Design, development and implementation of a technology enhanced hybrid course on molecular symmetry: Students' outcomes and attitudes
L. D.
Antonoglou
,
N. D.
Charistos
and
M. P.
Sigalas
Department of Chemistry, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece. E-mail: sigalas@chem.auth.gr
First published on 3rd October 2011
Abstract
A hybrid course of Molecular Symmetry and Group Theory which combines traditional face-to-face instruction with an online web enhanced learning environment within a Course Management System was designed, developed, and implemented with a purpose to establish an active and student-centred educational setting. Multi-representational educational material enclosing 3D interactive molecular visualization applets was designed, developed, and effectively integrated in the course in order to enhance students' learning and enable practice with molecular symmetry concepts. Moodle was utilized for content delivery in study blocks, formative online assessment, final examination, student tracking, communication and feedback. The hybrid instructional model provides an efficient mode of integration of visualization tools in the teaching and learning process. The findings over three years of implementation of the hybrid course reveal its positive impact on students' involvement with the course content during the whole semester, on students' performance as well as on their satisfaction with their educational experience.
Introduction
Molecular symmetry is a fundamental topic in Chemistry as the examination and identification of the symmetry of a molecular structure provide chemists with considerable insight into many of its chemical and physical properties such as bonding, reactivity, chirality, polarity and spectroscopy. This shift from molecular symmetry to molecular and electronic properties is mediated by the implementation of Group Theory, which enables the classification and algebraic treatment of structural configurations. However, chemists, chemistry teachers and educational chemistry researchers are traditionally less concerned about the mathematical formalism and focus more on the structural characteristics of molecular configurations.It is a common belief that molecular symmetry is a topic grounded in visualization (Tuvi-Arad and Blonder, 2010) as the identification of symmetry elements impels learners to create and manipulate static and dynamic three dimensional mental representations of molecular structures, usually by the observation of two dimensional symbolic representations. These tasks may overburden learners' cognitive system as they require extensive use of visuospatial thinking. Research has shown that chemistry students have difficulties in visualizing the interactive and dynamic nature of molecular properties by viewing 2D symbolic representations (Ferk and Yrtacnik, 2003; Wu and Shah, 2004).
In a conventional lecture-based teaching environment of molecular symmetry, students' involvement is restricted as educators usually employ teaching methods which focus on information delivery and knowledge acquisition rather than the construction of knowledge within a social context. A typical conventional lecture uses projections of static displays with symbolic representations of molecules and graphical depiction of molecular structure properties. Most of the time, the conventional lecture is conducted through a one-way communication, with limited opportunities for class discussion and active student involvement. In this setting students are passive listeners, taking notes from projected displays and from the lecturer's explanations (Talib et al., 2005). Drawing inferences from static illustrations in order to comprehend complex scientific conceptions may be time consuming. This is made more difficult if the conception is not only complex, but also abstract and dynamic such as in molecular symmetry.
Moreover, in traditional settings students have at their disposal conventional educational material, such as textbooks and lecture notes of molecular symmetry, which predominantly encompass static symbolic representations that do not support 3D visualization and do not give learners the opportunity to practice and actively explore these abstract concepts in order to construct their own conceptual understanding. In addition, many molecular symmetry concepts can not be depicted by 2D symbolic representations and the number of examples in textbooks is very limited.
Considering these issues, several educational chemistry researchers have developed molecular visualization websites and software applications to enhance learning of molecular symmetry concepts (Antonoglou, Charistos and Sigalas, 2008). These applications offer opportunities for extensive practice and active exploration by providing three dimensional visualization and modelling of symmetry elements and operations applied to molecular structures (Korkmaz and Harwood, 2004; Charistos et al., 2005; Cass and Rzepa, 2005; Tuvi-Arad and Gorsky, 2007; Meyer and Sargent, 2007; Johnston, 2009; Tuvi-Arad and Blonder, 2010). These tools can serve as learning objects that can be used and reused in different settings such as presentation tools to promote deeper understanding during instruction, or as self-pace tools in conjunction with classical textbooks, where the learners can actively explore the represented molecular concepts. Some of them are open-ended, accepting user data inputs and are appropriate for inquiry-based learning (Tuvi-Arad and Gorsky, 2007; Meyer and Sargent, 2007).
Although educators and students recognize the valuable capabilities of these tools as supportive learning materials (Ealy, 2004; Dori and Barak, 2001; Tuckey and Savaratnam, 1993), students often receive them as extra resources and the activities as additional or external to their principal activity which is the one to be evaluated or specifically required by the instructor (Korkmaz and Harwood, 2004; Orton-Johnson, 2009; López-Pérez et al., 2011). Other limitations of these applications concern their exclusive focus on specific aspects of the domain knowledge coverage and their paucity of integrated representational modalities and coding systems.
An innovative and effective integration of educational technologies for teaching and learning chemistry is essential (Barak, 2007). The innovative use of technologies refers to their pedagogically appropriate implementation in educational settings in order to stimulate and maintain active student involvement in a socially situated and highly interactive learning process (Vaughan, 2007).
In this study we present the design and development of interactive multi-representational educational material for learning and teaching Molecular Symmetry, as well as the development of a hybrid educational environment of Molecular Symmetry and Group Theory, which utilize the best features of traditional face-to-face instruction with effective implementation of innovative web-delivered educational technologies.
Design of multi-representational educational chemistry software
Representations in chemistry learning usually refer to the macroscopic, microscopic and symbolic levels (Johnstone, 1993; Treagust et al., 2003), while the molecular symmetry domain deals with the microscopic and symbolic level of representations. Chemistry learning requires the mastering of a plethora of symbolic representations. The set of skills that students have to develop for constructing, interpreting, transforming, and coordinating domain-specific external representations for learning and problem solving in chemistry has been identified as representational competence (Kozma and Russell, 1997; Kozma et al., 2000; Kozma and Russel, 2005; Stieff et al., 2011). The development of representational competence by chemistry students is a major goal in chemical education (Nakhleh and Postek, 2008). Learning with multiple representations has become a central topic in educational research and it is approached by several perspectives which examine the sensory channel and the modality of the representations as well as the way that multiple representations interact with each other and with prior knowledge (Schnotz and Kürschner, 2008; Cook et al., 2008).Ainsworth (1999, 2006), suggests that there are three main functions that multiple representations serve in learning situations: (a) to complement each other, where differences between representations may be either in the information that each contributes, or in the cognitive processes that each supports, (b) to constrain each other, where one familiar representation is used to constrain possible interpretations in the use of another and (c) to construct a deeper understanding in terms of promoting abstraction, encouraging generalisation and teaching the relation between representations. During a learning task with a multi-representational display all the above functions may be performed simultaneously.
Multi-representational learning environments are also approached by Cognitive Load Theory (Sweller, 1988; Sweller et al., 1998) and Cognitive Theory of Multimedia Learning (Mayer, 2001; 2003). Both theories share the same perspective of human cognitive architecture and focus on the nature of working memory, which is considered to be multiple and modality specific consisting of two independent slave systems: (a) the ‘phonological loop’ which processes auditory–verbal information and (b) the ‘visuospatial sketchpad’ which processes visual–pictorial information. Working memory is also limited in capacity and duration and interacts with an unlimited long-term memory. Learning with multiple representations requires the active processing of perceived information in each channel along with prior knowledge in order to construct a coherent knowledge structure. This process involves the selection of relevant information from each external verbal and pictorial representation, the formation of the corresponding verbal and pictorial mental models and their integration with prior knowledge into a coherent mental representation (Mayer, 2003). Working memory can be overloaded and learning can be hindered as the number of interactive elements that have to be simultaneously processed in each channel is increased. Both theories provide guidelines for the development of well-designed multimedia learning environments that aim to make optimal use of the two slave systems by presenting an ideal mix of static or dynamic verbal and pictorial information, without overburdening learners' working memory (Kirschner et al., 2011).
