the process of acquiring knowledge, we can predict that in the presence of multiple information, especially if it is complex, the limited capacity for handling information in a given time in working memory will lead to a mistreatment of such information. Some are forgotten, others will be distorted (perhaps incorrectly) to be linked to existing knowledge (Bodner, 2007) and others are still stored in long-term memory as isolated fragments. The terms “alternative conceptions” or “synthetic mental models” (Pope and Gilbert, 1983; Gentner and Stevens, 1983; Vosniadou, 1994), “quasi-theories” (Desautels and Larochelle, 1989), “pseudo- conceptions” (Vinner, 1997) or “alternative structures” (Driver and Easley, 1978; Taber, 1999) were used to describe a structure of thought, a simple explanatory model, logical, organized, built by the learner to think in a particular scientific field, which manifests itself in a given context. They are not always scientifically correct, because they are often deficient in a number of points, but generally defined functionally in that they allow a person to engage in the description, explanation and prediction of events (Gilbert and Boulter, 1998a, b; Hafner and Steward, 1995). Such alternative “knowledge structures” can act as impediments to learning rather than as an aid to more appropriate conceptualization (Taber, 2001a, 2003, 2005b). In her study, Nakiboglu (2003) notes that few prospective Turkish teachers (only 7.2%) gave an acceptable definition of hybridization and in some work done on understanding the concept of hybridization we note the following alternative conceptions. Hybridisation is:- A process of transformation (or mixture) of different types of orbitals to form a new set of energetically equivalent orbitals (Nakiboglu, 2003);
- A process whereby the reorganization/repositioning of the valence electrons (Nakiboglu, 2003; Papaphotis and Tsaparlis 2008b) form bonds;
- A process in which electrons are transferred/excited to an orbital/energy level/layer to another (Dumon and Sauvaitre, 1995; Taber, 2002b; Nakiboglu, 2003; Stefani and Tsaparlis, 2009) in the hope of obtaining a more stable structure (Taber, 2002b; Nakiboglu, 2003) or to transfer the electrons to a new state (Dumon and Sauvaitre, 1995);
- A mathematical operation (whose meaning is badly discerned), probably related to the atom, but mainly leads to the formation of MO (Dumon and Sauvaitre, 1995; Taber, 2002b; Stefani and Tsaparlis, 2009), and therefore, for some, the hybridization involves electrons of different atoms (Stefani and Tsaparlis, 2009);
- A mathematical concept (Dumon and Sauvaitre, 1995) or a simple game on paper with the shape of the orbitals (Stefani and Tsaparlis, 2009) that constructs a set of orbitals oriented along the bonding directions (or in connection with the geometry of the molecule) (Coll and Treagust, 2002; Nakiboglu, 2003; Papaphotis and Tsaparlis, 2008b);
- The formation of hybrid orbitals (having a real existence) which is a spontaneous process (Stefani and Tsaparlis, 2009).
The difficulties in understanding the hybridization concept can result from poor control of fundamental knowledge for its learning: the atomic orbital concept and the linking of the orbitals symbolization (s, p …) with their directional aspect (Zoller, 1990). Gillespie (1996) thinks that the common use of orbital representations to illustrate the orbital hybridization encourages students to think it is a physical process (with a redistribution of electron density) rather than a formal mathematical operation. For Taber (2002b), in the minds of students, the formation of hybrid orbitals proceeds in three stages: the starting point is the electronic configuration of the atom concerned in its ground state, followed by seeking a new set of orbitals (electronic configurations) that are best suited for recovery (hybridization), then they consider the formation of molecular orbitals or bonds (Papaphotis and Tsaparlis, 2008b). This leads to confusion between the set of hybrid orbitals and molecular orbitals.
The contextual framework
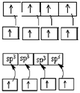
The quantum model is introduced in the second half of the first year as a common part of the curriculum for the preparation of a bachelor of physical sciences at the Kouba ENS (Algeria). It falls within the general chemistry module and follows the Lewis model in the description of the formation of covalent bonds, in the chapter on chemical bonding. The idea of sharing of electron pairs during the formation of covalent bonds in the Lewis model is replaced by the introduction of the atomic orbitals (AO) overlapping to form the MO. In order to give a more visual representation of the hybridization, a mathematical operation that involves a combination of the AOs of an atom belonging to the same electron shell to form new equivalent orbitals, is used as a representation of the hybridized and non-hybridized orbitals in quantum boxes, as shown in Fig. 1.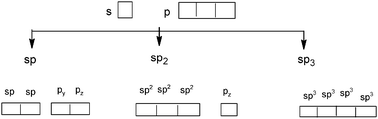 | ||
| Fig. 1 Schematic representation of hybridization states using quantum boxes. | ||
It is then possible to represent, after the construction of Lewis structures of the molecules, the σ (axial overlapping of atomic orbitals) and π (lateral overlapping of unhybridized p orbitals) bond formation representing the hybridization with quantum boxes as illustrated in Table 1 with examples of molecular oxygen and hydrogen cyanide.
| O2 | HCN | |
|---|---|---|
| Lewis representation |
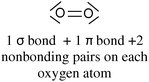
|
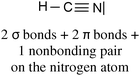
|
| Quantum boxes representation |
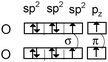
|
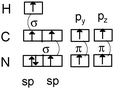
|
2. Research question and methodology
In this work, we were interested in the evaluation of knowledge integration by students belonging to the teacher training of future teachers of secondary schools, relating to the hybridization concept commonly used in organic chemistry. This investigation concerns two student groups (chemistry and physics options) of the “Ecole Normale Superieure” of Kouba (Algeria) preparing their diploma of bachelor of physical sciences.First, a paper and pencil questionnaire was given to the 140 3rd year students who had followed the same course of organic chemistry during the academic year 2009–2010, that is approximately 15 months after the topic had been taught in the first year. In this questionnaire, relating to the quantum model of the covalent bond, three questions concerned hybridization. The nature of the questionnaire was made clear to the students (it was anonymous, not used for assessment, seeking personal conceptions). The test items were developed from examination questions used in earlier years before the present research and which revealed students' misconceptions.
