Ultraflexible plasmonic nanocomposite aerogel†
Yi
Chen
a,
Khee Chaw
Ng
a,
Wenyi
Yan
b,
Yue
Tang
a and
Wenlong
Cheng‡
*a
aDepartment of Chemical Engineering, Monash University, Clayton, Victoria, 3800, Australia. E-mail: wenlong.cheng@monash.edu; Fax: +61 3 9905 5686; Tel: +61 3 9905 3147
bDepartment of Mechanical and Aerospace Engineering, Monash University, Clayton, Victoria, 3800, Australia
First published on 22nd September 2011
Abstract
A simple yet efficient approach to fabricate an ultraflexible plasmonic nanocomposite aerogel is described. High-quality gold nanoparticles with a wide size regime (13—101 nm) may be dispersed evenly into a viscous agarose aqueous solution at 60 °C. Gradually cooling the solution to room temperature lead to the formation of a nanocomposite hydrogel, which can be further converted into an aerogel by freeze-drying. Using this approach, particle loading as high as 92 nmol L−1 was achieved without any aggregation or agglomeration for the entire process. The resulting nanocomposite aerogel possesses well-pronounced surface plasmon resonance and exhibits superior mechanical strength yet remains ultraflexible. The maximum Young's modulus was estimated to be about 0.45 MPa in a typical compression test. In addition, an exceptionally high strain of more than 90% was observed, which is about two orders of magnitude larger than most brittle silica-based aerogels (i.e. only a fraction of a percent of strain-to-disintegration). Further structural characterizations demonstrated that the nanocomposite aerogel has a highly porous honeycomb structures, which is responsible for the unusually large strain observed. Only under sufficiently high pressing forces, the honeycomb structures collapse due to plastic deformation, leading to the formation of stiff nanocomposite paper with lamellar structures. This study demonstrates a robust approach to ultralow-density plasmonic aerogels with a myriad of potential applications in aerospace, foldable electronics and portable sensors.
1. Introduction
One of essential goals in nanotechnology is to design structurally well-defined multi-material composites to integrate multifunctionalities in a single system.1–6 Recently, substantial research efforts have been directed to integrate functional nanomaterials into aerogels to make nanocomposite aerogels.7–12 Aerogels are the world's lightest solid materials with unique functionalities, such as the lowest thermal conductivity and unmatched insulation properties of any known solids.13,14 Thus, incorporation of nano-objects, such as metallic nanoparticles,1,15 metal oxide nanoparticles,16carbon nanotube7,17–20 and graphene,21 into the nanocomposite aerogel constitutes a viable route to integrate the unusual optical, electronic, magnetic properties of nanomaterials with striking mechanical and thermal characteristics of aerogels.22–24 Such nanocomposite aerogels are a new class of advanced materials with a broad spectrum of applications in catalysis,25,26 supercapacitor devices,27 lightweight electronic devices,17,18 thermal/acoustic insulating materials 28 and energy storage devices.29Incorporation of gold nanoparticles into the nanocomposite aerogel is a particularly interesting topic due to their unprecedented light-confining abilities as a result of the localized surface plasmon resonance (LSPR). Notably, the modes and peak locations of LSPR can be tuned simply by adjusting structural parameters, such as size and shape, and interparticle distance without the need to change the chemical composition.30–32 Rational incorporation of these plasmonic gold nanoparticles into aerogels will allow for engineering optical properties of aerogels in a programmable way,33 with exciting applications in aerospace, nanophotonic devices and ultra-lightweight plasmonic biosensors.