In a literature review Wu and Shah (2004) summarize several design principles for effective educational chemistry software that helps students understand chemical concepts and develop representational skills through supporting their visuospatial thinking. Specifically, they propose that educational chemistry visualization tools should: (a) provide multiple representations and descriptions in order to enable students to make connections between the representations and the concepts that are represented, (b) provide students with opportunities to actively choose a representation suitable for different stages of understanding, (c) make linked referential connections visible, so that students can construct appropriate conceptual connections among multiple representations, (d) present the dynamic and interactive nature of microscopic chemical phenomena and concepts, in order to support visuospatial thinking, (e) promote the transformation between 2D and 3D representations of molecular structures, and (f) reduce cognitive load by making information explicit and integrating information for students.
The hybrid instructional model
Considering the promising benefits and positive impacts on learning by the implementation of Information and Communication Technologies (ICT), a common question which arises among the instructors is how to integrate the best features of ICT into a conventional classroom-based model of instruction. Marques et al. (1998), in response to that question, propose a hybrid instructional model. The hybrid approach to instruction is basically composed of face-to-face components such as lectures, in-class discussion and group work, with web components like online content, forum, chat rooms, web-mail, online assessment and feedback. Hybrid or blended learning is generally agreed to involve a mixture of instructional modalities, delivery media, instructional methods, and web-based technologies (Garrison and Kanuka, 2004; Graham, 2006; Graham and Dziuban, 2008). Bonk and Graham (2005) identify three functions of blended learning systems: enabling (access and convenience), enhancing (using technology to add value), and transforming (change to course design, learn through interactions and activities).It is important that hybrid courses are not confused with web-enhanced courses. A web-enhanced course may have a website or even a textbook online, but students attend class in the traditional lecture-based fashion. On the other hand the hybrid course is differentiated from a web-enhanced course in that the hybrid learning intends to thoughtfully integrate traditional face-to-face and fully online instructional components in order to take advantage of both learning environments and better address the diverse learning styles of the students (Dziuban et al., 2004; Garrison and Kanuka, 2004; Graham and Dziuban, 2008).
Graham and Dziuban (2008) suggest that the focus should be on the holistic nature of the learning experience, on how learning occurs and how it will be supported and enhanced, rather than on the instructional mode, the proportion between face-to-face and online components and the available state-of-the-art technologies. Williams et al. (2008) proceeded in redesigning a module in inorganic chemistry by adopting a hybrid instructional model. They concluded that students not only improved their performance when e-learning was added to traditional face-to-face teaching, but that students' interaction and satisfaction with the subject content, delivery and performance feedback improved as well.
Many universities have experienced success in implementing hybrid learning courses and have found a number of benefits and reasons for adopting the hybrid instructional model, that is: (a) better students' performance (Garnham and Kaleta, 2002; López-Pérez et al., 2011) (b) adjustment to greater range of learning and teaching styles (McCray, 2000; Olapiriyakul and Scher, 2006), (c) facilitation of student centered learning (Marques et al. 1998; Graham and Dziuban, 2008), (d) establishment of a community of learners (Rovai and Jordan, 2004; Lin, 2008), (e) student satisfaction (Lin, 2008; Williams et al., 2008; Delialioglu and Yildirim, 2008; López-Pérez et al., 2011) (f) increased engagement with course material (Riffell and Sibley, 2003) and (g) flexibility and greater cost effectiveness (Garnham and Kaleta, 2002).
Rationale of the project and research hypothesis
In our Chemistry department there is an undergraduate course covering the three topics of the domain: (a) Molecular Symmetry, (b) Group Theory and (c) Applications of Symmetry. Each topic refers to different conceptual and representational levels. In the first topic students have to conceptualize how symmetry operations transform molecular configurations and to classify molecules according to the spatial arrangement of atoms in three dimensional space. In the second topic they have to master the mathematical formalism of group theory and apply algebraic methods to molecular symmetry concepts that they learned in the first topic. In the third topic they have to combine concepts, strategies and techniques that they learned in both preceding topics in order to deduce meaningful chemical conclusions and predict chemical behaviour.Traditionally in our Department, the Molecular Symmetry and Group Theory course was taught three hours per week over the fifth (fall) semester of the third year of the Chemistry degree by means of lectures supported by multimedia presentations, textbooks, lecture notes and was terminated with a final, paper and pencil examination. Considering the limitations of traditional teaching with traditional media, we developed interactive multi-representational educational material for the first topic of the course and transformed the whole traditional course by adopting a hybrid instructional model. Our aim was to achieve a student centred learning environment that motivates and retains students' interaction during the whole semester with the course content and the learning process, enhances their performance in molecular symmetry subjects and satisfies them with their educational experience.
The objectives to accomplish this aim were the following:
• Increase students' engagement and interaction with study material.
• Encourage learners to take responsibility for their own learning at their own pace.
• Address diverse learning styles.
• Stimulate students' substantial and active participation in face-to-face lectures.
• Support the development of a learning community.
The accomplishment of the above objectives constitute the research hypothesis of the study which could be stated as: The hybrid educational environment is effective in increasing the quality and quantity of interaction with educational material and learning activities in an undergraduate chemistry course.
The hybrid course process and material
The molecular symmetry course in our Chemistry Department was redesigned and developed so as to incorporate traditional face-to-face teaching with effective pedagogical use of web-based distance learning technologies (Garrison and Kanuka, 2004; Graham and Dziuban, 2008), as well as molecular visualization educational software tools. The online part of the course was organized in study blocks and implemented in the free Content Management System (CMS) Moodle (Martin-Blas and Serrano-Fernandez, 2009). Each study block corresponds to a specific learning module of the chemistry subject. The course syllabus covers the following modules: (A) Molecular symmetry: (A1) Introduction to Symmetry, (A2) Symmetry and Chemistry, (A3) Symmetry elements and operations, (A4) Point groups, (B) Group Theory: (B1) Introduction to Group Theory, (B2) Representations of point groups, (B3) Character tables, (C) Applications of Symmetry: (C1) Techniques and methodology for symmetry applications, (C2) Applications of symmetry in Quantum Chemistry and (C3) Chirality, polarity and molecular symmetry.The basic features of the hybrid course are:
• The integration of novel molecular visualization educational software to support the teaching and learning of molecular symmetry concepts.
• The deliverance of the online course material in study blocks by the use of a CMS.
• The implementation of online learning activities and formative online assessment (quizzes).
• The provision of multiple forms of resources in different ways and locations, allowing students to select and utilize the materials that are most suitable to them.
• The provision of feedback to preceding and guidance to forthcoming study blocks.
• The provision of synchronous and asynchronous communication tools in conjunction with physical presence impelling students to face common learning objectives and practices in the setting of a learning community.
Interactive educational material
The educational material of the Molecular Symmetry and Group Theory course has been developed and delivered as Printable Lecture Notes in PDF format and as Interactive Lecture Notes in the form of a website. Both formats enclose the whole theory of the course in textual form accompanied by a large number of 2D symbolic representations in alignment with common educational books of the domain. Interactive lecture notes of the first topic (modules A1–A4) were enriched with eighty (80) 3D molecular visualization applets that visualize and model symmetry elements and operations. Two kinds of visualization applets were designed: (a) Concept Applets, in which a specific concept is depicted, and (b) Exploration Applets, in which many concepts are available for practice and exploration.Technology
Interactive lecture notes were developed within the HTML4/CSS2 framework. Molecular visualization applets were developed with Adobe Director authoring environment, which allows high quality and performance web delivered real-time native 3D graphics, utilizing the Shockwave plug-in. They were designed with an object oriented programming approach, allowing straightforward development of multiple modules that correspond to different learning scenarios.Learning with molecular symmetry visualization applets
In order to identify each symmetry element of a molecule the learner has to perform the following cognitive tasks: (a) form a 3D mental model of the molecular structure by the observation of a 2D symbol, (b) identify a possible symmetry element of the molecular structure, (c) perform the corresponding symmetry operation (rotation, reflection, inversion) to the 3D mental model, and (d) verify whether the final 3D molecular configuration is identical to the initial configuration. These tasks can overburden the visuospatial channel of working memory since they demand extensive use of visuospatial thinking (spatial visualization, spatial orientation and spatial relations) and hold a high intrinsic cognitive load as the 3D mental models of the initial, transforming and final structure have to be simultaneously processed.The 3D molecular visualization applets that we developed were designed to reduce cognitive load and facilitate learning by externalizing the above mental processes. They provide 3D visualization of molecular structures that the user can freely manipulate to any view point, visualization of symmetry elements and animations of symmetry operations. During the animation of a symmetry operation the molecule's initial geometry is displayed as a semitransparent “ghost” structure allowing the comparison of the configuration of the molecular structure before, during and after the application of the symmetry operation. Throughout the animation the user can still manipulate the molecule to reach the most convenient point from which to view the symmetry operation. The user can adjust the speed, pause and restart the animation at any time. In addition the user can manually perform the symmetry operation at her/his own pace by dragging a slider. After its completion the symmetry maps the molecule onto its “ghost” structure that is itself.