Then, in order to better assess student understanding of the hybridization concept and interpret the answers to the questionnaire, we used sequences of activities lasting 30 min. These sequences concern 32 of the students having answered the questionnaire, divided into four working groups made up of 8 students each. They are volunteers belonging to the top quarter of the class in terms of grades. For each asked question, a time for debate is allowed to all working groups. Naïve questions of a teacher “coach” will allow the discussion of each group to be guided so as to formulate step by step the reasoning followed during the resolution of the task: analysis of the problem, deductive reasoning and the logic of these deductions. The exchanges between students in each group have been recorded by video and fully transcribed.
It should be noted that the students were taught in Arabic and that this language was used for the collection of the data (questionnaire and activities). The answers to the questionnaire and the transcription of the exchanges between students were translated into French then into English for this report.
Presentation of the questionnaire
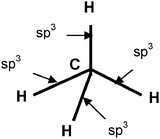
The aim of the first question, “What does sp3mean for you in terms of hybridization?”, is to assess the meaning given by students to the concept of hybridization and to the symbolic representation of a hybridization state and if they have an idea of the purpose of the construction of hybrid orbitals.With the second question, “Indicate on the following scheme, representing the methane molecule, the contributions of sp3hybrid AO of carbon in the formation of each bond”. We have tried to understand if the students confuse the formation of hybrid orbitals (spn) (AOs that belong to the same atom) and the molecular orbitals (MOs that belong to two atoms).
Finally, the aim of the third question, “Specify the number and nature of bonds, justify your response: (σ, π, covalent, non-covalent, etc.)” is to assess student's ability to describe the formation of σ covalent bonds in terms of the overlapping of atomic orbitals.
After reading the answers a categorization was undertaken independently by the two authors on the following principle: for questions 1 and 3, identification of key words appearing in descriptions (one description can contain several key words); for question 2, identification of the diagrams translating similar ideas. In the event of disagreement, a discussion took place to arrive at an agreed categorization.
Presentation of activities
By means of these activities, we looked, on one hand to evaluate to what extent the hybridization concept was assimilated by the students (first activity), on the other hand to clear up the students point of view on the way of combining atomic orbitals to form new hybrid equivalent orbitals (second activity). For the first activity we asked two groups of students (G1 and G2) to: “describe briefly the principle of the hybridization theory and the purpose of its use.” During the second activity, which also concerned two groups of students (G3 and G4), the students had to represent in the form of quantum boxes the states of hybridization of atoms in some molecules. In order to diversify the situations of application, each group had to work on different molecules. The task proposed to the group G3 consists of: “describe schematically, using quantum boxes, the different hybridization states sp, sp2, sp3based on the configuration of the ground state (s, p)” by applying it to molecules CH4, O2 and N2. In the task proposed to the group G4, students must “to represent, for the underlined atoms, the electrons distribution of different orbitals using quantum boxes:![[B with combining low line]](https://www.rsc.org/images/entities/i_char_0042_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) Cl2; H
Cl2; H![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif)
![[N with combining low line]](https://www.rsc.org/images/entities/i_char_004e_0332.gif) ;
; ![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2
H2![[N with combining low line]](https://www.rsc.org/images/entities/i_char_004e_0332.gif) H;
H; ![[B with combining low line]](https://www.rsc.org/images/entities/i_char_0042_0332.gif) H3; H
H3; H![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H
H![[O with combining low line]](https://www.rsc.org/images/entities/i_char_004f_0332.gif) ;
; ![[N with combining low line]](https://www.rsc.org/images/entities/i_char_004e_0332.gif) H3;
H3; ![[N with combining low line]](https://www.rsc.org/images/entities/i_char_004e_0332.gif) H4+”.
H4+”.
From the analysis of the students' exchanges during the solving of these tasks we hoped to be able to highlight possible knowledge structures which can appear in various situations.
3. Analysis of results
Analysis of questionnaire responses
- Question 1: Writing sp3 in terms of hybridization mean: linearly combining an s orbital with three p orbitals (px, py, pz), not equivalent, to create four new hybrid orbitals sp3, each describing the state of an electron and satisfying given conditions of equivalence and orientation in space.
- Question 2:
- Question 3: in the methane, the carbon atom of initial electronic configuration 1s2 2s2 2p2, has a valence of 4. The hybridization sp3 of carbon leads to 4 equivalent sp3AO. The four bonds of the methane molecule are σ-type covalent bonds derived from axial overlap between the carbon hybrid sp3 AOs and the s orbitals of hydrogens.
Q1. Significance of writing sp3. In Table 2 appear the different categories of key words identified in the answers relating to the significance of the notation sp3 given by students accompanied by their frequency.
| Category | Keywords contained in descriptions | N descriptions | % |
|---|---|---|---|
| A1 | combination of 1 s AO and 3 p AO | 26 | 22% |
| B1 | merger/mix/union of s and p orbitals | 28 | 23% |
| C1 | σ bond/overlap of s and p AO | 86 | 43% |
| D1 | Transition/excitation of an electron from sub-shell s to sub-shell p | 15 | 12,5% |
| Other | Reference to electron pairs/bonding electrons | 11 | 9% |
| Reference to the change in the electron distribution | 6 | 5% | |
| Reference to the molecular geometry | 4 | 3% |
The percentages are calculated relative to the total number of students who answered (120, i.e.; 86%). As in a given description several key words can appear, their total is higher than 100%. Among students who answered, only 22% (A1 category) mentioned the combination of different atomic orbitals to form new hybrid orbitals, mainly in the form “Combination of 1s+3p to give 4sp3”, without mentioning the equivalent nature of these hybrid orbitals.
The same proportion of students (23%) uses a common sense formulation (mixing, merger) of s and p orbitals (B1). In this description, they specify neither the number of hybridized orbitals formed, nor the equivalent nature of these orbitals: “a mixture of s+3p orbital; merger of s and p orbitals to give sp3hybridization”.