Nevertheless, gold nanoparticles (especially those synthesized by citrate-based approaches34,35) often aggregate in the gelation process, which is an indispensable step in typical aerogel fabrication process. For example, the formation of a silica aerogel involves a sol–gel reaction, which requires an acid or a base as a catalyst, however, such strong acidic or basic conditions often cause aggregation of pre-synthesized gold nanoparticles, especially with high particle loading. Here, we describe a simple yet efficient approach to fabricate an ultraflexible plasmonic aerogel based on an agarose biopolymer and gold nanoparticles. High-quality gold nanoparticles with a wide size regime (13—101 nm) may be dispersed evenly into a viscous agarose aqueous solution at 60 °C. Gradually cooling the solution to room temperature lead to the formation of a nanocomposite hydrogel, which can be further converted into an aerogel by freeze-drying. Using this approach, particle loading as high as 92 nmol L−1 was achieved without any aggregation or agglomeration for the entire process. The resulting nanocomposite aerogel possesses well-pronounced surface plasmon resonance, tunable by adjusting the particle size. In addition, the plasmonic nanocomposite aerogel exhibits superior mechanical strength yet remains ultraflexible. In typical compressive experiments, we obtained a Young's modulus of about 0.45 MPa and an exceptionally high strain of more than 90%. The strain is about two orders of magnitude larger than most brittle silica-based aerogels (i.e. only a fraction of a percent of strain-to-disintegration). Electron microscopy studies demonstrate that the ultralarge strain is due to the highly porous honeycomb structures, which collapsed by plastic deformation under high pressing forces leading to the formation of stiff nanocomposite paper. This work demonstrates a robust approach to ultra-lightweight plasmonic aerogels, which may find a wide spectrum of applications in nanoscience and nanotechnology.
2. Experimental section
Synthesis of gold nanoparticles
Gold nanoparticles with five different diameters ranging from ∼13 nm to ∼101 nm were synthesized according to the reported citrate-based method.34,35 Typically, 2 mL HAuCl4 (∼25 mM) was diluted by 170 mL Milli-Q water and heated to 100 °C until boiling. Then 6 mL sodium citrate solution (∼34 mM) was added into the above solution under vigorous stirring. 3 min later, the clear solution changed to a wine-red color, indicative of the formation of gold nanoparticles. After further boiling for 10 min, the heat source was removed and the dispersion was cooled down to the room temperature. By reducing the volume of sodium citrate solution to 3 mL, 2 mL, 1 mL and 680 μL, we could obtain gold nanoparticles with diameters of 24.2 nm, 52.1 nm, 72.5 nm and 101.4 nm, respectively. These nanoparticle solutions were further concentrated by repeating centrifugation, leading to stock solutions with concentrations of ca. 184 nM, 32 nM, 3.2 nM, 1.2 nM, 0.4 nM, respectively.Preparation of the plasmonic aerogel
A representative procedure for fabricating the plasmonic aerogel is as follows (using 13 nm gold nanoparticles as an example): 0.045 mg agarose was added into 1.5 ml Milli-Q water (3 wt%) and the mixture was then heated up to boiling in a microwave oven until the agarose was completely dissolved. The concentrated gold nanoparticle solution was subsequently mixed in an equal volume ratio with the 3% agarose solution at 60 °C, followed by agitation for 5 min to ensure that a homogeneous distribution was obtained. After pouring the viscous mixtures into vials and cooling down to room temperature, a dark red plasmonic hydrogel (1 cm × 1 cm × 3 cm) was obtained. The composite hydrogels were then put into a −70 °C freezer for 24 h before a typical freezing drying process, which led to the flexible plasmonic aerogel monolith with negligible volume shrinkage.An even more lightweight plasmonic aerogel foam was fabricated by simply adding 2 mL CTAB solution (0.1 wt%) into the above aqueous mixture at 60 °C, followed by violent agitation until bubbles filled the entire vessel and then gradually cooled down to ambient temperature. The freeze-drying of such solutions resulted in the formation of an ultra-lightweight aerogel foam without observable volume shrinkage.