Concept applets
Single representation of molecular symmetry concepts with 3D visualization does not ensure deeper understanding of the represented concepts unless they are combined with the corresponding content knowledge. Interactive lecture notes constitute a multi-representational educational environment since the same molecular concepts are simultaneously presented in three forms of external representation that differentiate in regard to the sign systems that they use, the level of abstraction and the operations that can be performed with them.Specifically, certain key concepts of molecular symmetry are presented with descriptive textual representations, depictive 2D symbolic representations and interactive 3D visualizations (Fig. 1). In this case concept applets are used, in which the content of the 3D environment is restricted to specific visualizations and its functionality is adjusted to certain tasks corresponding to the represented concept. Wherever it was applicable, textual labelling of atoms and symmetry elements were embedded in 3D applets and 2D symbolic representations in order to make referential connections visible. Within this setting the learner has the opportunity to choose a representation suitable for her/his stage of understanding, make connections across multiple representations and the concepts that are represented, make transformations between 2D and 3D representations, practice and actively explore the dynamic nature of the represented concepts.
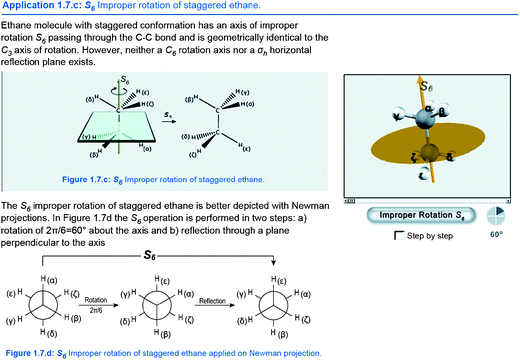 | ||
| Fig. 1 Detail from interactive lecture notes with descriptive textual, depictive 2D symbolic and interactive 3D representations of molecular symmetry concepts (translated from Greek original). | ||
The learning process with the multi-representational environment of interactive lecture notes could be described as follows. Except for reducing cognitive load and externalizing mental processes, 3D dynamic representations could complement and support the interpretation of static and abstract 2D symbolic representations and foster the construction of deeper understanding by establishing relations between representations, leading to the construction of a meaningful visual mental model. On the other hand, the learner could generate a propositional representation of the semantic content from the text, and construct a verbal mental model of the subject matter described (Schnotz and Kürschner, 2008). These two mental models could be combined together along with prior knowledge to result in meaningful learning.
Exploration applets
Apart from inherent cognitive demands of learning molecular symmetry concepts, another issue is the limited number of examples that can be depicted by 2D symbolic representations presented in traditional educational media as well as the restricted opportunities that a student has for practicing and exploring these concepts.Several exploration applets were embedded in interactive lecture notes (Fig. 2). These applets operate as molecular symmetry simulations and as searchable databases containing structural and symmetry data of more than 60 organic and inorganic molecules and shapes, spread to all symmetry point groups of chemical interest and covering all characteristic cases.
 | ||
| Fig. 2 Representative webpage with exploration applet (translated from Greek original). | ||
The user can search the database and filter the molecules according to the symmetry elements that they possess or the point groups that they belong to. Then she/he can select and visualize the molecule as an interactive 3D model as well as a 2D structural formula. When a molecule is selected, the textual representations of its symmetry elements are displayed in a list. Upon rolling the mouse over the list, the corresponding symmetry elements are temporarily displayed on the 3D molecular model. The user can display any combination or class of the symmetry elements, select a particular symmetry element and view animations or manually perform the associated symmetry operation.
These applets enable the user to actively explore and have individualized and extensive practice with a plethora of examples and molecular symmetry concepts, from simple molecules to highly symmetrical and complex molecular structures that are not accessible by other means.
Application in education
Interactive lecture notes can be used as presentation tools in class lectures to promote deeper understanding during instruction, as they provide novel visualizations that can complement the verbal representations of the tutor and exploit the dual processing capabilities of learners' cognitive systems. In addition the instructor can ask students questions about the theoretical significance of the representations and respond intelligently to their answers which often reveal alternative or incomplete conceptions. The instructor can then guide the students to use what they know to think more deeply about the issue, or to consider another case.Interactive lecture notes can also be used as self-pace tools, where the learners can read the theory, connect multiple representations, actively explore the represented molecular concepts, be engaged in extensive practice with these concepts and have experiences that help them create their own knowledge.
The hybrid course process
In the first meeting, students were informed that some course materials, activities and assignments would be delivered online. As proposed by Amrein-Beardsley et al. (2007), students become aware of the nature of the hybrid learning model which offers multiple forms of resources and learning tools in different ways and locations.Additionally, during this meeting, guidance was given to the students regarding how to register for this course, access the online part of the hybrid course, navigate in the Moodle learning environment and use the available tools in the platform. Only the authorized instructor and students had access to the online course material and activities in order to provide secured communication and e-education. Moreover, they were notified that their spending time and activities within the e-platform would be continuously recorded with a tracking system and that the instructor would have access to and check this piece of information. Furthermore, learning outcomes and course objectives were specified and students were informed about what was expected from them while using the online part of the course (distance activities), and during in-class time (physical presence).
In each face-to-face meeting, the instructor introduced a specific module of Molecular Symmetry, made a presentation of the corresponding web-posted study block and gave guidance for the embedded educational material and activities. In the time between two subsequent meetings, students were able to log into the Moodle, utilize all available course tools and access the current distributed study block.
Participants had the option to study the offered interactive lecture notes directly on their screen or print and study their pdf version, carry out available online learning activities and practice with enclosed molecular visualization applets. During the time interval after each face-to-face meeting, students also had the opportunity to ask questions in order to clarify specific aspects of the task and discuss the course content with their peers or the instructor, anytime, by using the synchronous or asynchronous online communication space.
By the completion of each module, students had to fill out and submit their answers in a relevant online knowledge and skill assessment quiz. The set of these quizzes had the character of a formative assessment and provided students with opportunities to reflect on their learning, identify weaknesses or gaps in their learning and to focus on improving these areas before proceeding to the next study block or before undertaking the final summative assessment. The quizzes consist of true/false, multiple choice, matching and short answer question types. Wherever it was applicable, 3D interactive visualization aids were embedded in quiz items (Fig. 3). On the other hand the analysis of students' responses in this set of quizzes allowed the instructor to monitor their learning progress during the whole semester and particularly to identify and address the topics where students encountered difficulties and misconceptions. Students had each quiz at their disposal for three days and the deadline had been defined just before the next scheduled in class meeting. After the submission, feedback was given to the students as well as their score in an online individual grade book.
 | ||
| Fig. 3 Representative quiz item (translated from Greek original). | ||
In each scheduled class meeting, students referred to difficulties and constraints that they had possibly confronted within the Moodle environment. There was also time for meaningful discussion about the online content and for elucidating students' misconceptions. The last part of these face-to-face meetings was devoted to the introduction by the instructor of the next module and the description of the corresponding study block distributed within Moodle.
Methods
Hybrid course implementation
The hybrid course of Molecular Symmetry and Group Theory has run during three winter semesters for the academic years 2008/09, 2009/10 and 2010/11. Each course implementation involved twelve (12) scheduled two-hour class meetings at the Department's computer lab in combination with the online study packs distributed by the CMS, Moodle. Feedback received from students and the instructor during the pilot implementation (2008/9) revealed aspects in hybrid course design for which improvement was necessary. Therefore in the subsequent implementations of the hybrid course in fall semesters 2009/10 and 2010/11 some refinements and enhancements were employed.In the pilot course implementation (2008/09), all quizzes became accessible by the completion of each module and remained available until the last day of the semester, which had been defined as the deadline for submitting them. In the two subsequent years, in an attempt to increase students' motivation and responsibility concerning the completion and submission of quizzes, the deadline had been defined just before the next scheduled in class meeting and each quiz was available for three days.