We note, in descriptions of type A1 or B1, a number of formulations showing that if the concept of hybridization is seen as needing to involve different kinds of orbitals, some misunderstandings arise about it. For example, saying that “sp3is the combination between the orbital 2 s2and orbital 2 p2to give sp3” (six students) or “it is the union between the two orbits (s+3p)” (4 students) suggests that for these students it is only one p orbital which combines with one orbital s. Moreover, the last group of students confuses orbits and orbitals, which shows the difficulty of understanding the concept of orbitals. For others (7 students), “It is the merger of one AO s with three AO p to give (spx+spy+spz), which is sp3”. Here is a conception of the meaning of sp3 writing that appears: the s orbital is combined with the three p orbitals to give three sp hybrid orbitals (hence the exponent 3).
For a significant proportion of students (43%) hybridization is σ bond formation by merger or overlap of orbitals s and p: “sp3 is an C–H bond”; “it is the merger between AO (s and p) to give a sigma bond”; “it is the axial overlap of s and p”, “Overlap of AO(s) containing two electrons with three p orbits containing two electrons, to form the molecule”, etc. We can deduce that these students confuse the hybridization (the combination of AO of the same atom) with the LCAO theory in the formation of MO (a combination of AO from two different atoms). A formulation such as “sp3 is the formation of three bonds like sp” (5 students) further confirms what was written above regarding the meaning given to the notation sp3. We also found, for two students, in a formulation showing a redistribution of electrons, but in the original AO, the idea that upon hybridization, only one p orbital combines with one s orbital: “Overlap of one AO s containing (1e−) with one AO p containing (3e−)/means that the atom involved in bond formation with one s orbital and three p orbitals”.
The idea that hybridization is the transfer or excitation of an electron from a sub-shell s to sub-shell p and not a redistribution of electrons in the equivalent hybrid AOs appears in 12.5% of the responses: “Transfer of an electron from a sub-shell (s) of lower energy to a sub-shell (p) of higher energy”; “sp3 is the axial overlap of s and p with an electron transfer from the sub-shell s to sub-shell p”, etc. This idea, according to which hybridization leads to a redistribution of electrons in pure AO of the atom is reflected in the expression referring to electron pairs or bond electrons: “sp3 means that AO s contains a electron pair, while p contains 3 pairs”; “Means that the orbit p must contain, under the rules of Pauli and Hund, 3 electrons”, etc. This last formulation, besides the fact that there is confusion between orbit and orbital, corresponds to another interpretation of the exponent 3 in sp3: the p orbital contains 3 electrons.
Finally, a few students (4) discussing the usefulness of hybridization in the linking with the molecular geometry: “sp3 is a hybridization for determining the geometry of the molecule and the angles, as it offers knowledge of the location of the orbitals in space based on the number of bonding pairs and nonbonding pairs”; “This is the overlap of bonds to form a tetrahedron, i.e. say, the merger of AOs with three p orbitals to give the sp3geometry”.
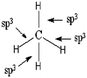
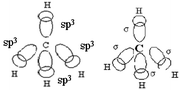
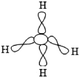
Q2. Representations of the sp3AO contribution to bond formation. It is worth noting that the various representations of the C–H bond formation appearing in the students' answers are often accompanied by a diagram of electron pairing (Fig. 2).
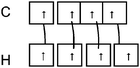 | ||
| Fig. 2 Students' representation of C–H bond formation with quantum boxes. | ||
Such drawing reflects a likening of hybridization to a simple redistribution of electrons in pure AO of carbon to obtain the four unpaired electrons necessary for the bonding pair formation.
The various representations provided by the 114 students (i.e., 81%) who responded, were classified into three categories characterized by representations showing similar ideas. The various identified representations are reported in Tables 3–5 and were accompanied by the number of students (N) having given such representations.
| Classic drawing | Hybrid AO in quantum boxes | Axial recovery of C hybrid AO and s AO of H | No drawing but one sentence |
|---|---|---|---|
|
|
|
“Methane is in a sp3 hybridization state” |
| N = 18 | N = 12 | N = 11 | N = 4 |
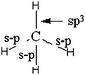
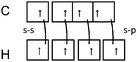
| 3 sp3 and 1 s–s | 3 s–p and 1 sp3 | Electron-pairing representation |
|---|---|---|
|
|
|
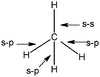
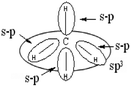
| N = 29 | N = 15 | (34 out of 44 responses) |
|
|
|
| N = 15 | N = 6 | N = 4 |
(1) Those who indicate the participation of four sp3 hybrid AO to the formation of four bonds (sometimes noted σ) drawing in different ways (Table 3: 45 responses).
These are acceptable answers in terms of formation of four bonds involving sp3 hybrid atomic orbitals of carbon and the s orbital of hydrogen. Some representations include sharing of unpaired electrons of hydrogen atoms with those resulting from the electronic redistribution in four equivalent quantum boxes of the carbon atom to obtain four bonding pairs. They are consistent with one representation of the hybridization given in teaching. On the other hand, the expressing “Methane is a sp3hybridization state” suggests that these students think it is the molecule that is hybridized and not the carbon atom AO. It is to be linked with the design involving hybridization to bond formation. Finally, for 22 students in this category, which is preceded by the diagrammatic representations of the redistribution of electrons leading to the existence of four unpaired electrons, we can say that the concept of hybridization, at least its representation in quantum boxes, is far from being mastered and certainly associated with bond formation.
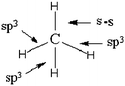
(2) Those which indicate both the participation in the bonds formation of sp3 hybrids AO and combination sH–sC or sH–pC (Table 4: 44 responses)
For students who represent the formation of four bonds by three sp3 and a combination s–s we find the conception of the meaning of the sp3 writing that s orbital is combined with three p orbitals to give three hybrid orbitals sp (hence the exponent 3). The fourth bonds (s–s) then results from the pooling of single electrons in the 2s AO of carbon and the 1s AO of hydrogen. This conception appears explicitly in the drawing of the electron pairing accompanying these two representations (for 34 out of 44 responses):
The 15 representations that include three combinations s–p and one AO sp3 could be explained in the same manner as in the previous case (3 s–p = sp3), the fourth bond would result from a combination of one AO of hydrogen and perhaps, because they believe that the methane molecule is in a state of sp3 hybridization, one carbon AO sp3, whose nature is different from previous ones.