Characterization methods
The nanocomposite aerogel was characterized by JEOL JSM-840A scanning electron microscopy (SEM) after coating a thin layer of Pt (about 2 nm in thickness). Transmission electron microscopy (TEM) was performed using a Philips CM20 at a 200 kV accelerating voltage. Small pieces of aerogel flakes were thoroughly cut into powders by doctor blade and deposited onto the surface of 300 mesh Formvar-carbon film-coated copper grids. Specimen of compressed aerogel paper was prepared by microtoming with a section of ∼30 nm thick (Reichert FCS Cryo-microtome) and examined under TEM. UV-visible spectra of aerogel flakes were recorded at room temperature using a J&M MSP210 microscope spectrometry system, which allowed for the acquisition of a localized spectrum from a single tiny flake, which was illuminated by high-intensity fiber light source under a 40× objective. Wide angle X-ray diffraction (WAXD) patterns were obtained at room temperature on a Philips PW1140/90 diffractometer with a Cu Kα radiation source (40 kV, 25 mA, λ = 1.54 Å). Samples were exposed at a scan rate of 2Theta = 4° min−1 between 2Theta = 4 and 90°. Indentation tests of the nanocomposite aerogel paper were performed by a TI 750 Ubi Nanomechanical System on three different areas. The compression tests of the aerogels were carried out on Instron 5860 mechanical tester equipped with a 100 N load cell. Cubic test pieces with a dimension of 1 cm × 1 cm × 1 cm were cut from the aerogel.3. Results and discussion
Fig. 1a illustrates the fabrication process of the plasmonic nancomposite aerogel. Briefly, the aqueous agarose solution (3 wt%) was obtained by heating an aqueous suspension of agarose powder with a microwave, which was followed by mixing the concentrated gold nanoparticle stock solution in an equal volume ratio at 60 °C. The dark hydrogels formed after gradually cooling the mixtures to ambient temperature. Subsequently, the hydrogels were freeze-dried, resulting in the generation of the nanocomposite aerogels without observable volume shrinkage.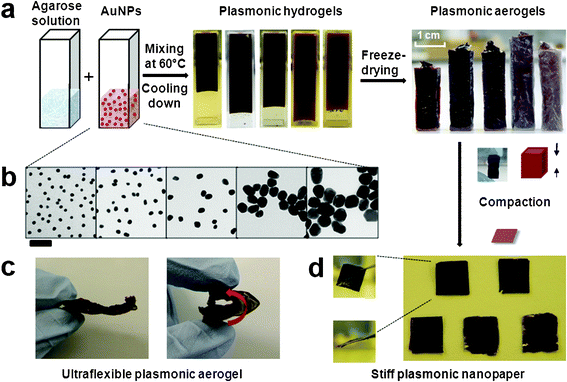 | ||
| Fig. 1 Fabrication of a flexible plasmonic aerogel and stiff nanopaper. (a) Schematic illustration of the synthetic steps. (b) TEM characterization of AuNPs with five diameters (scale bar = 100 nm). (c) A photograph demonstrating the striking flexibility of the plasmonic aerogel, which can be reversibly folded into a loop. (d) Representative images of the stiff plasmonic nanopaper loaded with a high-concentrated AuNPs with different diameters. Inset: surface and side-view of the nanopaper. | ||
Highly concentrated gold nanoparticles with five different diameters (i.e. 13.5 nm, 24.2 nm, 52.1 nm, 72.5 nm and 101.4 nm) are synthesized by following the reported strategy.34,35 Clearly, those particles show high size monodispersity, as confirmed by transmission electron microscopy (TEM) imaging (Fig. 1b). All these different sized nanoparticles were successfully incorporated into agarose aerogel without any aggregation. Each nanocomposite aerogel gives unique intense colors (from dark red to purple), specific to the particle sizes used. In all the five aerogels, agarose accounts for a weight fraction of 92% and nanoparticles account for a weight fraction of 8%. For 13 nm gold nanoparticles, this weight fraction corresponds to a molar centration of ca. 92 nmol L−1. To the best of our knowledge, such high concentrated citrate-protected nanoparticles have not yet been reported to disperse evenly into aerogels. Notably, the nanocomposite aerogel has a density of as low as 28 mg cm−3, but it exhibits surprising flexibility that can afford repeated bending over ±180° without appreciable deformation (Fig. 1c). Only with sufficiently high pressing forces, the nanocomposite aerogel experienced plastic deformation, leading to stiff plasmonic nanocomposite paper (Fig. 1d).
The structures of both the flexible nanocomposite aerogel and compressed composite paper were systematically investigated by means of SEM and TEM. Fig. 2a shows a representative cross-section micrograph of the plasmonic aerogel, revealing a honeycomb structure constructed of loosely stacked micro-flakes with proliferous morphology. The cell size ranges from 40–140 μm, whereas the thickness of the micro-flake is less than 1 μm. This unique internal structure may be due to the unidirectionally frozen nanocomposite hydrogels, similar to the modified ice-templating method using liquid nitrogen, which efficiently resulted in the pseudo-steady-state continuous growth of polygonal ice rods parallel to the freezing direction.36 This array provided templates for the formation of honeycomb structures and was consequently removed by freeze-drying to obtain the ordered porous honeycombs. In comparison, the aerogels without ice-templating showed an isotropic flake-like structure and no honeycomb morphology was formed (Fig. S1a, ESI†). Similar to the natural bee nests, the highly open honeycomb structures may be responsible for the striking mechanical flexibility and robustness (detailed mechanical description is given later in this report). Under conventional forces, the aerogel monolith can fully recover to its original shape upon force release (Fig. 1c), indicative of elastic deformation. Only under extremely high forces with a mechanical press, the honeycomb structures could experience irreversible plastic deformation. By continuously pressing the nanocomposite aerogel under a pressure of 30 MPa for 10 min, we obtained nanocomposite paper in which stacked lamellar structures are evident (Fig. 2b), which suggest the collapse of the honeycomb structure under such constant high pressing forces.