By the completion of the course, in 2009/10 and 2010/11 at the end of the semester a summative final assessment was added in order to evaluate how well students had acquired the knowledge and skills presented during the complete course. Particularly during the last meeting each student had to fill out a final assessment consisting of ten items, randomly chosen from the pool of questions, corresponding to each course module. On the contrary, in 2008/09, the final examination was not established. Therefore in 2008/09 each student's final course grade was based exclusively on their achievement in nine online quizzes (average score) while in 2009/10 and 2010/11 the course grade was a cumulative score over the available quizzes (30%) and the final on line test (70%). The only differentiation between the last two years is the absence of the sixth quiz in 2010/11.
For each of the three years the hybrid course was taught with the same course content and administered by the same instructor.
Quantitative and qualitative data for the effectiveness of the hybrid course
In order to evaluate the effectiveness of the hybrid course of Molecular Symmetry and Group Theory, we carried out an exit survey to elicit students' attitudes towards the hybrid instructional model, course material and their experience of the learning environment (Gall et al., 1996). Supplementary quantitative data from Moodle tracking and e-grade book utilities were retrieved and analyzed in order to examine the impact of the hybrid learning environment on students' involvement and performance. Statistical analysis of the data was conducted with the SPSS statistical package.Students' attitudes
By the completion of the hybrid course a pen and paper, five-point Likert type scale questionnaire, which typically asks for the extent of agreement with the attitude items, ranging from strongly agree (5) to strongly disagree (1) was distributed to the students. The survey consisted of new items that were written for the particular study and validated items from relevant existing instruments (Gall et al., 1996; Amrein-Beardsley et al., 2007; Ernst, 2008; Lin, 2008).The first part of the survey encompassed questions to access general demographic information, individual confidence with new technologies, as well as the frequency and purpose of using them in everyday life. The second part of the questionnaire was designed to gather information regarding students' attitudes towards the online part of the hybrid course (i.e., Moodle e-platform, content of interactive lectures notes, 3D molecular visualization applets and quizzes). In the third part of the survey students were asked to express their agreement with positive and negative statements regarding the hybrid instructional model and their experiences in the hybrid learning environment. We selected an equal number of positive and negative statements and arranged them in random order. Nevertheless in Table 2 all statements are presented as positive statements. Finally, the questionnaire also provided the opportunity for open ended comment and responses.
Coefficient alpha estimates of internal consistency reliability were computed for each section of the hybrid evaluation questionnaire. In the final implementation of the questionnaire all sections yielded acceptable alpha levels (acceptable reliability corresponds to α > 0.7) (Table 1).
| Sections of the Hybrid Course Evaluation Questionnaire | Cronbach's α | N of Items |
|---|---|---|
| Involvement and confidence with new technologies | 0.8 | 14 |
| Usability of e-platform and online tools | 0.7 | 9 |
| Quality of course content | 0.8 | 9 |
| Attitudes towards the hybrid model | 0.7 | 10 |
To ensure a high response rate, the instructor explained to the students that their feedback, though not obligatory, would help improve the overall program for future students. Moreover they were assured that there was anonymity in questionnaire responses and that their answers would not count toward their grade in the course.
Information retrieved from Moodle
Log files generated by a Course Management System consist of the source of data which is used to triangulate findings from the survey and to monitor students' engagement as well as their performance within the CMS. Students' involvement has been examined in terms of the frequency of access to the platform, their engagement with course content and participation in online quizzes. The impact on students' performance was drawn from their achievement in online quizzes (formative assessment) and from the scores in the final examination. Data were extracted in tabular format and imported to SPSS for statistical analysis.Student sample
The participants, who had initially registered in Moodle and eventually enrolled in the hybrid course of molecular symmetry, were 36 students (23 females and 13 males) in 2008/09, 30 students (22 females and 8 males) in 2009/10 and 39 students (26 females and 13 males) during the fall semester of 2010/11. According to the Department's curriculum, the course was compulsory. As happens with the conventional lectures, the hybrid course's attendance was not obligatory and the students had the option to attend lectures or not without reprimands. By the completion of the course all participants were asked to participate in an exit survey anonymously. The number of students who filled out and returned the questionnaire was 27 (19 females and 8 males) out of 36 participants in 2008/09, 26 (19 females and 7 males) out of 30 students in 2009/10 and 18 (13 females and 5 males) out of 39 students in 2010/11.Results
Involvement and confidence with new technologies
More than half of students accessed the internet through a high speed ADSL connection or dial up connection, while the rest of participants did not have internet access at home. To overcome the problem of access all students had network computers at their disposal at the university.The majority of students claimed to be comfortable with information and communication technologies and to enjoy working on computers (Table 2). It is notable that, despite the fact that the respondents were undergraduate students in chemistry in the third year of their studies, they stated that they rarely work on any chemical software for creating and modifying representations of chemical structures. On the whole, they are accustomed to using their personal computer primarily for communication and entertainment purposes rather than doing educational activities.
| 2008/09 (N = 27) % within group | 2009/10 (N = 26) % within group | 2010/11 (N = 18) % within group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Questions/Statements | Negative rank (1 & 2)a | Neutral (3)a | Positive rank (4 & 5)a | Negative rank (1 & 2)a | Neutral (3)a | Positive rank (4 & 5)a | Negative rank (1 & 2)a | Neutral (3)a | Positive rank (4 & 5)a |
| a Five point Likert Scale: 1 - Definitely disagree, 2 - Disagree, 3 - Neither Agree nor Disagree, 4 - Agree, 5 - Definitely agree. | |||||||||
| Involvement and confidence with new technologies | |||||||||
| I rely on my ability to use computers | 14.8 | 3.7 | 81.5 | 37.5 | 4.2 | 58.3 | 34.8 | 13.0 | 52.2 |
| I enjoy working with computers | 18.5 | 0.0 | 81.5 | 15.4 | 7.7 | 76.9 | 21.7 | 13.0 | 65.2 |
| Usability of e-platform and online tools | |||||||||
| I could always login to Moodle | 7.4 | 0.0 | 92.6 | 11.5 | 0.0 | 88.5 | 33.3 | 5.6 | 61.1 |
| Navigation throughout the online part is not complicated | 7.4 | 0.0 | 92.6 | 3.8 | 19.2 | 76.9 | 11.1 | 0.0 | 88.9 |
| I could always find easily how to manipulate 3D molecular models | 3.7 | 7.4 | 88.9 | 3.8 | 0.0 | 96.2 | 27.8 | 0.0 | 72.2 |
| I could easily select and display a symmetry element in 3D molecular visualization applets | 14.8 | 7.4 | 77.8 | 3.8 | 0.0 | 96.2 | 16.7 | 0.0 | 83.3 |
| I could easily perform a symmetry operation in 3D molecular visualization applets | 7.4 | 7.4 | 85.2 | 3.8 | 0.0 | 96.2 | 22.2 | 0.0 | 77.8 |
| Quality of course content | |||||||||
| Lecture notes are concise and clearly written | 7.4 | 0.0 | 92.6 | 4.0 | 4.0 | 92.0 | 11.1 | 11.1 | 77.8 |
| Symbols and representations are precise | 11.1 | 3.7 | 85.2 | 0.0 | 3.8 | 96.2 | 72.2 | 5.6 | 22.2 |
| 2D molecular representations enhanced my understanding of concepts relevant to “Molecular Symmetry” | 3.7 | 0.0 | 96.3 | 0.0 | 3.8 | 96.2 | 5.6 | 5.6 | 88.9 |
| Interactive 3D molecular visualization applets enhanced understanding of concepts relevant to “Molecular Symmetry” | 14.8 | 0.0 | 85.2 | 0.0 | 0.0 | 100 | 0.0 | 11.1 | 88.9 |
| Exercise and practice through molecular visualization applets improved my ability to mentally transform 2D molecular representations into 3D and operate symmetry operations. | 3.7 | 0.0 | 96.3 | 0.0 | 0.0 | 100 | 0.0 | 11.1 | 88.9 |
| Attitudes towards the hybrid model | |||||||||
| The hybrid instructional model accommodated my learning style or learning preferences | 7.4 | 0.0 | 92.6 | 3.8 | 0.0 | 96.2 | 11.1 | 0.0 | 88.9 |
| The hybrid instructional model met my expectations | 0.0 | 0.0 | 100 | 11.5 | 0.0 | 88.5 | 16.7 | 0.0 | 83.3 |
| The hybrid instructional model increased understanding of the course content | 7.4 | 0.0 | 92.6 | 0.0 | 0.0 | 100 | 5.6 | 5.6 | 88.9 |
| In a hybrid learning environment the sense of joining learning community was improved | 14.8 | 3.7 | 81.5 | 11.5 | 3.8 | 84.6 | 27.8 | 11.1 | 61.1 |