(3) Those which represent the formation of four bonds from combinations s–p and s–s (Table 5: 25 responses).
Nineteen students in this category are preceded their representations by the drawing showing the redistribution and pairing of electrons. For the 15 students who offered the first representation, it appears clearly in these drawing the formation of two types of bonds: 1 by sharing electrons s of C and H atoms (s–s bond), 3 by formation of pairs between carbon AO px, py, pz and three hydrogen AO s (s–p bond).
The representation showing four combinations of sp can be interpreted by considering that from the s and p configurations of the valence state of carbon (represented by quantum boxes), students will consider that 4 s–p type bonds are created, as in the form shown in the table, the regrouping of three of them is noted sp3, the fourth result on the combination of p carbon AO and s AO of one hydrogen. This also shows the type of representation given by 4 students: overlapping of p carbon orbitals represented by their lobes and AO hydrogen atoms.
Q3. Nature and number of bonds in CH4. Almost all the students (129, i.e. 92%) answered question 3. In these answers the keywords σ and ‘covalent’ characterizing the nature of the 4 C–H bonds were identified, sometimes specified as equivalent. The number and the percentage of occurrence of these key words appear in Table 6.
| Keywords | Number | % |
|---|---|---|
| σ bonds | 52 (including 2 ‘equivalent’) | 40 |
| covalent bonds σ | 45 (including 2 ‘equivalent’) | 35 |
| covalent bonds | 29 | 23 |
| 2 covalent and 2 σ bonds | 3 | 2 |
| (‘equivalent’ bonds) | (4) | (3) |
| Total | 129 | 100 |
While referring to the frequency of appearance of the various key words, one can say that, for students having answered, the four CH bonds are σ bonds (40%) or covalent bonds (23%), and that they are covalent bonds σ for 35% of them. Very few students (4) specify that they are equivalent. Finally, for three students, there are two types of bonds, two covalent and two σ bonds.
If we look at closely the students' responses, we find that the denomination ‘covalent’ and ‘σ bond’ hide misconceptions. For example, for 13 students, “There are four covalent bonds because there are only single bonds and no double bonds”. The covalent term is explicitly linked only to a single bond, then the existence of a single electron pair of binding (see previous studies). With regard to the σ bonds, for 37 students they result from a combination of s and p carbon orbitals with s orbitals of hydrogen. Regarding the three students that differentiate covalent and σ bonds, starting from s and p configurations of the ground state of carbon (represented by quantum boxes), they build with the four hydrogen, two sigma bonds (s–s) from the two electrons in the carbon sub-shell s and two other covalent (s–p) from unpaired electrons in the sub-shells px and py. A covalent bond would be the sharing of two electrons of different atoms (but pertaining to AO of different nature: s and p) to form a bonding pair, as the σ bond it would results from the overlap of two s orbital, regardless of the number of electrons involved, showing their difficulty in building bonds starting from the appropriate valence state.
Among students with an idea of the usefulness of hybridization, in addition to students mentioning the formation of four equivalent bonds, 3 students indicated formation of four σ bonds with tetrahedral geometry.
Analysis of the discussions between students relating to hybridization
In Group 1, students begin to cite known hybridization states: “the hybridization of the orbitals… it is sp2, sp3 etc., it is a mix of orbitals s and p?” And then they wonder about how this mixture “but how to get it, this is the question.” They try to recall the principle by applying a familiar example, the methane: “In CH4, carbon is hybridized sp3, it is known”. Assuming that carbon is hybridized sp3, they try to form the four bonds of the molecule: “Yes I remember that established the electronic configuration of atoms each time before forming bonds”. To form the four C–H bonds of methane must be that carbon has 4 unpaired electrons. To do this, it should excite/pass one electron s toward one vacant p orbital: “for carbon… it is necessary to excite 1 e−s to p for obtain 4 e−unpaired”, which amounts according to them to realize a mixture/merger of orbitals s and p electrons. It is then possible to form four sp3 bonds, which reveals confusion between the hybridization of the AO and the formation of the MO (which confirms the analysis of responses to the questionnaire). The meaning of the sp3 denomination is the result of hybridization, carbon has “3 e− owned by p and 1e− by s”, and it is thus associated with the new distribution of electrons in the valence shell (which also confirms the analysis of responses to the questionnaire). To summarize the conception of hybridization for this group we can say that: hybridization consists in transferring one electron from a sub-shell (or orbital) to another, in order to respect the valence of the element in question, to effect the merger (mixture) of electron s and p AOs in order to describe the bond formation.
Students of group 2 begin to connect the different states of hybridization with π and σ bonds: hybridization sp3 corresponds, to the image of CH4, at 4 σ bonds, hybridization sp2 to two bonds (σ + π) and hybridization sp to 3 bonds (σ + 2π). To connect hybridization to the bonds formation, students establish new carbon valence shell electron distribution of carbon in methane: 2s1 2p3, and then they form 4 σ bonds with hydrogen. They also consider hybridization as resulting from the transfer of an electron from a sub-shell s towards a p sub-shell and seem to confuse the hybridization AOs with the MO formation: “is evident, we mixed electrons s and p to obtain 4 unpaired e−and form 4 bonds sp3with 4 hydrogen” or “methane is in a sp3state of hybridization”. However these students prefer to talk about mix/merger of AO (4 occurrences) to form hybrid orbitals, that mix of electrons (1 case). Finally, a relationship between the different states of hybridization and the geometry of molecules ends their discussion: “a hybridization type sp3is tetrahedral; a sp2is plane while sp is linear”. In summary, for these students, hybridization is by transferring one electron from a sub-shell (or orbital) to another, in order to respect the valence of the element in question, making a mix of s and p AOs of different atoms, in order to relate the σ and π bond formations to the geometry of molecules (which confirms the analysis of responses to the questionnaire).