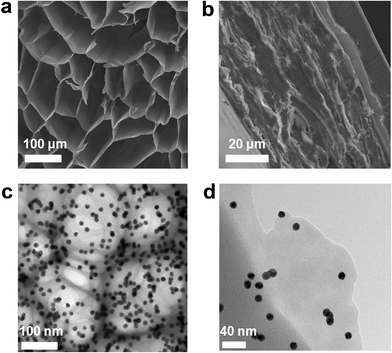 | ||
| Fig. 2 SEM characterization shows (a) the vertical section of aerogel–AuNP24nm, indicating a honeycomb structure constructed by loosely stacked micro-flakes and retains a poriferous aerogel skeleton, (b) cross section of the plasmonic nanopaper–AuNP24nm after drying and compression; TEM characterization of aerogel-AuNP13nm indicates (c) monodispersed AuNPs well-incorporated in the honeycomb structures and (d) a mono-layer flake. | ||
To further prove the presence of the nanoparticles within the aerogel, we prepared a TEM specimen by slicing the aerogel samples into thin pieces and transferring them onto a copper grid. Fig. 2c shows a representative TEM image clearly demonstrating a uniform distribution of gold nanoparticles within the honeycomb networks. Particles are kept separated without aggregation. Consistent with SEM characterization, the thin flakes were also observed by TEM (Fig. 2d). The back-scattered electron imaging could give the compositional contrast. Evenly distributed gold nanoparticles are evident in both the nanocomposite aerogel and compressed composite paper (Fig. S1 and S2, ESI†). The absence of particle aggregation was also proved by wide angle X-ray diffraction (WAXD, Fig S3, ESI†). WAXD analysis of the plasmonic aerogel shows characteristic diffraction peaks at 38.2°, 44.4°, 64.6°, and 77.6°, which could be indexed to (111), (200), (220) and (311) Bragg's reflections of the face-centered cubic (FCC) lattice of gold (JCPDS 04-0784). The crystallite sizes estimated from WAXD using Scherrer's equation are also consistent with those measured by TEM (Fig. 1b) for pre-synthesized particles, therefore, further proving the absence of aggregation.
All the nanocomposite aerogels exhibit well-pronounced plasmon resonance peaks dependent on the particle size. We used microscope UV-visible spectrophotometer under the transmission mode to measure the spectra from a single thin flake peeled off from the bulk aerogel. Notably, it is not possible to acquire spectra from the aerogel samples with a conventional UV-visible spectrophotometer due to the high particle concentrations. Instead, we used a microscope UV-visible spectrophotometer to focus on a single flake and acquire the spectra simultaneously. The microscope spectrometer enables the measurement of localized spectra with strong local light-illumination intensity. Since the particles are evenly distributed within aerogel, it is expected that the spectra from the flakes represent the plasmonic properties of the entire aerogel. Fig. 3a shows pictures of thin flakes peeled off from the surface layer of the aerogels, which are dark red in color due to the presence of gold nanoparticles in high concentration.
