Students' experience in a general chemistry cooperative problem based laboratory
Santiago
Sandi-Urena
a,
Melanie M.
Cooper
b,
Todd A.
Gatlin
a and
Gautam
Bhattacharyya
c
aDepartment of Chemistry CHE 205, University of South Florida, Tampa, FL 33620, USA. E-mail: ssandi@usf.edu; tgatlin@mail.usf.edu
b259 Hunter Laboratories Department of Chemistry, Clemson University, Clemson, SC 29634, USA. E-mail: cmelani@clemson.edu
c363 Hunter Laboratories Department of Chemistry, Clemson University, Clemson, SC 29634, USA. E-mail: gautamb@clemson.edu
First published on 4th October 2011
Abstract
Most educators and scientists would agree that science laboratory instruction has the potential of developing science practices fundamental to achieving scientific literacy. However, there is scant evidence to support that this potential is realized, particularly in tertiary level education. This paper reports qualitative results from a sequential explanatory mixed methods study aimed at investigating the effectiveness of a college, cooperative, project-based General Chemistry Laboratory in promoting student learning. Quantitative findings showed that students in this programme increased their ability and metacognitive strategies in solving online ill-structured chemistry problems. For the current study, we used a phenomenological approach to investigate the essence of students' experiences in this learning environment. Eleven participants volunteered to take part in in-depth, open-ended interviews. Phenomenological data reduction, analysis and interpretation produced an outcome space composed of three dimensions (Affective Response, Understanding of the Learning Experience, and Strategic Response). The need to ‘take charge of learning’ emerged as the interconnecting factor bringing cohesiveness to the experience. These findings are consistent with the previous quantitative report. Additionally, they support the notion that immersion in a lab environment appropriately designed to constantly challenge students to think through problems and figure out solutions does indeed promote metacognition and problem solving skills. Situating learning in this context facilitates meaningful self-discovery and internalization of strategies as opposed to externally introducing them. We believe that these findings contribute evidence of the relationship between experiences in the laboratory and student effective learning and can inform and guide curricular development.
Introduction
Scientists and science educators alike defend with fervour the role of the academic laboratory in learning science, yet controversy abounds concerning the benefits that students actually accrue from engaging in laboratory activities. Already in 1915, E. B. Spear asserted that the study of chemistry was well adapted to develop students “mentally”—beyond just offering factual information. He viewed attitudes of mind such as “the desire to find out by himself and the willingness to examine the evidence” worthy of teachers' most dedicated efforts. Nevertheless, the title of his article—Problems in the Experimental Pedagogy of Chemistry—suggested a question that still persists today: Is the potential of laboratory instruction being effectively realised in practice? Thus, Spear proposed the use of experimental investigation to gather evidence that might contribute in enhancing the effectiveness of chemistry teaching.Almost a century after Spear's observations, laboratory instruction continues to be a focus for most chemistry education researchers and practitioners. Although the research agenda has centred on secondary school teaching and learning of the subject, we contend that findings regarding the laboratory as a medium of instruction extend to first year college chemistry. The hands-on experiences in the lab are an ideal setting to develop meaningful conceptual understanding of chemistry, to access understanding about the nature of science and to engage in doing science (Hofstein and Lunetta, 1982; Tobin, 1990; Hodson, 1996; Hofstein and Lunetta, 2004). However, research evidence does not support the assumption that the traditionally structured laboratory is accomplishing learning objectives that parallel Spear's attitudes of mind and that are desirable and significant outcomes for science literacy (Gabel, 1999; Hart et al., 2000; Hofstein and Mamlok-Naaman, 2007), such as, problem solving skills, critical thinking, experiment design and implementation (Kirschner and Meester, 1988; Lagowski, 1990; Domin, 1999; Herrington and Nakhleh, 2003; Reid and Shah, 2007). Researchers have called attention to this apparent contradiction and in recent years, awareness has resulted in a surge in research aimed at gathering empirical evidence to support the implementation of adequate college level chemistry laboratory instructional methods (Cooper, 1994; Suits, 2004; McCreary et al., 2006; Suits, 2004; Tien et al., 2007).
In pursuing this goal, we recently reported findings from a quantitative study that investigated the effectiveness of a college cooperative problem based laboratory programme in developing students' use of more metacognitive strategies and in their ability to solve online ill-structured chemistry problems (Sandi-Urena et al., 2011). In our approach we employed an automated, web-based platform, IMMEX, for the deployment of online, ill-defined problems that are framed in a real life type scenario. In the problem selected for this study, Hazmat, participants identify inorganic unknowns by navigating a problem space that presents background information (solubility tables, flame test colour key, etc.) as well as physical and chemical tests specific to the unknown. Researchers have used IMMEX extensively in gathering student performance and problem solving strategy information (Stevens et al., 2004; Cooper et al., 2008a, 2008b). Artificial neural networks (ANN) and Hidden Markov Models (HMM) modelling affords IMMEX the capability to rapidly analyse and classify students' strategies into one of three previously characterised metacognition levels: Low, Intermediate, and High (Cooper et al., 2008b). Similarly, we modelled IMMEX performance data using Item Response Theory (Hambleton et al., 1991) to produce a measure of students' ability to solve the online IMMEX problems. The ability to solve open-ended, ill-structured problems is associated with metacognition (Cooper et al., 2008b), thus we proposed this parameter, along with the IMMEX classification of metacognitive strategy level, as indicators of metacognitive behaviour. The level of automation of IMMEX allowed the participation of hundreds of students in each of the three independent replicates within that study. Consistent results from the three replicates produced quantitative evidence that suggests that, as implemented, cooperative project-based laboratory instruction exerts a positive effect on students' ability to solve ill-structured problems and on the metacognitive level of their solution strategies. Findings from that quantitative work are in accordance with those from other researchers who have used qualitative inquiry methods to probe metacognition in chemistry laboratory environments rich in social interaction (Case et al., 2001; Larkin, 2006). Similarly, these findings are in agreement with a report presented by Kipnis and Hofstein (2008) that investigated a high school inquiry-based, chemistry laboratory programme. In their qualitative work, these authors provided evidence to support that “an inquiry-type laboratory that is properly planned and performed can give students an opportunity to practice metacognitive skills” (p. 601). In this paper we present qualitative research conducted to complement our prior quantitative study of the cooperative, problem-based general chemistry programme (Sandi-Urena et al., 2011). Hence, using a sequential explanatory approach (Creswell, 2009), we set out to gather evidence reflecting the processes taking place in the learning environment that could advance our understanding of the causes leading to the quantitative effects observed in our previous work, that is, an increase in use of more metacognitive strategies and higher ability in solving ill-structured, open-ended, online IMMEX chemistry problems.