In group 3, we found strategies for solving the problem that were identical to those already identified. Students begin their discussion by recalling the link between the states of hybridization and the number of π and σ bonds: “we know that sp3is 4 σ bonds and sp2is one σ + one π bond, while sp state is a triplet (σ + 2π)”. They then try to recall the principle, by application to a familiar example (CH4): “Right, I remember, the process in CH4it was find electronic configuration and then see how to form bonds C–H” or “it seems to me that one must first represent the ground state (while drawing the quantum boxes) 1s2, 2s2, 2p2of carbon.” Then it is appropriate to excite an electron s toward one p orbital: “must excite 1e−s to p for obtain 4 unpaired electrons”. Finally, “we count the valence electrons and build bonds that represents schematically the hybridization”. They thought to apply this procedure to dioxygen and dinitrogen molecules, “we can proceed similarly to represent the sp state in N2and sp2state in O2”, but because they focused their discussion on the establishment of the valence state of the atoms, ignoring the non binding pairs, as the number of electrons unpaired in the basic configuration allows the formation of the number of bonds corresponding to the Lewis formula, there is finally no need to consider hybridization: “obviously, each orbital containing 1 unpaired e−overlaps axially and laterally with the second atom to give a doublet (σ+π) in the oxygen molecule O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O”. It observes, while a student speaks of axial and lateral overlapping, this does not show a mastery of the necessary conditions for the formation of π and σ bonds. However, when ground state does not justify the valence of the atom, they used hybridization, as in the case of methane.
O”. It observes, while a student speaks of axial and lateral overlapping, this does not show a mastery of the necessary conditions for the formation of π and σ bonds. However, when ground state does not justify the valence of the atom, they used hybridization, as in the case of methane.
In conclusion, the answer given by the group to represent states of sp, sp2 and sp3 hybridization in terms of quantum boxes is given in Table 7.

|
It appears that for these students, the description of hybridization states is possible only once the bonds formed. Hypothetical modification of the electronic distribution at the atomic orbital level before the bonds formation is not taken into account. This representation of the different states of hybridization in 2, 3 and 4 boxes, each containing two paired electrons, confirms the confusion existing in the mind of students between the linear combination of the different AO of the same atom for hybridization and those of the AO belonging to different atoms to form bonds (MO). If the need to reorganize atomic orbitals to hybridize them is present in their minds, it is once again the question of how to combine them which poses difficulties.
The explanations relating to hybridization states as related to (σ + π) and (σ + 2π) bond formation from the unpaired ground state electrons in molecules N2 and O2, and the σ bonds in CH4 from the valence state of carbon, suggests that the difference in nature between σ and π bonds is not clearly recognized and that understanding is superficial.
Group 4 students had to treat the hybridization state of AO belonging to various atoms of the neutral and charged molecules. These atoms (belonging to the second row of the periodic table) include boron and beryllium that are exceptions to the octet rule. In proposing these formulas, we sought to establish whether alternative conceptions that students have constructed are of the same type as those previously encountered, for example does the hybridization correspond to the establishment of the state of valence or to the formation of bonds?
In the treatment of this task, students adopt the following procedure:
- Establishment of the electronic configuration of each atom in the ground state: this is done correctly for all atoms.
- Representation of the Lewis structure, but taking into account only the number and nature of the bonds to be formed. The non-binding pairs are forgotten.
- Hybridization of atomic orbitals (represented in a quantum box): If the number of unpaired electrons does not allow the formation of the identified number of bonds, then a hybridization state by reorganization or excitation of electrons must be considered, otherwise it is not useful. Two examples of reasoning illustrate this process: “in HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N: it recognizes (1σ) + (2π) between C and N. Formation of these three bonds can be explained from the configurations of these two atoms. C (1s22s22p2) and N (1s22s22p3), AOs of C are reorganized in 2s12p3for having 4 unpaired e−whilenitrogen (which has 3 unpaired e−plus one lone pair) need not be hybridized to participate in the formation of these bonds”, while “To Be in BeCl2; with Z = 4, the configuration is: 1s22s22p0. As it is linked to 2 Cl must form two bonds it suffices to excite an e−from s to p for a new electronic distribution and form two bonds with two chlorines
N: it recognizes (1σ) + (2π) between C and N. Formation of these three bonds can be explained from the configurations of these two atoms. C (1s22s22p2) and N (1s22s22p3), AOs of C are reorganized in 2s12p3for having 4 unpaired e−whilenitrogen (which has 3 unpaired e−plus one lone pair) need not be hybridized to participate in the formation of these bonds”, while “To Be in BeCl2; with Z = 4, the configuration is: 1s22s22p0. As it is linked to 2 Cl must form two bonds it suffices to excite an e−from s to p for a new electronic distribution and form two bonds with two chlorines ”. It is the operation which involves forming bonds between atoms by pairing their unpaired electrons, which is then considered to be hybridization.
”. It is the operation which involves forming bonds between atoms by pairing their unpaired electrons, which is then considered to be hybridization.
A particular conception is noted when it is a question of specifying the hybridization states of beryllium (sp) and boron (sp2) which require a reorganization of their valence electrons to form respectively 2 or 3 bonds, reorganization effectively suggested by students: for Be in BeCl2, “but there is not π bond in the molecule so… I think that Be is not hybridized”, and for B in BH3, initially, a student said “In the BH3molecule there's formation of 3σ bonds between the 3 unpaired e−of B after reorganization and the 3H: 1s22s22p1 → thus 2s12p2”, then, to the question “which is then the hybridization state of boron”, another student answers: “there is no π bond, it is the same case that Be, one cannot speak about hybridization”. The hybridization states sp and sp2 are therefore only related to the existence of π bond in the molecule and not to the redistribution of electrons in equivalent AO.