 | ||
| Fig. 3 (a) Individual flakes peeled off from the bulk aerogel, (b) Representative images of thin-sheets from the aerogel acquired in transmission mode under the optical microscope, (c) UV-vis spectra of plasmonic aerogels monitored by the microscope spectrometry system over a tiny area above the pieces of the thin-flakes. | ||
The recorded UV-visible spectra shows that all the thin flakes have well-pronounced LSPR bands with adjustable peak positions from 531 nm to 633 nm dependent on the particle diameters (Fig. 3c). The larger the particle diameters are, the longer the LSPR wavelengths are. This result demonstrates a reliable route to tailor the plasmonic properties of the nanocomposite aerogel in a quantitative way. Consistent with TEM results, the strong LSPR bands also prove that the nanoparticles are free of aggregation and deterioration despite high nanoparticle concentration. Compared with LSPR bands in water (Fig. S4, ESI†), all the LSPR peaks in the aerogel shift to longer wavelengths (Fig. 3c). Notably, the hydrogelation only led to negligible spectral shifts and it was the dehydration that led to the significant red-shifts (Fig. S4, ESI†).30,31 The LSPR red shifts for the nanoparticles with diameters of 13 nm, 24 nm, 52 nm, 72 nm and 101 nm, were estimated to be 13 nm, 31 nm, 30 nm, 48 nm, 50 nm, respectively. It appears that the larger the particles are, the more significant the red-shifts are (Table S1, ESI†). This result may indicate the greater sensitivity of larger particles in response to surrounding dielectric environments. Unlike the gold cluster/silica aerogel that exhibited a broad and weak LSPR absorption band owing to the peripheral distribution,15 our plasmonic nanocomposite aerogel showed narrow LSPR bands due to uniform particle distribution in the aerogel matrix.
Despite the low densities and highly porous structures across the macroscopic dimensions, the plasmonic nanocomposite aerogels are mechanically robust and flexible. Strikingly, the nanocomposite aerogel could survive the compression down to 10% of its volume and rapidly recovers to its original volume upon force release. Such a process can be repeated over hundreds of cycles without appreciable damage to the materials (Fig. 4a and supplementary videos, ESI†). To evaluate the relationship between internal structures and the final mechanical property, compression tests was performed with the stress loaded parallel and perpendicular to the honeycomb cell axis direction (Fig. S6a and b, ESI†), respectively. The stress–strain (σ-ε) diagram from compression along the cell axis of the nanocomposite aerogel exhibits three regions, owing to the elastic deformation and buckling of the cell wall at the beginning, then resulting in impinging upon each flake. In comparison, the compression normal to the cell axis shows only two regions corresponding to a linear-elastic region followed by a densification region, which has a smaller compression stress at the same strain (273 vs. 99 KPa at ε ∼95%) due to the anisotropic internal structure of the plasmonic aerogels. The maximum modulus calculated from the stress–strain diagram is 0.45 MPa, with an exceptionally maximum strain of more than 90%. The strain is about two orders of magnitude larger than most brittle silica-based aerogels (i.e. only a fraction of a percent of strain-to-disintegration).13,14,37 From a mechanical perspective, the excellent compression recovery property of the plasmonic aerogel is critical for their integration into flexible, lightweight devices for many technical applications.
 | ||
| Fig. 4 (a) The compression recovery property of aerogel-AuNP13nm, (b) Ultralight Aerogel-AuNP13nm with CTAB induced bubbles before and after freeze-drying, right image shows its flexibility by bending and compression, (c) Force-displacement diagram of the nanopaper-AuNP24nm by indentation testing. | ||
We further show that the plasmonic composite aerogel can be made even more lightweight. In a typical experiment, we introduced hexadecyltrimethylammonium bromide (CTAB) into the agarose solution and generated a bubbled solution with vigorous agitation followed by routine freeze drying. This process led to a highly porous plasmonic aerogel with an ultralow density (5 mg cm−3), which is comparable to the lowest aerogel density reported (i.e. 3 mg cm−3).39 The obtained CTAB–bubbled aerogel foam was resilient and could sustain bending under strong compression forces and recover to its original shape upon force release. Such a process was highly reversible over hundreds of cycles without any observable damage to the material (Fig. 4b).
On the basis of the aforementioned analysis, the striking flexibility and compression recovery properties can be attributed to the strong and thin honeycomb-shaped flakes with a lateral thickness of less than 1 μm across the aerogel monolith. Notably, theoretically the flexibility is inversely proportional to the third power of the film thickness,38 hence, our honeycomb-shaped thin flakes are responsible for all the striking mechanical properties observed (Fig. 2a). When the compressing stress is applied to the aerogel, the bending of numerous thin-flakes stored the strain energy within the plasmonic aerogel and then release this energy inducing the recovery of the compressed aerogel to its original volume. This also shows that porous honeycomb structures within plasmonic nanocomposite aerogels can sustain large elastic deformation. The permanent plastic deformation could only occur while constantly applying the very high compression forces (for example, under pressure of 30 MPa for 10 min), in which a composite paper was formed.