Cooperative project based laboratory
The US National Science Education Standards, NSES, (National Research Council, 1996) outline what pre-college students “need to know, understand, and be able to do to be scientifically literate” (p. 2). They unequivocally highlight the centrality of achieving scientific literacy by promoting skills such as asking questions, constructing and testing explanations against current scientific knowledge and alternate explanations, communicating ideas, and reasoning and thinking skills (National Research Council, 1996). While no such comprehensive standards exist for higher education, it is our contention that most scientists and science educators would find these science practices of paramount relevance in tertiary education, as well. Cooperative learning lends itself as an ideal pedagogical method to address most, if not all, of the skills aforementioned. As an instructional approach, cooperative learning places the students at the centre of the learning experience and creates an environment in which they are actively engaged (Johnson et al., 1991). Typically, students work in fixed small groups and interact non-competitively towards completion of a common goal in the context of structured activities. This approach allows students to become engaged cognitively, physically, and emotionally in constructing their own knowledge which is a fundamental step in assisting them in becoming active agents in their learning experience (Johnson et al., 1991). Springer et al. (1999) provided meta-analysis evidence showing that varied forms of small group learning are effective in promoting student science learning including academic achievement, attitudes and persistence. Thurston et al. (2010) performed a longitudinal two-year study to explore retention of gains from a cooperative learning project as pupils transitioned from primary school to high school. These authors reported, amongst other findings, that “attainment gains that accrued during the original study persisted over time, and gains were still observable in the experimental group 18 months” after the initial assessment (p. 5150). Springer and collaborators (1999) argue that although the body of research is primarily based on pre-college education, it clearly suggests that cooperative learning is well established as an effective instructional approach conducive to student learning in general. Consistent with this stance, a meta-analysis that investigated the influence of cooperative instruction on learning using 15 studies, 30 learning outcomes and a total of 437 high school students and almost 1100 college students reported a mean size effect of 0.37 (standard deviation of 0.39) in chemistry achievement (Bowen, 2000).Cooperative, project-based laboratory instruction is one of the many possible ways to implement cooperative learning and it was chosen as the pedagogical intervention in the present study. Full conversion to the cooperative format at the participating institution occurred in 1994 making it a firmly established programme at the time of the study. According to the instructor's manual (Cooper, 2012b) this laboratory course
“emphasizes experimental design, data analysis, and problem solving. Inherent in the design is the emphasis on communication skills, both written and oral. Students work in groups on open-ended projects in which they are given an initial scenario and then asked to investigate a problem. There are no formalized instructions and students must plan and carry out their own investigations.” (p. 3)
The program appoints postgraduate students in all divisions of chemistry as graduate teaching assistants (GTAs). Besides being in charge of facilitating the weekly laboratory meetings, these GTAs are responsible for the evaluation of students' written reports and oral presentations. They participate in a training programme at the beginning of the academic year. The laboratory coordinator/instructor holds weekly meetings to support GTAs teaching and to assure adequate adherence to the guidelines of cooperative work project-based instruction. During the first meeting of the laboratory class, GTAs introduce students to the instructional format and assign them to teams of four that are fixed for the extent of the semester. Laboratory sections accommodate between four and six teams and meet weekly for three hours. The projects typically last four weeks and teams engage in analysing the problem, setting goals, planning strategies, designing and implementing experiments, learning necessary lab techniques, discussing and evaluating processes and outcomes, answering guiding and planning questions and so forth. Meetings outside the scheduled lab time occur at the team's discretion and are normally used to continue with planning and reviewing. During the final oral presentations, the teams communicate their procedure and rationales behind their decisions and conclusions, and their findings, and respond to questions from peers and the GTA. In addition, students submit individual preliminary reports that are returned with extensive feedback before the final reports are due. The learning experience is designed to explicitly require students to exercise an array of varied regulatory metacognitive skills that fall in the fundamental categories of planning, monitoring and evaluating. Details about the laboratory characteristics and workings can be found in the student's laboratory manual and the instructor's manual to the course (Cooper, 2012a, 2012b).