Table 8 is given by students in response to the question
| Molecule | Electronic configuration (ground state) | Hybridization state (represented in quantum boxes) | Bond angle and molecular geometry |
|---|---|---|---|
| BeCl2 | Be 1s22s22p0 |
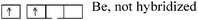
|
180°, linear |
| HCN | C 1s22s22p2 |

|
180°, linear |
| N 1s22s22p3 |

|
||
| BH3 | B 1s22s22p1 |
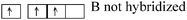
|
120°, triangular |
| CH2NH | N 1s22s22p3 |

|
90°, planar |
| C 1s22s22p2 |
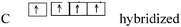
|
||
| HCHO | C 1s22s22p2 |
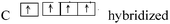
|
90°, planar |
| O 1s22s22p4 |

|
||
| NH3 | N 1s22s22p3 |

|
109°, tetrahedral |
| [NH4]+ | N 1s22s22p3 |

|
109°, tetrahedral |
It is noted that the representation of different hybridization states of C always results in the redistribution of electrons in order to obtain 4 unpaired electrons, which is represented by four quantum boxes (1s and 3p). Here is how the proposed hybridization of carbon in the case of H–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: “In the molecule H–CH
O: “In the molecule H–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O there's (1σ) + (1π) between the C and O, thus 4 bonding pairs around C and two around the oxygen, which requires a sp2hybridization.
O there's (1σ) + (1π) between the C and O, thus 4 bonding pairs around C and two around the oxygen, which requires a sp2hybridization.
From the electronic reorganization at level of AO of O, 1s22s22p4 (2s2, 2px22py12pz1) and C, 1s22s22p2 (2s12px12py1, 2pz1), we can form a σ bond by axial overlapping and π bond by lateral overlapping, oxygen having two non-bonding pairs (2s22px2)”. Bond formation by axial or lateral overlapping is considered, but these overlaps occur between py and pz orbitals of oxygen and carbon, the electron of carbon orbitals 2s and 2px making complete the tetravalency, and lone pairs of oxygen are localized on the 2s and 2px orbitals. The sp2 hybridization is simply related to the formation of a π bond (it requires two p orbitals overlap laterally) rather than the need to form three equivalent hybrids AO may lead to three σ bonds by axial overlapping. It is the same for the sp hybridization in the case of HCN, the “overlapping” of 2px, 2py, 2pzAO of carbon and nitrogen can form one σ bond and two π bonds.
The values of the angles between the bonds are systematically linked to hybridization states, but with a doubt for the sp2 hybridization state, as shown in this exchange between three students: “We have always said that sp3is tetrahedral (109°) so that sp2is uh… I think 90° (plane) and sp is 180° (linear)”;/“It seems to me that sp2is 120°… uh… well I'm not a hundred percent sure but…”/“I'm a lot more to 90 degrees because the sp2state is made with 3 AO p which are 90° between them”. This, in accordance with the conception of hybridization described above, induces them to provide an angle of 90° between the bonds for molecules CH2NH and HCHO, molecules to which they assign a planar geometry (which is the case for hybridization sp2) without wondering how, with angles of 90° between the three bonds, geometry may be planar. On the other hand, in the case that hybridization is not taken into account, one wonders if the correct values proposed for the angles between bonds (and thus the geometry of molecules) is not based solely on counting σ bounds in molecules: BeCl2, 180° because the molecule has only two bonds; BH3, 120° because the molecule contains three bonds. If tetrahedral geometry proposed for the molecular ion NH4+ is in accordance with proposed sp3 hybridization for nitrogen, the same geometry attributed to ammonia without hybridization can result, without calling into question our previous assumption, a simple remembering of knowledge: CH4 and NH3 have a tetrahedral molecular structure.
Moreover, in the case of [NH4]+, as shown in schematic form of quantum boxes, positive charge (+) is attributed to the loss of an electron from the s orbital to allow it to have four unpaired electrons and form four σ bonds, as in methane. However, they wondered about the fate of the lost electron. The difficulty to imagine a dative bond formation had already been established during the activities relating to the formal charges determination of charged structures, in the case of Lewis model (Salah et al., 2011).
4. Discussion
Integration of the hybridization concept
The target knowledge that students were taught during their first year at university is: hybridization is the construction of a new set of atomic orbitals resulting from a linear combination of initial AO to give new hybrid equivalent AO characterized by a new spatial orientation of their axes. The purpose of the idea of hybridization is to relate bond formation from the overlapping of two different atoms AOs (hybrid or not) to obtain MO σ and π in accord with known molecular geometry. From the analysis of the results we observe a difference between this target knowledge and the present state of the students' knowledge and understanding of the hybridization concept.It seems that the majority of students know that:
- hybridization consists of the combination of different orbitals (s and p) in order to form the new set of orbitals, but the equivalent character of the hybrids orbitals are forgotten. Moreover, in contrary to certain previous studies, the energy aspects (Taber, 2005b) or mathematical aspect (Dumon and Sauvaitre, 1995) of hybridization does not appear.
- hybridization leads to a modification of the distribution of valence electrons compared to the ground state, but for that, electrons are transferred/excited from orbital/energy level/layer to another (Dumon and Sauvaitre, 1995; Taber, 2002; Nakiboglu, 2003; Papaphotis and Tsaparlis, 2008; Stefani and Tsaparlis, 2009) in order to obtain the number of unpaired electrons corresponding to the valence of the atom;
- hybridization makes it possible to describe the bond formation between atoms, but by the pairing of electrons to form bond pairs and not by AOs overlapping leading to MO formation;
- the axial overlapping of two AO of different atoms gives a σ bond and the lateral overlapping of two p AO, a π bond, but the molecular orbital concept does not appear.
Lastly, for some students, hybridization is a transformation process or AO mixture in relation to the molecular geometry (Dumon and Sauvaitre, 1995; Coll and Treagust, 2002; Nakiboglu, 2003; Papaphotis and Tsaparlis, 2008).
Moreover, some confusions and misconceptions have been highlighted:
- confusion between (spn) hybrid orbitals formation (AO combined belong to the same atom) and molecular orbitals formation (AO combined belong to two different atoms) leading to bond formation (Sauvaitre and Dumon, 1995; Taber, 2002; Tsaparlis and Stefani, 2009);
- conception according to which the hybrid orbital symbolism (sp, sp2, sp3) is associated, either with the fact that in the “new” electronic configuration of the atom there is an electron belonging to the s orbital and 1, 2, or 3 electrons to p orbitals, or with the pairing of the electrons of one s AO with one p AO of another atom: for example the carbon atom in CH4 is sp3 in conformity with 3 sH-pC bond pairs;
- for some students, conception according to which the covalent term is explicitly associated to a single bond, i.e., the existence of a single bonding pair; for others, only if the bond results from the pairing of electrons from s and p AO of different atoms; if not it is a σ bond.