| The conventional educational setting is in no way inferior compared with the hybrid learning environment | 22.2 | 3.7 | 74.1 | 53.8 | 7.7 | 38.5 | 44.4 | 5.6 | 50.0 |
| In a hybrid learning environment I could inspect my progress and level of knowledge | 7.4 | 0.0 | 92.6 | 7.7 | 7.7 | 84.6 | 0.0 | 11.1 | 88.9 |
| In a hybrid learning environment I learn at my own pace and in my own time | 3.7 | 0.0 | 96.3 | 0.0 | 0.0 | 100 | 16.7 | 5.6 | 77.8 |
| I think that more courses should be redesigned by adopting a hybrid instructional model | 0.0 | 0.0 | 100 | 0.0 | 7.7 | 92.3 | 11.1 | 16.7 | 72.2 |
| In a hybrid learning environment the communication and interaction with the instructor was enhanced | 14.8 | 3.7 | 81.5 | 11.5 | 15.4 | 73.1 | 33.3 | 0.0 | 66.7 |
Students' attitudes
Students' attitudes toward the usability of the online part of the hybrid course
In the exit survey specific features of the hybrid course, relating to online tools, course content, instructional design and navigation within Moodle were also investigated. It is essential to conduct usability testing to ensure that the material is appropriate for all users.Interpreting students' responses on survey items concerning usability reveals that students did not confront any significant procedural or technical problems during the hybrid course. Students, after the treatment of initial problems concerning registration and log in to Moodle, were always aware of their possible actions and the available utilities and tools in the e-platform. Participants seemed to categorize the Moodle platform as a flexible learning tool, allowing either rapid scanning or reviewing of course material, and amenable to eclectic and selective use. Therefore students' attitudes towards its usability and ease of navigation were positive, as the great majority agreed or even strongly agreed with statements such as “Navigation through the platform is not complicated” and “Information and resources can be found easily” (Table 2).
Students did not have any significant problems concerning technical aspects, such as the software compatibility and the set up of some of the required plug-in. However, more than half of the students in 2008/09 said that it took a long time to download interactive lecture notes containing more than one visualization applet. Only a few students considered molecular visualization applets difficult to use, with the rest of the respondents finding that they could readily manipulate 3D molecular representations and could easily interact with the applet and conduct symmetry operations (Table 2).
Students' attitudes towards quizzes were also retrieved. The great majority stated that the instructions for answering quiz questions were clear and accurate and that they could easily find how to interact with online questionnaires and how to submit their responses.
Students' attitudes toward the course content
The great majority of respondents ranked the content of interactive lecture notes positively. They agreed that it was accurate, well worded, with coherent implementation of symbols and molecular representations. Responses to the survey show that the visual elements of the module, such as interactive 3D molecular visualizations and 2D molecular representations, were appreciated warmly. Students unanimously agreed that both representations enhance the conceptualization of molecular symmetry. They also respond that exercise and practice with molecular visualization applets proved very helpful and improved their ability to mentally transform 2D molecular representations into 3D and then manipulate the latter (Table 2).Students' attitudes towards the hybrid instructional model and their experience in a hybrid learning environment
The results have demonstrated positive attitudes of students toward hybrid instruction and a satisfactory experience within a hybrid learning environment. The great majority of respondents in the three academic years ranked the hybrid mode as a beneficial learning environment, which addresses students' needs and their expectations.Additionally, the survey data indicated that the great majority of students claimed that the hybrid instructional model favoured active learning and that students felt that this mode accommodated their learning style and competencies in new technologies. There was a clear consensus among them that the hybrid learning environment provided flexibility and convenience as they could study at their own pace, without time and place constraints (Table 2).
Almost all students believed that the combination of computer-based and face-to-face learning increased understanding of the course content. As indicated by their responses to relevant statements this was attributed to the integrated ICT technologies. More than 85% of the respondents agreed or definitely agreed that visualization applications and other educational technologies support instruction and enhance learning.
The survey data showed that online quizzes were well received by the majority of respondents. Students found quizzes to be a useful self-assessment tool to monitor their learning progress and diagnose their weaknesses over the course content (Table 2).
Another significant point is that a great number of participants perceived a sense of community during the hybrid course as the collaboration with fellow students was improved. Moreover students claim that the combination of multiple forms of communication (online and face-to-face), improved interaction, open and free dialogue with the instructor. Mutual interaction with the instructor was enhanced (Table 2).
Findings from the survey revealed that all students reap benefits of the hybrid course model, and consequently expressed an interest and preference in taking more hybrid courses in the future. Students in high percentages positively ranked the statement that more courses should be redesigned as hybrid courses (Table 2).
On the other hand, despite the students' favourable opinion towards the hybrid instructional model, 71.4% of respondents in 2008/09 found that the conventional educational setting is in no way inferior compared with the hybrid learning environment. In 2010/11 half of the students ranked this statement positively, while in 2009/10 the percentage of students who were in favour of conventional teaching was restricted to 38.4%.
Finally, at the end of the exit survey there was space for students to write down their comments on the hybrid course they had already attended. From all received comments, we selected to present two most representative and controversial ones.
A student who seemed to be in favour of the hybrid course wrote “Congratulations for your effort to upgrade the educational process! Many instructors should imitate the work employed in this course. Misconception in other chemistry courses in previous years were completely covered”.
On the other side, a student commented, “Personally I am in favour of the conventional teaching as I believe that in traditional educational settings, students are closer to each other and have better chances of comprehending the educational material. Moreover, I think that the computer is a medium unable to enhance meaningful relationships between teacher and student”.
Students' outcomes
Students' performance in the hybrid course of molecular symmetry
An insight into the impact of a hybrid learning environment on students' performance was obtained by monitoring the mean scores in online quizzes (formative and summative assessment), the overall success rate and by comparing the average final course grade within these three years 2008/09, 2009/10 and 2010/11 with those achieved in a representative year, 2007/8, when a traditional lecture approach was employed. Both hybrid and traditional courses were administered by the same instructor and covered the same curriculum. The traditional course had been delivered as three-hour lectures per week and concluded in a single final overall examination which encompassed questions from the whole course content.In the three successive years when the course was delivered in the hybrid mode the percentage of students who achieved a pass grade more than 4.5 was high. In 2008/09 the success rate was 80.6% (29/36), in 2009/10 it was 86.7% (26/30) while in the most resent implementation in 2010/11 the percentage was 84.6% (33/39).
It is also important that the average final course grade within the students who passed the course was more than adequate. Particularly the average final grade was 7.0 (N = 29, S.D. = 0.2) in 2008/09 while students in 2009/10 and 2010/11 obtained on average a better final course grade, 9.0 (N = 26, S.D. = 0.2) and 8.2 (N = 33, S.D. = 0.2) respectively. On the other hand in 2007/08 the mean grade achieved in the final examination was 6.1 (N = 35, SD = 0.21) and the percentage of students who succeed in passing the traditional delivered course was 71.4%.
Considering that the four groups of students were drawn from different cohorts, a reliable comparison of students' achievements based on statistical analysis cannot be achieved, though the repeatability of positive effects of the hybrid course as well as its greater learning outcomes compared to the traditional setting are manifested.