Research goal
The purpose of this study was to use a phenomenological approach to generate a rich description of the cooperative, project-based laboratory learning environment lived by general chemistry students. By means of this description, we intended to explain the findings obtained quantitatively, namely that upon participating in lab instruction a larger proportion of students used more metacognitive strategies and showed an increased ability in the solution of online, ill-structured, open-ended IMMEX chemistry problems.The study
Design and data collection
We share Nakhleh, Polles, and Malina's view that “The goal of research is to thoroughly understand what occurs in the laboratory and then work on revising curriculum and pedagogy” (2003, p. 78). Moreover, to reach that thorough understanding researchers need to realise that “the effect or value of the laboratory experience might not be measurable in a quantitative sense” (Nakhleh et al., 2003, p. 78). From this perspective, we proposed a mixed methods sequential explanatory, quantitatively driven design to investigate learning in and from the academic general chemistry laboratory. In utilising this deductive approach, we first gathered quantitative evidence to explore changes that occurred as consequence of the intervention (i.e. what occurred) and then collected qualitative data with the intention of explaining how those changes came about (i.e. how and why they occurred) (Creswell and Plano Clark, 2007). We decided to investigate the laboratory experience as lived by the students, that is, through their participant-observer lens. Fundamental strengths of this approach are the length of time the students played their role as observers and the intensity of their engagement as participants. Multiple traditions of qualitative inquiry have developed in the past century and, in some cases, they have built on each other, producing methodological overlaps that impose conscientious choosing of analytical procedures. We make the methodological case here for a phenomenological approach based on its distinct ability to provide access to understanding of “the meaning of a chosen human experience by describing the lived experience or phenomenon as perceived by the participants” (Casey, 2007, p. 118). In synthesizing this idea van Manen (1990) proposes that “phenomenology asks for the very nature of a phenomenon, for that which makes a some-”thing” what it is—and without which it could not be what it is”. Furthermore, phenomenological methods seek to uncover the internal and invariant structure of the experience not in its empirical individuality but in its essence (Patton, 2002, p. 482). Previously, Casey (2007) suggested the potential of phenomenology (Moustakas, 1994; Patton, 2002) as a research tool to study the academic laboratory experience; however, it has seldom been utilised (Sandi-Urena et al., 2011). Moreover, in view of the dimension of “researcher as instrument” in qualitative research (Patton, 2002, p. 14) we consider the expertise that our research group has accumulated in this methodological tradition a significant factor in this decision. Data collection utilised a semi-structured interview protocol designed to promote participants' reflection about and reconstruction of their laboratory experience. This type of interview involves the use of a small number of broad, open-ended, predetermined questions that prompt participants to express their spontaneous opinions and views about the experience of interest. A significant characteristic of these questions is that they provide the participants with an opportunity to introduce and discuss topics of their interests and that, we maintain, reflect those aspects that are truly essential to their experience. The interviewer then has the opportunity to further probe beyond the initial comments thereby accessing a deeper understanding of the topics brought up by the interviewee. Interview prompts included: How was your experience in the chemistry lab? How was it similar or different from the other labs you've taken? A few initial background questions, intended to develop rapport with the participants and to put them at ease, preceded those related to the lab experience. Likewise, some general, casual comments followed the main interview questions to afford wrapping up.Participants were students in large enrolment (over 1500 students) General Chemistry I Laboratory at a US research-intensive institution where the entire first year chemistry lab programme is cooperative project based. Therefore, the intervention did not impose any additional burden on the students who were merely doing the work as part of their course assignments. The only portion of the study that was not actually part of the course curriculum was the interview session. Volunteer recruiting was accomplished by sending students a mass electronic message indicating the General Chemistry Programme's interest in giving students a voice in the improvement of laboratory instruction. Students were made aware that the person in charge of the interviews was not involved with their laboratory assessment and that their participation would not have any impact on their lab grade. No compensation was offered and confidentiality and anonymity were strongly emphasised. This self-selection, convenience sampling technique produced eleven participants (five females, six males) who were all given pseudonyms. The interviews were conversational in nature and lasted from 40 to 75 min, approximately. The researcher who functioned as interviewer had previous training in conducting semi-structured interviews. Shortly after completion of each of the first five interviews, they were briefly discussed with one other researcher to fine-tune the interviewing process and monitor consistency. All interviews took place during the last two weeks of classes and finals' week of the semester the students were enrolled in the laboratory course.
Analysis and interpretation of data followed the general phenomenological guidelines proposed in the literature (Creswell and Plano Clark, 2007; Creswell, 2009; Patton, 2002). Interviews were recorded and later transcribed and independently coded by two members of the research team without the use of pre-established codes. During subsequent sessions of data analysis, the same two coders used a consensus approach to identify emergent themes. The coders jointly created brief thematic descriptions and at this point, a third researcher with extensive experience in qualitative inquiry was integrated to the discussion in the role of peer examiner (Creswell, 2009). The iterative analysis of the resulting themes led to the emergence of dimensions that constitute the core of the description of the essence of the laboratory experience. The interconnection amongst the dimensions was established at the final analysis stage. Upon its completion, the model was informally introduced to the instructor in charge of coordinating GTA's and the General Chemistry Laboratory Programme, and to a GTA with four semesters of teaching experience at the same institution. The coordinator had over eight years of experience supervising GTAs using the instructional approach, and had facilitated the same curriculum as teaching assistant for several semesters. Although these two individuals could not judge the accuracy of the model when contrasted with the data collected, we believed their experience and involvement with the learning environment made their views relevant for validity purposes.
Neither the GTA coordinator nor the GTAs were part of the research team and even though they were aware of the data collection, they were unaware of the scope and nature of the research questions. Additionally, researchers did not have any instructional contact with participants; neither did they supervise the graduate students serving as GTAs. We find appropriate separation between the implementation of the intervention and its assessment to be of utmost relevance in supporting the trustworthiness of the study. Researchers' participation in direct or indirect instruction of participants may bias their responses and performance, and/or researchers' data interpretation.