So, we can affirm that the conceptual integration of hybridization is not carried out by our students. They have built up only piecemeal knowledge (Taber, 2005b) without making the link between them. With the typology of learning impediments proposed by Taber (2005b), this incapacity to build up correct chains of reasoning about hybridization can be considered as “within-topic fragmentation learning impediments”. This failing to make a connection between parts of knowledge leads the students to use an alternative conceptual framework based on the Lewis model, i.e., the sharing of electron pairs. With this “electron pair framework” they consider that:
- As was already noted by Taber (2005b) in another context, the bonding electrons are in an atomic orbital (s, p or hybrids) rather than in a molecular orbital: “sp3 is a C–H bond”; “… we mixed electrons s and p to obtain 4 unpaired e−and form 4 sp3bonds with 4 hydrogens”, etc.
- The scheme for forming hybrid orbitals is: electronic structure in ground state → new electronic structure → electron pair formation → hybrid orbitals;
- The hybridization state determination of an atom in molecules is based on this reasoning: Lewis structure of the molecule → number and nature of bonds to form (the non-bonding pairs are forgotten) → if the number of unpaired electrons does not allow the formation of the identified number of bonds, then a hybridization state must be considered, otherwise it is not useful. For example, to the formation of the double bond in the dioxygen molecule or triple bond in dinitrogen, hybridization is not necessary because there are two unpaired electrons on AO 2pz and 2py of oxygen and 3 electrons on AO 2px, 2py and 2pz of nitrogen.
The answer of one student to a question about the sp3AO contribution to bond formation illustrates well the students' level of understanding of the hybridization concept: “As the configuration of C is 1s22s22 p2, we can excite an electron from the subshell s to subshell p to have 4 unpaired e−. What justifies the carbon tetravalence by hybridization sp3 (1AOs + 3AOp), that is to say 2s12px12py12pz1. These four unpaired electrons are formed with four hydrogen atoms, 4 σ bonds. So methane is a sp3hybridization state”.
Hybridization, the nature of bonds and molecular geometry
σ and π bond formation is not directly related to the hybridization state of atoms but to the nature - single, double or triple - of the bonds to be formed. For example, a double bond is one σ bond and one π bond → π bond result from a lateral overlapping of p orbitals of two neighboring atoms → it requires sp2 hybridization → σ bond results from the sharing of electrons located on unpaired s or p AOs of the two atoms in their ground state. The need to form three equivalent hybrid AOs, which will be occupied by bonding or non-bonding pairs, resulting from the neighboring atoms axial overlapping of AO, is not mentioned. Moreover, as the non-binding pairs are not taken into account when considering a hybridization state, it is systematically associated with a number of σ bond which can be formed: 4 σ bonds to sp3, 3 σ and one π for sp2, 2 σ and 2 π for sp. Linking hybridization states to the number of bonds, such as the existence of 4 σ bonds for sp3, suggests that all sp3 hybridized atoms should form four σ bonds, contrary to what we find in the case of NH3 (3 σ) and H2O (2 σ) for example.The molecular geometry and the angles value between bonds are also systematically linked with hybridization states: sp = linear (180°) sp2 = triangular planar (120°) or simply planar (90°)?; sp3 = tetrahedral (109°). In addition to the fact that no reference to the repulsion of the electron pairs is mentioned to indicate a possible difference of angles from the theoretical values, suggesting a planar geometry with bond angles of 90° show a certain ignorance of the possible geometry of molecules in space. Otherwise, it is the number of bonds which will systematically fix geometry: 2 bonds, linear; 3 bonds, planar; 4 bonds, tetrahedral.
5. Conclusion
We can infer that the knowledge structure that the vast majority of students was built up on hybridization is: electronic configuration of the atom in its ground state → search for an electronic configuration in conformity with the valence of the atom → bond formation (single, double or triple) without considering the symmetry conditions for overlapping → hybridization if the number of unpaired electrons does not allow the formation to the identified number of bonds. This is consistent with and extends the observation made by Taber (2002b). To explain this knowledge structure we have considered that students used an alternative conceptual framework: the “electron pair framework”. If we used the typology of learning impediments proposed by Taber (2005b), we can think of it as “octet framework”. Taber (1999), considered that this framework is a “pedagogical learning impediment” in the sense that this idea present in cognitive structure derives from teaching. Not only from the teaching of the Lewis model of molecules but also from the visual representation of the atomic electronic structure in each hybridized and not hybridized AO in quantum boxes, used in Algerian teaching with a purely symbolic purpose. This representation leads students to retain only the reorganization of electrons in quantum boxes, representing hybrid or pure AO, to obtain a number of unpaired electrons in accord with the atomic valence and favor the idea of electron pairs formation to make bonds. The linear combination of AO, the overlapping of AO to form MO, is not present in this representation.Another possible explanation of the inadequate conceptual integration, taken from an impromptu interview with students who have completed the sequence of activity, is that as teachers do not put much emphasis on the usefulness of hybridization and the need for orbital hybrid equivalent, the students do not take this into account. As a result they have difficulties in making sense of the concept.
Starting from the idea that hybrid orbitals are very useful in organic chemistry to explain the molecular geometry, it would seem more appropriate to introduce the mathematical concept of hybridization, without the symbolism of quantum boxes, only after the introduction of the different molecular geometries with one central atom from the VSEPR method and discuss how to obtain, from the s and p orbitals of different symmetries of an atom, new equivalent orbitals to find, after MO formation, the bond angles consistent with experience.
References
- Agrebi S., (2004), Les mécanismes en chimie organique, Thèse, Université Lyon 2.
- Barlet R. and Plouin D., (1997), La dualité microscopique- macroscopique un obstacle sous jacent aux difficultés en chimie dans l'enseignement universitaire, Aster, 25, 143–174.