The nanocomposite paper is extremely stiff, as demonstrated by the indentation test. For each nanocomposite paper, a load was applied to drive the indenter into three random areas and the corresponding load–displacement curves were recorded. A typical curve is shown in Fig. 4C. Using the Oliver–Pharr method (Fig. S5, ESI†), the Young's modulus and hardness could be estimated as ∼2 GPa and ∼0.12 GPa, respectively. These values are comparable to that for the magnetic nanopaper fabricated by the cellulose nanofibrils.16
4. Conclusion
In summary, we have demonstrated a facile route to construct structurally well-defined, customizable, and property-tunable plasmonic composite aerogel. The porous honeycomb structure constructed by robust and flexible micro-flakes provides striking mechanical attributes, such as striking flexibility and compression recovery properties. Both electron imaging and plasmonic resonance measurements demonstrate that high-quality particles with a wide range of sizes are free of aggregation, even with high particle loading of 92 nmol L−1. We believe that our approach is a model system extendable to anisotropic plasmonic nanoparticles, such as rods, prisms, cubes, polyhedral and branched/hollow structures. Hence, the simple yet efficient fabrication methodology reported here can combine the tailorable optical properties of plasmonic nanomaterials with the appealing mechanical characteristics of argarose, which will render such highly flexible and porous materials as promising candidates for a plethora of applications including ultra-lightweight plasmonic sensors, foldable electronics, aerospace, etc.Acknowledgements
This work is funded by New Staff Member Research Fund (2437404), Faculty of Engineering and by Department of Chemical Engineering, Monash University. The authors acknowledge use of facilities in the Monash Centre for Electron Microscopy (MCEM). Y.C. acknowledges the graduate student scholarships (MGS & IPRS) from Monash University.References
- C. A. Morris, M. L. Anderson, R. M. Stroud, C. I. Merzbacher and D. R. Rolison, Science, 1999, 284, 622 CrossRef CAS
.
- C. Boissiere, D. Grosso, A. Chaumonnot, L. Nicole and C. Sanchez, Adv. Mater., 2011, 23, 599 CrossRef CAS
.
- K. Y. Chun, Y. Oh, J. Rho, J. H. Ahn, Y. J. Kim, H. R. Choi and S. Baik, Nat. Nanotechnol., 2010, 5, 853 CrossRef CAS
.
- W. L. Cheng, M. J. Campolongo, J. J. Cha, S. J. Tan, C. C. Umbach, D. A. Muller and D. Luo, Nat. Mater., 2009, 8, 519 CrossRef CAS
.
- W. L. Cheng, N. Park, M. T. Walter, M. R. Hartman and D. Luo, Nat. Nanotechnol., 2008, 3, 682 CrossRef CAS
.
- W. L. Cheng, M. R. Hartman, D. M. Smilgies, R. Long, M. J. Campolongo, R. Li, K. Sekar, C. Y. Hui and D. Luo, Angew. Chem. Int. Ed., 2010, 49, 380 CAS
.
- X. Zhang, J. Liu, B. Xu, Y. Su and Y. Luo, Carbon, 2011, 49, 1884–1893 CrossRef CAS
.
- M. L. Anderson, R. M. Stroud and D. R. Rolison, Nano Lett., 2002, 2, 235 CrossRef CAS
.
- L. Martin, J. O. Ossó, S. Ricart, A. Roig, O. Garcia and R. Sastre, J. Mater. Chem., 2008, 18, 207 RSC
.
- J. L. Monahan, I. U. Arachchige and S. L. Brock, Science, 2005, 307, 397 Search PubMed
.
- S. Bag, P. N. Trikalitis, P. J. Chupas, G. S. Armatas and M. G. Kanatzidis, Science, 2007, 317, 490 CrossRef CAS
.
- N. Leventis, N. Chandrasekaran, A. G. Sadekar, C. Sotiriou-Leventis and H. Lu, J. Am. Chem. Soc., 2009, 131, 4576 CrossRef CAS
.
- N. Hüsing and U. Schubert, Angew. Chem., Int. Ed., 1998, 37, 22 CrossRef
.
- A. C. Pierre and G. M. Pajonk, Chem. Rev., 2002, 102, 4243 CrossRef CAS
.