Results
The leading question for this phenomenological study was: What is the essence of the students' experience in the cooperative problem based chemistry laboratory project? Consistent with our overall mixed-methods design, our premise was that accessing this information would allow us to elucidate how the lived experience led to the findings derived from the quantitative study (Sandi-Urena et al., 2011). Those findings were: (a) an increase in the ability to solve ill-structured, online IMMEX chemistry problems, and (b) an increase in metacognitive behaviour during the solution of the same chemistry problems. Iterative analyses of the interview transcripts generated the outcome space shown in Fig. 1. In this model, three dimensions contribute to describe the experience. We propose that the interrelating factor that brings these three dimensions together is the need to take charge of the learning experience in the laboratory. The laboratory GTA coordinator found the proposed model accurate in describing students' experience in the laboratory; furthermore she confirmed that the utterances quoted in this paper are typical of students' comments and end-of-course evaluations. The dimensions are described separately below.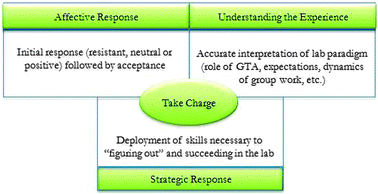 | ||
| Fig. 1 Dimensions in the outcome space. | ||
“[In high school] we were told exactly what to do and how to do it, with what supplies to use, etc. In this lab [general chemistry] we have to figure out what we need, figure out how to use it, figure out what's going on in the process and come to conclusions all on our own. It's not as guided, which can be a good or a bad thing, so… I can understand why they would want that in the aspect we're learning it more thoroughly but it does get frustrating at times.”
Some students may be more resistant than others to the challenging environment, but regardless of the initial response, there is an implicit realisation that to be functional in the laboratory the dynamics of the work to be done must be accepted: reality sinks in. Zoey's reflections are an exemplar of the kind of comments that support this dimension:
“I was very intimidated the first, uh, project that we had, just because. I felt like we were kind of thrown in, and we had to try to swim to the surface to try to figure out what to do…But after about the…halfway through the first project, I started to get in my groove and did pretty good after that.”
Just as Tyler did, Zoey found herself out of her comfort zone during the first weeks, but progressively she reached a state of understanding that enabled her to function comfortably in the learning environment:
“Once I figured out the way it [the chemistry lab] worked, I guess the way it was gonna progress, um, it did help me.”
Pete demonstrates that implicit realisation: He clearly identifies the way things are and the way he would like them to be but he accepts he cannot change them:
“I mean, I know they don't want to give you, ok here's this step and then this step but it would be nice to, like, have it clear: this is the goal…”
“The biology lab is, you have pre-described steps and you follow them and you have already little tables and stuff for you to fill out… what, what your answers are that you get from the test. Um, chemistry lab, you have a concept and you're supposed to read through before you go into it and know kinda what you're supposed to do and get some directions from the TA and then just go from there. There isn't, you know, step one do this, step two do this, step three do this. […] With the chemistry labs, I don't have the steps but I understand what I'm supposed to do and what I'm doing, so therefore, I understand the reasoning behind it.”
As Tam does, Ted not only compares labs but also through a simple and almost casual statement, he informs us of his awareness about the lab expectations. While in his other labs Ted is expected to follow directions, in the chemistry lab environment he is expected to understand and his lab work team is expected to be autonomous:
“In, um, the other labs I'm a part of, there they give us directions on what we're supposed to be doing. But, um, in this chem lab, um they don't really give us concise spoken directions. They're primarily expecting us to understand what is in the lab manual so it's, um, we're pretty much going on our own.”
Students' ability to accurately describe the role of the GTA and the team, and the absence of direct procedural instruction (written or oral) is evidence of their understanding of the laboratory operative demands. Moreover, as introduced above, students report awareness of the expectations held by the “course”, i.e., their having to reach understanding as opposed to just doing, and their having to figure out and to think through. In this sense, Pedro identifies his GTA not as a source of answers to his questions or simply a source of information but as a source of guidance:
“He [the GTA] doesn't like, outright give us the answer ‘cause I guess he's not allowed to do that, but he kinda guide us in the right direction of, like, this is what you need to look for, this is what you need to kinda set up and then once we do that, we understand what we're doing.”
Even when she was “beyond frustration” Zoey, too, saw her GTA as a resource and not as a provider of answers:
“He [the GTA] could tell that I was, like, beyond frustration, he was like ok, let's think about this for a minute […] He kinda helped me connect the dots, but um, just sitting down and calmly trying to figure out a logical way to go about what I had to do.”
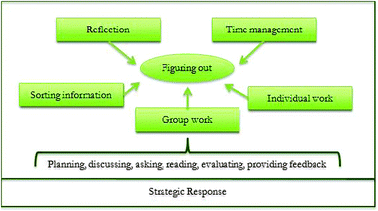 | ||
| Fig. 2 Detailed description of the strategic response dimension. | ||
| Theme | Exemplar quote |
|---|---|
| Reflection | “actually in a way it was kinda helpful to have both [Chemistry and Biology labs] because I got to see some of the differences in ways that I like to learn, um, it was kind of constricting to have all those certain steps [Biology lab] that I have to do and I don't necessarily know why I'm doing them. With the chemistry labs, I don't have the steps, but I understand…” |
| Time management | “…it's like a two part process, we'll do this other part, you know, kinda like just utilizing our time. So that we don't waste it and stay…have to finish it the next lab or something.” |
| Sorting information | “I looked to the lab or the lecture book that I bring to class. So I would use that information to put me on the right path.” |
| Individual work | “And I kinda know that…it's our responsibility to make a plan, and figure it out so. I'm more prepared coming in for these labs than I was at the very beginning. “In this lab we have to figure out what we need. Figure out how to use it. Figure out what's going on in the process and come to conclusions all on our own.” |
| Group work | “Um well we do the lab manual and we read it and we figure that stuff out and we see as much information and we can gather from that, and then the information the TA gives us and then if we and the we kinda like, devise a plan to go by and then, when that starts either not working, you know, we do something wrong, miscalculation, um, you know, we go to the TA or we just keep looking in our lab manual so it kinda, I mean, [it] gives you that, too, just kind of you don't really know what to do, just use your resources kind of thing. |
As cited above, Tam enjoyed not having to follow pre-determined steps but she also says that:
“…it was just a little bit difficult sometimes to figure out, I guess the exact road I'm supposed to take, in the respect of, you know, what's the end result supposed to look like.”