Table 3 summarizes the participation and the mean scores achieved in each of the online assessment quizzes on a scale of 0 to 10 by the three groups of participants. The mean scores of the final overall examination for 2009/10 and 2010/11 are also presented.
| 2008/09 (N = 36) | 2009/10 (N = 30) | 2010/11 (N = 39) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Course Topic | Quiz | % Part/iona | Mean Score | S.D. | % Part/iona | Mean Score | S.D. | % Part/iona | Mean Score | S.D. |
| a Percentage of students' participation within group. | ||||||||||
| Molecular Symmetry | q1 | 91.7 | 8.4 | 1.7 | 93.3 | 8.9 | 0.7 | 92.3 | 8.4 | 1.3 |
| q2 | 94.4 | 7.8 | 1.9 | 96.7 | 8.9 | 1.2 | 97.4 | 8.1 | 1.4 | |
| q3 | 89.2 | 9.0 | 1.4 | 96.7 | 9.6 | 0.9 | 84.6 | 8.7 | 2.0 | |
| q4 | 83.3 | 8.9 | 1.0 | 93.3 | 8.0 | 1.8 | 84.6 | 8.3 | 2.5 | |
| Group Theory | q5 | 94.4 | 7.3 | 1.9 | 96.7 | 9.3 | 0.8 | 89.7 | 8.7 | 0.9 |
| q6 | 91.7 | 5.3 | 1.9 | 93.3 | 8.2 | 1.8 | — | — | — | |
| q7 | 88.9 | 5.4 | 2.2 | 96.7 | 9.1 | 1.1 | 92.3 | 9.2 | 0.6 | |
| Application of symmetry | q8 | 88.9 | 5.4 | 2.3 | 93.3 | 8.1 | 1.2 | 84.6 | 7.1 | 2.9 |
| q9 | 86.1 | 4.0 | 1.7 | 93.3 | 5.5 | 1.6 | 79.5 | 6.4 | 3.1 | |
| All topics | Final exam. | — | — | — | 86.7 | 9.5 | 1.2 | 84.6 | 8.4 | 1.6 |
It is apparent that the participation in all quizzes as well as in the final examination was high in all the years the hybrid course ran. It is important to mention that in 2009/10 and 2010/11 students who had not submitted more than one online quiz could not take part in the final examination. Instead these students had to take the conventional exams of the previous years and therefore to respond to questions which examined exclusively 2D molecular representations. In 2008/09 there were not a prerequisite number of participations in the nine quizzes as the aim was an average cumulative score that surpassed the border fail line of 4.5. In all years the untaken quizzes were counted as zero.
Another notable point in Table 3 is that students in 2008/09 scored less after the sixth quiz. A possible explanation is the observed gradual decrease of students' frequency of access to CMS within the period that quizzes 6 to 9 were distributed. Question items for the quizzes q6, q7 and q8 were slightly modified for the next academic years in order to be more accurately and clearly written for students. Therefore in 2009/10 and 2010/11, an improved performance was observed in all quizzes with the exception of the last quiz (q9). The quiz q9 concerns applications of molecular symmetry and group theory to quantum chemical problems, while in the first five quizzes (q1 to q5), students' understanding on basic concepts of molecular symmetry such as symmetry elements, operations, point groups and group representations were examined. A possible explanation is that the tasks examined in the ninth quiz were more demanding as it sought their prior content knowledge and required students to apply their conceptions about molecular symmetry to the properties of molecular structure. However, according to the tutor's experience during the past years of traditional teaching, students always had problems in recognizing symmetry elements and applying symmetry operations within molecules. Therefore, the improved performance in quizzes q1 to q5 is likely attributable to the beneficial contribution of interactive 3D molecular visualizations and other applets on students’ understanding and perception of concepts related to molecular symmetry. It is characteristic that the majority of interactive applications are embedded in the first modules. Finally, a remarkable observation was the high performance of the students in the final overall examination.
Quantitative data regarding students’ frequency of access to the online part of the hybrid course
In Fig. 4, the frequency or otherwise the daily access to the available educational material over the whole period of the hybrid course is presented. The detailed analysis of log data reveals that the students use all the educational material of each module in both formats, either as a printable text in pdf or in interactive html format, with an increased frequency of access to interactive lecture notes enclosing concept and exploration visualization applets (topic 1).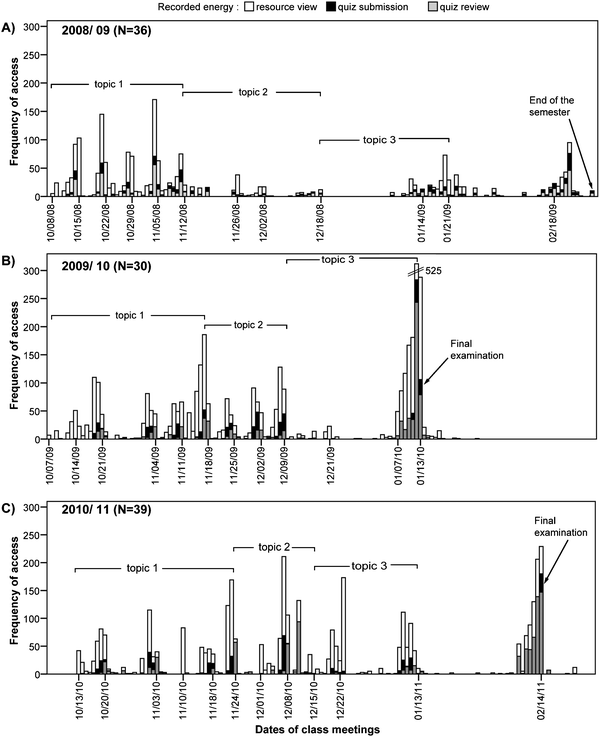 | ||
| Fig. 4 Frequency of accessing and interacting with the hybrid course's resources/material, submitting quizzes and reviewing quizzes' feedback during the fall semester of A) 2008/09, B) 2009/10, C) 2010/11. | ||
In the diagram corresponding to 2008/09 a differentiation from the other years is indicated. Fig. 4a shows that students in the first four weeks in the fall semester 2008/09 were engaged with course content, submitted quizzes and checked the accuracy of their answers (quiz review). In the rest of the semester the frequency of their access to the course resources as well as their engagement with quizzes became occasional and decreased until the last week of the course, in which an increased number of quiz submissions before the overall deadline was observed. This was attributed to the fact that students during this semester 2008/09 had at their disposal all quizzes that opened periodically during the course until the end of the semester.
A regular participation in the available online quizzes as the instructor proceeded to course modules during the semester was observed in 2009/10 and 2010/11. Students had each quiz at their disposal for three days and the deadline was defined just before the next in class meeting (Fig. 4b and c). Additionally a frequent access to the online grade book as well as to a review of correct and wrong answers for each quiz is evident, as both of these actions were recorded in the log file of the platform as “quiz review”.
Dates close to class meetings showed an increased student engagement with the corresponding online delivered course resources and activities. The same is also true for the dates before quiz submission. Thus, students seem to return to lecture notes in order to confirm their responses or to restructure conceptions and build new ones that could be used in the posed question. In Fig. 4 one can observe a distinct high frequency of recorded resource views just the day before the submission of the 4th quiz (the last quiz submission in the first topic), which examines the identification of all symmetry elements and operations of a given molecular structure. The further analysis of log data reveals that in these cases the recorded resource views correspond to interactive lecture notes which integrate exploration applets for practice and exercise.
The bar chart in Fig. 4 also indicates that during the semester students systematically filled out the embedded online quizzes and most students reviewed the feedback of their responses just before the next scheduled face-to-face meeting. A probable explanation is that students wanted to be aware of their progress and thus supposedly to be prepared for meaningful discussion in the classroom. Furthermore, at the end of 2009/10 and 2010/11 semesters an increased engagement with interactive lecture notes and quizzes' reviews of right and wrong answers (feedback) referring to all modules was observed and attributed to revision purposes. Finally, it is worth mentioning that the discernible gaps in the three frequency diagrams attributed to weeks without in class meetings.