- Y. Tai, M. Watanabe, K. Kaneko, S. Tanemura, T. Miki, J. Murakami and K. Tajiri, Adv. Mater., 2001, 13, 1611 CrossRef CAS
.
- R. T. Olsson, M. A. S. Azizi Samir, G. S. Alvarez, L. Belova, V. Ström, L. A. Berglund, O. Ikkala, J. Nogués and U. W. Gedde, Nat. Nanotechnol., 2010, 5, 584 CrossRef CAS
.
- A. E. Aliev, J. Oh, M. E. Kozlov, A. A. Kuznetsov, S. Fang, A. F. Fonseca, R. Ovalle, M. D. Lima, M. H. Haque, Y. N. Gartstein, M. Zhang, A. A. Zakhidov and R. H. Baughman, Science, 2009, 323, 1575 CrossRef CAS
.
- J. H. Zou, J. H. Liu, A. S. Karakoti, A. Kumar, D. Joung, Q. Li, S. I. Khondaker, S. Seal and L. Zhai, ACS Nano, 2010, 4, 7293 CrossRef CAS
.
- T. Bordjiba, M. Mohamedi and L. H. Dao, Adv. Mater., 2008, 20, 815 CrossRef CAS
.
- M. B. Bryning, D. E. Milkie, M. F. Islam, L. A. Hough, J. M. Kikkawa and A. G. Yodh, Adv. Mater., 2007, 19, 661 CrossRef CAS
.
- M. A. Worsley, P. J. Pauzauskie, T. Y. Olson, J. Biener, J. H. Satcher, Jr. and T. F. Baumann, J. Am. Chem. Soc., 2010, 132, 14067 CrossRef CAS
.
- N. Gaponik1, A. Wolf, R. Marx, V. Lesnyak, K. Schilling and A. Eychmüller, Adv. Mater., 2008, 20, 4257 CrossRef
.
- L. Sorensen, G. F. Strouse and A. E. Stiegman, Adv. Mater., 2006, 18, 1965 CrossRef CAS
.
- E. Bekyarova and K. Kaneko, Adv. Mater., 2000, 12, 1625 CrossRef CAS
.
- D. R. Rolison, Science, 2003, 299, 1698 CrossRef CAS
.
- M. Bonnet, L. Schmid, A. Baiker and F. Diederich, Adv. Funct. Mater., 2002, 12, 39 CrossRef CAS
.
- G. R. Li, Z. P. Feng, Y. N. Ou, D. C. Wu, R. W. Fu and Y. X. Tong, Langmuir, 2010, 26, 2209 CrossRef CAS
.
- M. Schmidt and F. Schwertfeger, J. Non-Cryst. Solids, 1998, 225, 364 CrossRef CAS
.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28 CrossRef CAS
.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS
.
- P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Nano Today, 2007, 2, 18 CrossRef
.
- W. L. Cheng, S. J. Dong and E. K. Wang, J. Phys. Chem. B, 2004, 108, 19146 CrossRef CAS
.
- D. D. Smith, L. A. Snow, L. Sibille and E. Ignont, J. Non-Cryst. Solids, 2001, 285, 256 CrossRef CAS
.
- G. Frens, Nature, Phys. Sci., 1973, 241, 20 CAS
.
- S. D. Perrault and W. C. W. Chan, J. Am. Chem. Soc., 2009, 131, 17042 CrossRef CAS
.
- S. R. Mukai, H. Nishihara and H. Tamon, Chem. Commun., 2004, 7, 874 RSC
.
- M. Pääkkö, J. Vapaavuori, R. Silvennoinen, H. Kosonen, M. Ankerfors, T. Lindström, L. A. Berglund and O. Ikkala, Soft Matter, 2008, 4(12), 2492 RSC
.
-
R. Solecki, R. J. Conant, in Advanced Mechanics of Materials, Oxford University Press 2003, Search PubMed
Ch. 10.
- T. M. Tillotson and L. W. Hrubesh, J. Non-Cryst. Solids., 1992, 145, 44 CrossRef CAS
.
Footnotes |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c1ra00532d/ |
| ‡ Present address: Wenlong Cheng, Associate Professor, Department of Chemical Engineering, Monash University, Room 230, Building 36, Clayton, Victoria 3800, Australia, Website: http://users.monash.edu.au/~wenlongc/ |
| This journal is © The Royal Society of Chemistry 2011 |