In her comment Tam reflects not only about how the laboratory challenged her team to map out a plan to accomplish their lab goal but she also touches upon the need to be able to evaluate their results by having an idea of what they should look like. To overcome the difficulty, Tam resorts to metacognitive skills: planning and evaluating. Unlike Tam, Anna dislikes the chemistry laboratory and she is anything but shy to let the interviewer know when she says:
“Um, honestly, I hate chemistry lab. Uh, I really like my lab group and I like my TA a lot, but the chemistry lab sucks.”
To better understand this emotional stance, it may be helpful to consider Anna's epistemological world-view, her understanding of knowledge. The following quotes will help in this purpose:
“I mean, I don't like to put something in my head that I'm not sure if it's right and kinda like, … I like to know it's right and then I'll, you know, go with it […] And so, I like to be taught something and learn it, and be like, well this is why happens, ok, now go do it.”
Anna thinks of knowledge as a transferable object that she can “put in her head” and that has unalterable qualities (right or wrong). Besides, she likes “to be taught”; she rather efficiently describes a transfer-only, vertical model of instruction where her responsibility is to perform by following procedures and verifying the correctness of knowledge. Her assumptions about knowledge are coherent with what Baxter Magolda (2004) calls an absolute knower and it is reasonable to expect that these assumptions will influence Anna's expectations about learning. It is no surprise that she hates cooperative problem-based laboratory projects! But what makes Anna's contribution so informative is that, despite her dislike of the laboratory, she renders a description of the lab and the activities with which she engages that are consistent with those of other students. For example, when prompted about what she is getting out of the lab she remarks that:
“It kinda shows you the chemist perspective of chemistry. You know, like you have, when you think of like a scientist, you know, exploring stuff.”
In the above quote, she shares her views of the lab approximating what she believes scientists do: exploring. The team collects information from several sources and:
“we kinda like, devise a plan to go by and then, when that starts either not working, you know, we do something wrong, miscalculation, um, you know, we go to the TA or we just keep looking in our lab manual so it kinda, I mean, [it] gives you that, too, just kind of you don't really know what to do, just use your resources kind of thing.”
Anna's team sorts out information and then engages in planning and monitoring as part of their lab routine. In the last sentence in the quote above Anna wraps up her experience in the lab by paraphrasing a common definition of problem solving being “what you do when you don't know what to do” (Wheatley, 1984). In her own words, those of a student with a dislike for the lab and an approach to learning that emphasises transfer of knowledge from the instructor, she describes the environment as one in which you learn to solve problems.
The role of social interaction goes beyond serving as a supporting frame for the practice of metacognitive skills, and its impact on learning is underscored by some of the participants. For instance, after declaring that “I really like my chemistry lab”, and telling the interviewer that he learns from others in the lab, Tim is asked what is it in the interaction with others that helps him learn to what his response is:
“a lot of my peers really do know quite a bit and I guess they've had a better background in chemistry than I have, but they get in there and they understand what's happening and they can go and they are on, they, they know what they're doing but they are on the same level as I'm on, so they can work better to relate it to me where a person, say a graduate student, like my TA, he kinda forgot what it's like to be right there […] there are the students down at my level, can be like, oh, oh well, I know what you are missing, you don't understand this part. And then they can go back and understand it and, uh, explain that to me so I can understand it.”
Interestingly, these candid statements constitute a sound contribution to support the relevance of the social mediation of learning as described by Vygotsky's framework in this particular laboratory environment. They constitute Tim's experiential explanation of scaffolding and how it helps him achieve higher levels of autonomous competence in the context of the laboratory problem solving activities (Reigosa and Jimenez-Aleixandre, 2007). Tim acknowledges the GTA's function but at the same time realises that his ‘more able peers’ are closer to him in understanding, they can relate better to him and can support him in his learning task. Reigosa and Jimenez-Aleixandre (2007) have previously proposed collaborative open problem-solving laboratory activities as mediators to promote scaffolding learning and have studied implementation difficulties.