Additionally, the instructor reported high retention rates in the hybrid course. Particularly, the instructor mentioned that during the hybrid course there was a notable rise in student attendance to the face-to-face part as well as substantial interaction between students and the instructor. It was observed that students came to in class meetings well prepared, which enabled them to involve in free and open dialogue, critical debate and meaningful discourse between their peers and the instructor. It was also noted that during the intermediate weeks, the department's computer lab became a meeting place for students' collaboration regarding online learning activities, as asserted by data retrieved from the Internet Protocol address (IP) tracker utility of Moodle (Table 4).
| 2008/09 | 2009/10 | 2010/11 | |
|---|---|---|---|
| Frequency of visits (course view) during the semester | 1942 | 5215 | 5710 |
| Frequency of accesses through university's IP addresses (%) | 766 (39.4%) | 1660 (31.8%) | 2167 (24.2%) |
Discussion and conclusions
This case study provides evidence that the adoption of a hybrid instructional model for demanding undergraduate chemistry courses such as Molecular Symmetry and Group Theory is beneficial and capable of improving the quantity and quality of students' involvement with the course content throughout the whole semester, not just before the final examination. The findings of these three years of research reveal several factors that contribute to the effectiveness of the hybrid course in supporting students' learning process, on improving their performance and increasing their satisfaction with their educational experience.The organization and deliverance of the hybrid course content in study blocks gives students flexibility for action and reflection in order to enhance their performance and preparedness for the forthcoming assessment as well as for the upcoming in class meeting. The quantitative data retrieved from Moodle provides evidence that students devote time to access the online part of the hybrid course in diverse hours outside of class meetings during the semester.
Another asset of the hybrid course is the efficient integration of molecular visualization applets for the topic of Molecular Symmetry. The purposely designed educational software, which was also utilized for demonstration during instruction, aided students throughout self-regulating study of the subject, as well as for both practice and exercise. Students' responses and quantitative data reveal that they appreciate the existence of both 3D and 2D molecular representations and they frequently interact with molecular visualization applets. They recognize that such applets helped them to become familiar with conceptualization of 3D molecular structure. Moreover the increased engagement with the embedded exploration applets in interactive lecture notes and the high success rates in the corresponding assessment quizzes indicate their positive impact on students' learning process.
The course material was designed taking into consideration arguments and design principles derived from a cognitive approach to learning, aiming to reduce cognitive load and support visuospatial thinking as well as to support the construction of deeper understanding by providing multiple representations. However, no research was conducted regarding the cognitive processes of learning with this material. Many researchers support that, except for studies conducted in a natural learning environment, there is a necessity for carefully controlled cognitive experiments to examine specific learning processes that occur with specific educational designs and specific learning tasks (Kozma and Russel, 2005; Cook et al., 2008; Urhahne et al., 2009; Stieff, 2011). Although there is no doubt that visuospatial thinking plays a critical role in chemistry learning and problem solving, recent research has focused on a more fine-grain analysis, revealing that a variety of task-specific alternative cognitive processes and problem solving strategies are available to students and applicable to chemistry tasks related to molecular structure, such as imagistic, diagrammatic and algorithmic reasoning strategies (Stieff et al., 2010; Stieff and Raje, 2010; Stieff, 2011; Stieff et al., 2011). Hence, more detailed and task-specific research on cognitive processes and strategies that are employed by learners during problem solving and reasoning about molecular symmetry concepts, as well as research on cognitive processes while learning with multi-representational material, is needed in order to evaluate the impact of this educational material on students' reasoning and learning and to develop more efficient task-specific educational chemistry tools and practices.
It is worth mentioning that undergraduate students in this study reported that they were not accustomed to use their computer for chemistry learning activities but mainly for recreation purposes. Therefore the hybrid instructional model, apart from the learning and teaching benefits, contributes to students' awareness of situated use of technologies for educational activities.
In addition to that, students seem to take responsibility for their own learning as they are regularly engaged in distributed online learning activities and assessment. Students take greater responsibility for their own learning when they regularly assess themselves (Shepard, 2001). In the hybrid course, students' assessment occurs during the teaching and learning process rather than after it, and has its primary focus as the ongoing improvement for all students.
In the pilot implementation of the hybrid course there were no time constraints for the submission of the nine distributed quizzes. Although the cumulative score of this set of quizzes counted exclusively towards the students' final grade, the students were not motivated and they did not put a great deal of effort into all quizzes until the completion of the course. Data retrieved from Moodle shows a decline in the frequency of students' access to online course material after the distribution of the sixth study pack as well as on their performance after the sixth quiz.
On the other hand, in the two subsequent years the addition of distinct deadlines for each quiz and the restriction of their availability to students to three days contributed to the observed increase and maintenance of students' interaction and engagement with the whole course content during the semester. The set of quizzes in both academic years seemed to gain more formative assessment character as students regularly participated, submitted their answers and reviewed their achievement before the scheduled face-to-face meeting. Although the scores that students achieved in the available quizzes counted for only thirty percent of their final course grade, data retrieved from Moodle revealed that students were more motivated and achieved higher scores than in the first year of the hybrid course implementation. Therefore, we conclude that the effective use of formative assessment increases students' engagement with course content and can help students to be more autonomous in their learning, to reflect on their performances, to take responsibility for their learning and address their efforts within virtual and real class towards the clarification of their misconceptions and the recovery of their weaknesses before undertaking the final summative assessment. Additionally the fact that students receive feedback just before the scheduled meetings reinforces their participation in meaningful face-to-face discussions and gives them opportunities to reflect, monitor and communicate their learning progress to their peers during the course.
It is evident that the establishment of the final examination in 2009/10 and 2010/11 had a positive impact on the teaching and learning process as the great majority of students who attended the hybrid course proceeded to the final examination, and achieved high success rates and performance. On the contrary, in previous years, when traditional lecture-based instruction was employed, significant percentages of withdrawals as well as reduced success rates were observed.
Students' responses and observations of face-to-face meetings reveal an enhancement in student–student and student–instructor interaction. However the interaction through online communication was restricted to tasks concerning the announcements, the schedule and the use of distributed course material. The main reason for limited meaningful interaction through the web is attributed to our omission in placing the appropriate emphasis on utilising computer mediated communication for purposely designed learning activities.
Results derived from this project indicate that, although the development of a hybrid learning environment initially requires extra time and effort, it is repeatable, upgradable and adaptable to adjust to more learning and teaching styles. The hybrid instructional model introduces an efficient mode of integration of visualization tools in multiple ways in order to enhance students' active involvement with chemistry tasks and their satisfaction with their experience in the educational environment.
References
- Ainsworth S., (1999), The functions of multiple representations, Comput. Educ., 33(2–3), 31–152.
- Ainsworth S., (2006), DeFT: A conceptual framework for learning with multiple representations, Learn. Instr., 16, 183–198.
- Amrein-Beardsley A., Foulger T. S. and Toth M., (2007), Examining the development of a hybrid degree program: using student and instructor data to inform decision-making, J. Res. Technol. Educ., 39(4), 331–357.
- Antonoglou L. D., Charistos N. D. and Sigalas M. P., (2008), Design of molecular visualization educational software for chemistry learning. In T. B. Scott and J. I. Livingston, Leading-edge educational technology (pp. 105–131). New York: Nova Publishers.
- Barak M., (2007). Transition from traditional to ICT-enhanced learning environments in undergraduate chemistry courses, Comput. Educ., 48, 30–43.
- Bonk C. J. and Graham C. R., (2005), Handbook of blended learning: Global perspective, local designs. San Francisco: Pfeiffer.
- Cass M. E. and Rzepa H. S., (2005), An animated interactive overview of molecular symmetry, J. Chem. Educ., 82, 1742–1743.
- Charistos N. D., Tsipis C. A. and Sigalas M. P., (2005), 3D molecular symmetry shockwave: three-dimensional perception of molecular symmetry, J. Chem. Educ., 82, 131–132.
- Cook M., Wiebe E. N. and Carter G., (2008), The influence of prior knowledge on viewing and interpreting graphics with macroscopic and molecular representations, Sci. Educ., 92, 848–867.
- Delialioglu O. and Yildirim Z., (2008), Design and development of a technology enhanced hybrid instruction based on MOLTA model: its effectiveness in comparison to traditional instruction, Comput. Educ., 51, 474–483.
- Dori Y. J. and Barak M., (2001), Virtual and physical molecular modelling: fostering model perception and spatial understanding, Educ. Technol. Soc., 4, 61–74.
- Dziuban C. D., Hartman J. L. and Moskal P. D., (2004), Blended learning, ECAR Research Bulletin, 7. Retrieved from http://net.educause.edu/ir/library/pdf/erb0407.pdf on 28 June 2011.
- Ealy J. B., (2004), Students' understanding is enhanced through molecular modelling, J. Sci. Educ. Technol., 13, 461–471.