- Bodner G. M., (2007), Strengthening conceptual connections in introductory chemistry courses, Chem. Educ. Res. Pract., 8, 93–100.
- Champagne A. B., Klopfer L. E., Desena A. and Squires D. A., (1981), Structural representations of student's knowledge before and after science instruction, J. Res. Sci. Teach., 18, 97–111.
- Coll R. K. and Treagust D. F., (2002), Exploring tertiary students' understanding of covalent bonding, Res. Sci. Tech. Educ., 20, 241–266.
- Desautels J. and Larochelle M., (1989), Qu'est-ce que le savoir scientifique?, Les Presses de l'Université Laval, Québec.
- Driver R. and Easley J., (1978), Pupils and paradigms: a review of literature related to concept development in adolescent science students, Stud. Sci. Educ., 5, 61–84.
- Dumon A. and Sauvaitre H., (1995), Comment les étudiants s'approprient-ils le modèle quantique de la liaison chimique?, Actualité Chimique, 4, 13–22.
- Gentner D. and Stevens A. L. (ed.), (1983), Mental models, Hillsdale, NJ: Erlbaum.
- Gilbert J. K. and Boulter C., (1998a), Models in explanations, Part 1: Horses for courses?, Int. J. Sci. Educ., 20, 83–97.
- Gilbert J. K. and Boulter C., (1998b), Models in explanations, Part 2: Whose voice? Whose ears?, Int. J. Sci. Educ., 20, 187–203.
- Gillespie R. J., (1996), Bonding without orbitals, Educ. Chem., July, 103–106.
- Gold M., (1988), Chemical education: an obsession with content, J. Chem. Ed., 65, 780–781.
- Hafner R. and Steward J., (1995), Revising explanatory models to accommodate genetic phenomena: Problem solving in the “context of discovery, Sci. Educ., 79, 111–146.
- Hassan A. K., Hill R. A. and Reid N., (2004), Ideas underpinning success in an introductory course in organic chemistry, Univ. Chem. Educ., 8, 40–50.
- Johnstone A. H., (1991), Thinking about thinking, Int. News. Chem. Educ., 36, 7–11.
- Nakiboglu C., (2003), Instructional misconceptions of Turkish prospective chemistry teachers about atomic orbitals and hybridization, Chem. Educ. Res. Pract., 4(2), 171–188.
- Nakiboglu C., (2008), Using word associations for assessing non major science students' knowledge structure before and after general chemistry instruction: the case of atomic structure, Chem. Educ. Res. Pract., 9, 309–322.
- Papaphotis G. and Tsaparlis G., (2008a), Conceptual versus algorithmic learning in high school chemistry: the case of basic quantum chemical concepts, Chem. Educ. Res. Pract., 9, 323–331.
- Papaphotis G. and Tsaparlis G., (2008b), Part 2. Students' common errors, misconceptions and difficulties in understanding, Chem. Educ. Res. Pract., 9, 332–340.
- Pope M. and Gilbert J., (1983), Personal experience and the construction of knowledge in science, Sci. Educ., 67, 193–203.
- Rivera-Huizar A., (1997), Etude didactique et épistémologique de l'enseignement expérimental de la chimie organique, Thèse, Université Joseph Fourier, Grenoble.
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9, 122–130.
- Salah H., Dumon A. and Said Z., (2011), Etude des connaissances relatives au modèle de Lewis chez de futurs enseignants de sciences physiques algériens, Rev. Elec. Ens. Cien., 10, 307–333.
- Stefani C. and Tsaparlis G., (2009), Student's levels of explanation, models and misconceptions in basic quantum chemistry: a phenomenographic study, J. Res. Sci. Teach., 46, 520–536.
- Taber K. S., (1999), Alternative frameworks in chemistry, Educ. Chem., 36, 135–137.
- Taber K. S., (2001), Building the structural concepts of chemistry: Some considerations from educational research, Chem. Educ. Res. Pract., 2, 123–158.
- Taber K. S., (2002a), Conceptualizing quanta: illuminating the ground state of student understanding of atomic orbitals, Chem. Educ. Res. Pract., 3, 145–158.
- Taber K. S., (2002b), Compounding quanta: probing the frontiers of student understanding of molecular orbitals, Chem. Edu. Res. Pract., 3, 159–173.
- Taber K. S., (2003), The atom in the chemistry curriculum: fundamental concept, teaching model or epistemological obstacle?, Found. Chem., 5, 43–84.
- Taber K. S., (2005a), Conceptual integration and science learners – do we expect too much? Invited seminar paper presented at the Centre for Studies in Science and Mathematics Education, University of Leeds, February.
- Taber K. S., (2005b), Learning quanta: barriers to stimulating transitions in student understanding of orbital ideas, Sci. Edu., 89, 94–116.
- Treagust D. F., (2004), Students' Understanding of the descriptive and predictive dature of teaching models in organic chemistry, Res. Sci. Edu., 34, 1–20.
- Tsai C. C., (1998), An analysis of Taiwanese eight graders' science achievement, scientific epistemological beliefs and cognitive structure outcomes after learning basic atomic theory, Int. J. Sci. Edu., 20, 413–425.
- Tsaparlis G., (1997), Atomic orbitals, molecular orbitals and related concepts: conceptual difficulties among chemistry students, Res. Sci. Edu., 27(2), 271–287.
- Tsaparlis G. and Papaphotis G., (2002), Quantum-chemical concepts: are they suitable for secondary students?, Chem. Edu. Res. Pract., 3(2), 129–144.
- Vinner S., (1997), The pseudo-conceptual and the pseudo-analytical thought processes in mathematics learning, Edu. Stud. Math., 34, 97–129.
- Vosniadou S., (1994), Capturing and modelling the process of conceptual change. Learn. Instr., 4, 45–69.
- Zoller U., (1990), Students' misunderstanding and misconceptions in college freshman chemistry (general and organic), J. Res. Sci. Teach., 27, 883–903.
| This journal is © The Royal Society of Chemistry 2011 |