Discussion
The results from this study document the experience of general chemistry laboratory students engaged in cooperative problem-based project instruction. Our phenomenological analysis approach aimed at elucidating “the meaning, structure, and essence of the lived experience” (Patton, 2002, p. 482) for this group of students. Deep textual analysis of the interviews shed light to allow construction of an outcome space (Fig. 1) that describes students' experience in terms of three fundamental dimensions. There is a chronological aspect to these dimensions and they may initiate sequentially: First, students start the process of acceptance of the laboratory format as the solution to the affective conflict. This affective conflict arises, along with the cognitive imbalance, from entering an instructional environment that carries unexpected demands, and that cannot be easily related to previous experiences. The nature of the interactions with peers and instructor, and the lab dynamics are unfamiliar. Not surprisingly, in such a situation, students may be overwhelmed, develop a sense of loss of control and be prone to frustration. Secondly, students start the engagement in understanding the laboratory operative level and its expectations. We believe the events that advance this process are not necessarily premeditated or conscious but occur naturally within the culture that develops in the lab. For example, after approaching the GTA with direct content questions, the students soon come to realise that the instructor is not a source of straight answers. This understanding is acquired by participating and contributing in the establishing of the laboratory culture, and constitutes the initial step in regaining control. Thirdly, empowered by their gradual increase in understanding of the lab paradigm, students start implementing and/or further developing the skills needed to complete the task successfully. Initially, they may be hesitant to participate in adaptive learning behaviour such as sharing and refuting ideas, asking questions, attempting to learn and do new things that may lead to “undesirable consequences” such as facing failure or public ridicule (Beghetto, 2009). This kind of behaviour, intellectual risk taking, is considered a key attribute of scientific reasoning (Bransford and Donovan, 2005) and has been associated with learning and academic identity development (Beghetto, 2009). Progressively, students become more comfortable engaging in this kind of behaviour. For example, they rely less on the GTA for help and look more at each other for support and discussion; in accordance with their reports they assume more responsibilities as their own, such as finding and sorting information. Our findings constitute supporting evidence for Thurston and collaborators' suggestion that in cooperative environments “learning may be encoded with meta-cognition” (Thurston et al., 2010). These authors maintain that cooperative group work promotes reflective learning were participants become aware of how learning relates to prior learning, and self-regulation is induced and facilitated by peer-to-peer immediate and sustained feedback. Or as Larkin (2006) states in reference to the effect of collaborative environments in the development of metacognitive processing in children: “Asking question of oneself can begin by being questioned by others” (p. 23). This skilfulness dimension is of paramount relevance for the larger research work in which this qualitative analysis is nested. It brings forth evidence indicating that as a result of their immersion in the laboratory environment the students engage actively and deeply in metacognitive behaviour. Figuring out becomes a social norm within the laboratory experience that encourages engagement in reflection and argumentation, and promotes feedback and reciprocal explaining. The ability to explain is “a basic property of human beings, one that influences many aspects of cognition, including memory, problem solving, and conceptual understanding” (Siegler and Lin, 2009). The learning experience is therefore enhanced by having multiple opportunities to articulate ideas to peers and to hear and discuss others' ideas (Singer et al., 2006, p. 81). It is evident from several of the quotes above that despite beginning sequentially, the dimensions that emerged do not necessarily follow a linear trajectory but overlap and mutually inform one another. For instance, students develop skilfulness while still consolidating their understanding of the lab environment and negotiating their affective stance. Likewise, their understanding of the learning environment and how they function in it may influence their affective stance. The progress made in these processes, in terms of time and depth, is individual. This fluidity and interrelationship is represented by the dimensions sharing edges in Fig. 1 which portrays students' transitions amongst the dimensions once they are established.The final stage in our data analysis moved beyond the descriptive outcome space to identify additional structural qualities. We argue here that taking charge is the overarching, interconnecting factor that can be seen as the feature that holds the model together. Initially, learning in the cooperative laboratories is facilitated for the students but ultimately it becomes their responsibility. Students are empowered in that they are in control of their learning but in order to access this control and take charge of the situation, they have to continually elaborate on the three dimensions describing the outcome space. Taking charge of their learning is the overarching requisite of success and their actions and decisions need to be concerted in that direction. This directly addresses Gunstone's contention of the challenge being “to help learners take control of their own learning in the search for understanding” (Gunstone, 1991, cited in Hofstein and Lunetta, 2004, p. 32).
Conclusions and implications for teaching
The laboratory programme studied was the product of a reform effort in the early 1990s (Cooper, 1994). It was designed on the premise that such experiences improve problem solving skills and metacognition, a premise strongly supported by the quantitative findings reported previously (Sandi-Urena et al., 2011). The phenomenological study presented here posed an obvious path to reach a deeper understanding of the processes taking place in the laboratory. The qualitative inquiry produced a description of the learning environment that confirms that it effectively creates an experience rich in opportunities for metacognitive practice. Participants' need to use and develop metacognitive strategies was evidenced particularly by the Strategic Response dimension in the outcome space in which a toolbox of related skills is described. This finding resonates with Kipnis and Hofstein's (2008) observations derived from their long-term comprehensive series of investigations in high school inquiry-type chemistry laboratory. Especially so with their assertion that:“Since the students that participate in the inquiry laboratory activities are obliged to act according to the activities that are typical of students with developed metacognition, it is logical to assume that during the study of the inquiry laboratory unit the students can practice and develop their metacognitive skills.” (p. 604)
Though it may seem trivial at first, and for sure hard to quantify, students engage in metacognitive processes as they begin to solve the affective conflict and to try to understand how the lab operates. Their object of reflection here is different from the lab project itself and refers to their own cognitive processes and products: How to function and succeed in this instructional environment? This problem may be more readily recognized as such by the participants than the problem related to the actual lab project. It appears that the solution to this conundrum—even though students would not verbalize it this way—is to be and act more metacognitive. Although instruction was metacognitive and metacognition-promoting in nature (Sandi-Urena et al., 2011), instruction of metacognition was not explicit, that is, participants were not directly instructed about metacognition. We believe that situating learning in this context facilitates internalization of strategies since they are self-discovered or generated for a meaningful purpose as opposed to externally introduced. This adaptive behaviour may explain in part the persistence of gains reported by other researchers (Thurston et al., 2010).