- Ernst J. V., (2008), A comparison of traditional and hybrid online instructional presentation in communication technology, J. Technol. Educ., 19(2), 40–49.
- Ferk V. and Yrtacnik M., (2003), Students' understanding of molecular structure representations, Int. J. Sci. Educ., 25, 1227–1245.
- Gall M. D., Borg W. R. and Gall J. P., (1996), Educational Research: An introduction (6th ed.). New York: Longman Publishers.
- Garnham C. and Kaleta R., (2002), Introduction to hybrid courses, Teaching with Technology Today, 8(10), Retrieved from http://www.wisconsin.edu/ttt/articles/garnham.htm on 28 June 2011.
- Garrison D. R. and Kanuka H., (2004), Blended learning: uncovering its transformative potential in higher education, Internet High. Educ., 7(2), 95–105.
- Graham C. R., (2006), Blended learning: Definition, Current trends and future directions. In C. J. Bonk and C. R. Graham, Handbook of blended learning: Global Perspectives, local designs (pp. 3–21). San Francisco, CA: Pfeiffer Publishing.
- Graham C. R. and Dziuban C., (2008), Blended learning environments. In M. J. Spector, D. M. Merril, J. Merrienboer and M. P. Driscoll, Handbook of Research on Educational Communications and Technology (pp. 269–276). New York, Taylor & Francis.
- Lin H., (2008), Blending online components into traditional instruction in pre-service teacher education: the good, the bad, and the ugly, Int. J. Scholarship Teach. Learn., 2(1), Retrieved from http://academics.georgiasouthern.edu/ijsotl/v2n1/articles/Lin/index.htm on 28 June 2011.
- Johnston D. H., (2009), Visual-spatial learning: development of an interactive web-based symmetry tutorial, Fall 2009 CONFCHEM Conference: Excellence in Education with CCLI, Retrieved from http://www.ched-ccce.org/confchem/2009/Fall2009/P4-Johnston.html on 28 June 2011.
- Johnstone A. H., (1993), The development of chemistry teaching, J. Chem. Educ., 70, 701–705.
- Kirschner F., Kester L. and Corbalan G., (2011), Cognitive load theory and multimedia learning, task characteristics and learning engagement: The Current State of the Art, Comput. Hum. Behav., 27, 1–4.
- Korkmaz A. and Harwood W. S., (2004), Web-supported chemistry education: design of an online tutorial for learning molecular symmetry, J. Sci. Educ. Technol., 13(2), 243–253.
- Kozma R. and Russell J., (1997), Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Kozma R. B., Chin E., Russell J. and Marx N., (2000), The role of representations and tools in the chemistry laboratory and their implications for chemistry learning, J. Learn. Sci., 9, 105–144.
- Kozma R. and Russel J., (2005), Multimedia Learning of Chemistry. In R. E. Mayer (Ed.), Cambridge Handbook of Multimedia Learning (pp. 409–428). New York: Cambridge University Press.
- López-Pérez V. M., Pérez-López C. M. and Rodríguez-Ariza L., (2011), Blended learning in higher education: Students' perceptions and their relation to outcomes, Comput. Educ., 56(3), 818–826.
- Marques O., Woodbury J., Hsu, S. and Charitos S., (1998), Design and development of a hybrid instruction model for a new teaching paradigm, In Proceedings of Frontier in Education Conference, Retrieved from http://fie-conference.org/fie98/papers/1229.pdf on June 28, 2011.
- Martin-Blas T. and Serrano-Fernandez A., (2009), The role of new technologies in the learning process: Moodle as a teaching tool in physics, Comput. Educ., 52, 35–44.
- Mayer R. E., (2001), Multimedia learning (1st ed.). New York: Cambridge University Press.
- Mayer R. E., (2003), The promise of multimedia learning: using the same instructional design methods across different media, Learn. Instr., 13, 125–139.
- McCray G. E., (2000), The hybrid course: merging on-line instruction and the traditional classroom, Inform. Technol. Manag., 1, 307–327.
- Meyer D. E. and Sargent A. L., (2007), An interactive computer program to help students learn molecular symmetry elements and operations, J. Chem. Educ., 84, 1551–1552.
- Nakhleh M. B. and Postek B., (2008), Learning Chemistry Using Multiple External Representations. In J. K. Gilbert, M. Reiner and M. Nakhleh (ed.) Visualization: Theory and Practice in Science Education (pp. 209–231). The Netherlands: Springer.
- Olapiriyakul K. and Scher J.M., (2006), A guide to establishing hybrid learning courses: employing information technology to create a new learning experience, and a case study, Internet High. Educ., 9, 287–301.
- Orton-Johnson K., (2009), ‘I’ve stuck to the path I'm afraid': exploring student non-use of blended learning, Brit. J. Educ. Technol., 40(5), 837–847.
- Rovai A. P. and Jordan H. M., (2004), Blended learning and sense of community: a comparative analysis with traditional and fully online graduate courses, International Review of Research in Open and Distance Learning, 5(2), Retrieved from http://www.irrodl.org/index.php/irrodl/article/view/192/795 on 28 June 2011.
- Riffell S. K. and Sibley D. F., (2003), Using web-based instruction to improve large undergraduate biology courses: an evaluation of a hybrid course format, J. Coll. Sci. Teach., 44(3), 217–235.
- Schnotz W. and Kürschner C., (2008), External and internal representations in the acquisition and use of knowledge: Visualization effects on mental model construction, Instr. Sci., 36(3), 175–190.
- Shepard, L. A. (2001), The role of classroom assessment in teaching and learning. In V. Richardson (Ed.), Handbook of research on teaching (4th ed., pp. 1066–1101). Washington, DC: AERA.
- Stieff M., Ryu, M. and Dixon B. L., (2010), Students' use of multiple strategies for scientific problem solving. In K. Gomez, L. Lyons and J. Radinsky (ed.), Proceedings of the Ninth International Conference of the Learning Sciences (ICLS) (Vol. 1, pp. 765–772). Mahwah, NJ: Erlbaum.
- Stieff M. and Raje S., (2010), Expert algorithmic and imagistic problem solving strategies in advanced chemistry, Spat. Cognition Computat., 10(1), 53–81.
- Stieff M., (2011), When is a molecule three dimensional? A task-specific role for imagistic reasoning in advanced chemistry, Sci. Educ., 95, 310–336.
- Stieff M., Hegarty M. and Deslongchamps G., (2011), Identifying Representational Competence with Multi-Representational Displays, Cognition Instruct., 29(1), 123–145.
- Sweller J., (1988), Cognitive load during problem solving: effects on learning, Cognitive Sci., 12, 257–285.
- Sweller J., van Merrienboer J. and Paas F., (1998), Cognitive architecture and instructional design, Educ. Psychol. Rev., 10, 251–296.
- Talib O., Matthews R. and Secombe M., (2005), Computer-animated instruction and students' conceptual change in electrochemistry: Preliminary qualitative analysis, Int. Educ. J., 5(5), 29–42.
- Treagust D. F., Chittleborough G. and Mamiala T. L., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25, 1353–1368.
- Tuckey H. and Selvaratnam M., (1993), Studies involving three-dimensional visualization skills in chemistry: a review, Stud. Sci. Educ., 21, 99–121.
- Tuvi-Arad I. and Gorsky P., (2007), New visualization tools for learning molecular symmetry: a preliminary evaluation, Chem. Educ. Res. Pract., 8, 61–72.
- Tuvi-Arad I. and Blonder R., (2010), Continuous symmetry and chemistry teachers: learning advanced chemistry content through novel visualization tools, Chem. Educ. Res. Pract., 11(1), 48–58.
- Urhahne D., Nick S. and Schanze S., (2009), The effect of three-dimensional simulations on the understanding of chemical structures and their properties, Res. Sci. Educ., 39(4), 495–513.
- Vaughan N., (2007), Perspectives on Blended Learning in Higher Education, Int. J. E-Learn., 6(1), 81–94.
- Williams N. A., Bland W. and Christie G., (2008), Improving student achievement and satisfaction by adopting a blended learning approach to inorganic chemistry, Chem. Educ. Res. Pract., 9, 43–50.
- Wu H.-K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88, 465–492.
| This journal is © The Royal Society of Chemistry 2011 |