In our study design, the assessment of the effectiveness of the environment in developing metacognition does not rely on student evaluations or surveys directly addressing the intervention. Our data gathering methods were not specific to the construct under scrutiny and subjective aspects such as engagement, morale, or participation are not included as measures of effectiveness. We have strived to place and maintain distance between researchers and participants during the intervention and to not influence the workings of the learning environment; we have tried to actively avoid direct instruction of any kind that may bias students' responses and performance, and/or researchers' data interpretation. We maintain that the use of a phenomenological approach to investigate the academic chemistry laboratory suits this array of purposes.
We propose that the applicability and usefulness of the findings drawn from this work may not be exclusive to this laboratory course. Their consistency with other reports (Case et al., 2001; Larkin, 2006; Case et al., 2007; Poock et al., 2007; Cooper et al., 2008; Kipnis and Hofstein, 2008) sheds light about the possibilities of instruction in the science laboratory—particularly at the college level. Two fundamental components of this type of learning experience are the intense purposeful social interaction—which must be clearly differentiated from the activity for the activity's sake—and an environment that is conducive to the exercise of metacognitive skilfulness. We postulate that the combination of these factors significantly promotes the development of problem solving skills and learning. We believe that the evidence presented supports the relationship between experiences in the laboratory and student effective learning, and can inform curricular development based on sound research evidence. The science laboratory is an ideal environment to promote the development of metacognition as an underlying constant across a variety of social learning and teaching tasks (Yore and Treagust, 2006).
One final note, from our interviews and observations it was clear that not all students “liked” the laboratory format. For instance, Anna believed “the lab sucks”, yet still articulated descriptions of the lab that were consistent with other students. Even though she was unwilling to admit it, Anna was beginning to use skills and thought processes that contradicted her conception of knowledge as a transferable object. It has been proposed that maximal learning takes place when students are challenged with new experiences in a supportive environment. However, learning is difficult, challenging and may be uncomfortable, particularly for students who have not made the transition to self directed learning and especially so in large introductory courses. Student satisfaction should not be the only metric used to evaluate teachers and learning environments; in fact, we believe that is it possible to provide fruitful learning environments even, and especially, for those resistant students.
References
- Baxter Magolda M. B., (2004), Evolution of a constructivist conceptualization of epistemological reflection, Educ. Psychol., 39, 31–42.
- Beghetto R., (2009), Correlates of intellectual risk taking in elementary school science, J. Res. Sci. Teach., 46, 210–223.
- Bowen C., (2000), A quantitative literature review of cooperative learning effects on high school and college chemistry achievement, J. Chem. Educ., 77, 116.
- Bransford J. D. and Donovan S. M., (2005), Scientific inquiry and how people learn, in S. M. Donovan and J. D. Bransford (ed.), How students learn: History, mathematics, and science in the classroom, Washington, DC: The National Academies Press, pp. 397–420.
- Case E. L., Cooper M. M. and Stevens R. H., (2007), Is collaborative grouping an effective instructional strategy?, J. Coll. Sci. Teach., 36, 42–47.
- Case J., Gunstone R. and Lewis A., (2001), Students' metacognitive development in an innovative second year chemical engineering course, Res. Sci. Educ., 31, 313–335.
- Casey K., (2007), Phenomenology, in G. M. Bodner and M. Orgill (ed.), Theoretical frameworks for research in chemistry/science education, Upper Saddle River, NJ: Pearson Education, Inc., pp. 122–131.
- Cooper M. M., (1994), Cooperative chemistry laboratories, J. Chem. Educ., 71, 307.
- Cooper M. M., (2012a), Cooperative chemistry laboratory manual, (6th ed.), New York, NY: McGraw-Hill.
- Cooper M. M., (2012b), Instructor's manual to accompany cooperative chemistry laboratory manual, (4th ed.), New York, N.Y.: McGraw Hill.
- Cooper M. M., Cox C. T., Nammouz M., Case E. and Stevens R. H., (2008a), An assessment of the effect of collaborative groups on students' problem solving strategies and abilities, J. Chem. Educ., 85, 866–872.
- Cooper M. M., Sandi-Urena S. and Stevens R. H., (2008b), Reliable multi method assessment of metacognition use in chemistry problem solving, Chem. Educ. Res. Pract., 9, 18–24.
- Creswell J. W., (2009), Research design: Qualitative, quantitative, and mixed methods approaches, (3rd ed.), Thousand Oaks, CA: Sage Publications, Inc.
- Creswell J. W. and Plano Clark V. L., (2007), Designing and conducting mixed methods research, Thousand Oaks, CA: Sage Publications.
- Ding N. and Harskamp E., (2010), Collaboration and peer tutoring in chemistry laboratory education, Int. J. Sci. Educ., DOI:10.1080/09500693.2010.498842.
- Domin D., (1999), A review of laboratory instruction styles, J. Chem. Educ., 76, 543–547.
- Gabel D., (1999), Improving teaching and learning through chemistry education research, J. Chem. Educ., 76, 548–544.
- Hambleton R. K., Swaminathan H. and Rogers H. J., (1991), Fundamentals of item response theory, Newbury Park, CA: Sage Publications.
- Hart C., Mulhall P., Berry A., Loughran J. and Gunstone R., (2000), What is the purpose of this experiment? Or can students learn something from doing experiments?, J. Res. Sci. Teach., 37, 655–675.
- Herrington D. G. and Nakhleh M. B., (2003), What defines effective chemistry laboratory instruction? Teaching assistant and student perspectives, J. Chem. Educ., 80, 1197–1205.
- Hodson D., (1996), Laboratory work as scientific method: Three decades of confusion and distortion, J. Curriculum Stud., 28, 115–135.
- Hofstein A. and Lunetta V. N., (1982), The role of the laboratory in science teaching: Neglected aspects of research, Rev. Educ. Res., 52, 201–217.
- Hofstein A. and Lunetta V. N., (2004a), The laboratory in science education: Foundations for the twenty-first century, Sci. Educ., 88, 28–54.
- Hofstein A., Shore R. and Kipnis M., (2004b), Providing high school chemistry students with opportunities to develop learning skills in an inquiry-type laboratory: A case study, Research report, Int. J. Sci. Educ., 26, 16.
- Hofstein A. and Mamlok-Naaman R., (2007), The laboratory in science education: The state of the art, Chem. Educ. Res. Pract., 8, 105–107.
- Johnson D. W., Johnson R. T. and Smith K. A., (1991), Active learning: Cooperation in the college classroom, Edina, MN: Interaction Book Company.
- Kipnis M. and Hofstein A., (2008), The inquiry laboratory as a source for development of metacognitive skills, Int. J. Sci. Math. Educ., 6, 601–627.
- Kirschner P. A. and Meester M. A. M., (1988), The laboratory in higher science education: Problems, premises and objectives, High. Educ., 17, 81–98.
- Lagowski J. J., (1990), Entry-level science courses: The weak link, J. Chem. Educ., 67, 185.
- Larkin S., (2006), Collaborative group work and individual development of metacognition in the early year, Res. Sci. Educ., 36, 7–27.
- McCreary C. L., Golde M. F. and Koeske R., (2006), Peer instruction in the general chemistry laboratory: Assessment of student learning, J. Chem. Educ., 83, 804–810.
- Moustakas C., (1994), Phenomenological research methods, Thousand Oaks, CA: Sage Publications.
- Nakhleh M. B., Polles J. and Malina E., (2003), Learning chemistry in a laboratory environment, in J. K. Gilbert, O. De Jong, R. Justi, D. Treagust and J. H. Van Driel (ed.), Chemical Education: Towards Research-Based Practice, Dordrecht, Kluwer Academic Publishers, pp. 69–94.
- National Research Council, (1996), National science education standards: Observe, interact, change, learn, Washington, D.C.: National Academy Press.
- Patton M. Q., (2002), Qualitative research and evaluation methods, (3rd ed.), Thousand Oaks, CA: Sage Publications.
- Pienta N., Cooper M. and Greenbowe T. (ed.), (2005), Chemists' guide to effective teaching, Washington, DC: National Academy Press.
- Poock J. R., Burke K. E., Greenbowe T. J. and Hand B. M., (2007), Using the science writing heuristic in the general chemistry laboratory to improve students' academic performance, J. Chem. Educ., 84, 1371–1379.
- Reid N. and Shah I., (2007), The role of laboratory work in university chemistry, Chem. Educ. Res. Pract., 8, 172–185.
- Reigosa C. and Jimenez-Aleixandre M., (2007), Scaffolded problem-solving in the physics and chemistry laboratory: Difficulties hindering students' assumption of responsibility, Int. J. Sci. Educ., 29, 307–329.
- Sandi-Urena S., Cooper M. and Gatlin T., (2011), Graduate teaching assistants' epistemological and metacognitive development, Chem. Educ. Res. Pract., 12, 92–100.
- Sandi-Urena S., Cooper M. and Steven R., (2011), Effect of cooperative problem based lab instruction on metacognition and problem solving skills, submitted.
- Schraw G., Crippen K. J. and Hartley K., (2006), Promoting self-regulation in science education: Metacognition as part of a broader perspective in learning, Res. Sci. Educ., 36, 111–139.
- Siegler R. S. and Lin X., (2009), Self-explanations promote children's learning, in H. S. Waters, W. Schneider and J. G. Borkowski (ed.), Metacognition, strategy use, and instruction. New York: Guilford Press.
- Singer S. R., Hilton M. L., Schweingruber H. A. and National Research Council (ed.), (2006), America's lab report: Investigations in high school science, Washington, D.C: National Academies Press.
- Springer L., Stanne M. and Donovan S. S., (1999), Effects of small-group learning on undergraduates in science, mathematics, engineering, and technology: A meta-analysis, Rev. Educ. Res., 69, 21–51.
- Stevens R., Soller A., Cooper M. M. and Sprang M., (2004), Modeling the development of problem solving skills in chemistry with a web-based tutor, Intelligent Tutoring Systems 7th International Conference Proceedings, Berlin, Germany, 580–591.
- Suits J. P., (2004), Assessing investigative skill development in inquiry-based and traditional college science laboratory courses, School Sci. Math., 104, 248–257.
- Thurston A., Topping K., Tolmie A., Christie D., Karagiannidou E. and Murray P., (2010), Cooperative learning in science: Follow-up from primary to high school, Int. J. Sci. Educ., 32, 501–522.
- Tien L. T., Teichert M. A. and Rickey D., (2007), Effectiveness of a MORE laboratory module in prompting students to revise their molecular-level ideas about solutions, J. Chem. Educ., 84, 175–181.
- Tobin K., (1990), Research on science laboratory activities: In pursuit of better questions and answers to improve learning, School Sci. Math., 90, 403–418.
- Wheatley G. H., (1984), MEPS technical report 84.01.
- Yore L. D. and Treagust D. F., (2006), Current realities and future possibilities: Language and science literacy—empowering research and informing instruction, Int. J. Sci. Educ., 28, 291–314.
| This journal is © The Royal Society of Chemistry 2011 |
