A cell compatible fluorescent chemosensor for Hg2+ based on a novel rhodamine derivative that works as a molecular keypad lock
Yinghui
Wang
ab,
Yanqi
Huang
c,
Bin
Li
*a,
Liming
Zhang
a,
Hang
Song
a,
Hong
Jiang
a and
Jie
Gao
a
aKey Laboratory of Excited State Processes, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, Changchun, 130033, P. R. China
bGraduate School of the Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing, 100039, P. R. China
cSchool of Materials Science and Engineering, Shandong University, Jinan, 250061, P. R. China. E-mail: lib020@nenu.edu.cn; Fax: +86-431-86176935
First published on 27th September 2011
Abstract
A novel fluorescent chemosensor based on rhodamine derivative (Rh1) has been designed and synthesized for detection of Hg2+ ions, which exhibits high sensitivity and selectivity over other metal ions in aqueous solution and living cells. Moreover, this “Off–On”-type fluorescent sensor could successfully mimic a molecular level keypad lock in the presence of Cu2+ ions. Stimulated by the two chemical inputs (Hg2+ and Cu2+), Rh1 undergoes transformation of the structure between spirocyclic and ring-opened spirocyclic, which results in the change of fluorescence. Significantly, the outputs of the system depend on not only the proper combination but also on the correct order of the input signals, which is the most important feature of the keypad lock system. Only a specific sequence of inputs, i.e. the correct password, results in strong fluorescence emission at 555 nm which can be used to “open” this molecular keypad lock. Therefore, this molecular keypad lock has the potential for application in security devices, which could be used to authorize a user, to verify authentication of a product, or to initiate a higher process.
Introduction
In recent years, various molecular switches, logic gates and logic circuits based on chemical systems capable of elaborating binary (Boolean) information have been proposed because of the wide variety of organic molecular designs, synthesis, and light-emitting properties that are available.1 The combination of these molecular logic gates and logic circuits has been used for mimicking various electronic devices at the molecular level. The primary motivation for this is to develop novel paradigms for information processing that, by moving beyond silicon-based technology, could lead to electronic devices of extremely small size, low power consumption and unprecedented performance. Implementation of various mimicking electronic devices, such as memory units,2 comparators,3 and demultiplexers,4 performing digital operations with numerous chemical systems is now possible. Recently, a keypad lock, which is an important electronic logic device, has been mimicked at the molecular level.5 This device can be used for numerous applications in which access to an object or data is to be restricted to a limited number of persons having the exact password to open the keypad lock. The most important advantage of a keypad lock system over simple logic gate is that its output signals are dependent not only on the proper combination of inputs but also on the correct order by which these inputs are introduced. Therefore, the development of such molecular devices, capable of authorizing password entries, is a particularly attractive research goal as it represents a new approach for protecting information on molecular scale.To mimic various electronic devices, numerous chemical systems responding to a large variety of activating input signals (e.g., light, electrical, magnetic, and chemical) have been developed.1k,6 Among these chemical systems, the most important one is fluorescent chemosensors with high selectivity and sensitivity for a specific analyte, such as heavy metal ions, anions, and pH. In particular, a fluorescent chemosensor for heavy metal ions not only can be used to detect the presence of the heavy metal ions (such as Hg2+ ions) in aqueous solution and living cells, but also to integrate these heavy metal ions as chemical-driven molecular machines in future molecular computing. Therefore, there is an urgent need to develop innovative and convenient fluorescent chemosensors for heavy metal ions (such as Hg2+ ions) because of the dual important application of a single molecule.
For fluorescent chemosensors, suitable fluorescence indicators that are sensitive to heavy metal ions (such as Hg2+ ions) concentrations and exhibit changes in fluorescence intensity have been used as molecular recognition materials. Among the numerous indicators, rhodamine derivatives are an excellent candidate due to their high fluorescence sensitivity to heavy metal ions, in addition to the excellent spectroscopic properties of large molar extinction coefficients, high fluorescence quantum yields and visible wavelength excitation. They can change their geometric structures among two states as a result of stimuli. Typically, in the absence of metal ions, the molecules prefer their spirolactam ring-closed geometry,7 which shows little absorption and fluorescence, whereas upon the addition of specific metal ions, the chelation or reaction of metal ions with sensor molecules will simultaneously open the spirolactam ring, which gives rise to a strong fluorescence emission.8 Simultaneously, they display a strong color development against the colorless blank during the sensing event, which is an important feature that would facilitate “naked-eye” detection. Therefore, many molecular switches and logic gates using rhodamine derivatives as indicator have been proposed.9 However, up to now, there has been no report on molecular keypad locks based on rhodamine derivatives as far as we know.
Herein, we intend to report the synthesis and characterization of a novel fluorescent chemosensor to detect Hg2+ both in aqueous solution and in living cells with rhodamine derivative 1 (Rh1) as fluorescent indicator. Moreover, Rh1 has been identified to respond as On and Off state of fluorescence depending upon the sequence of addition of Hg2+ and Cu2+ ions into the solution. Based on this property, a molecular keypad lock based on rhodamine derivative Rh1 was constructed whose fluorescence is in the On state in response to a specific sequence of chemical inputs (Hg2+ and Cu2+ ions).
Experimental section
Reagents and materials
Rhodamine 6G and hydrazine hydrate were supplied by Shanghai Chemical Reagents (Shanghai) and used as received. Lawesson's Reagent (2,4-bis(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane-2,4-disulfide) and 2-hydroxy-1-naphthaldehyde were purchased from Alfa Aesar. Toluene was treated by sodium chips and then distilled. Ethanol was treated by magnesium ribbon and then distilled. Other chemicals are of analytical reagent grade and used without further purification except when specified. All inorganic metal salts were analytical grade and used without further purification. The water used in our present work was deionized. NaAc-HAc buffer solution was prepared using proper amount of NaAc and HAc (analytical grade) under adjustment by a pH meter.Characterization
Absorption spectra were recorded with a Shimadzu UV-3000 spectrophotometer. Fluorescence spectra were measured with a Hitachi F-4500 fluorescence spectrophotometer with a 10 mm quartz cuvette. The excitation and emission wavelength bandpasses were both set at 5 nm. 1H and 13C NMR spectra were recorded using a mercury-300BB spectrometer (Varian, USA) operated at 300 MHz with tetramethylsilane (TMS) as internal standard. Mass spectra were performed on Agilent 1100 MS series and AXIMA CFR MALDI/TOF (Matrix assisted laser desorption ionization/Time-of-flight) MS (COMPACT). All of the measurements were done at room temperature, about 298 K.Synthesis of the materials
Cell culture and imaging
The Rat Schwann cells (RSC 96) were provided by Norman Bethune College of Medicine Jilin University (China). Cells were grown in H-DMEM (Dulbecco's Modified Eagle's Medium, High Glucose) supplemented with 10% FBS (Fetal Bovine Serum) in an atmosphere of 5% CO2, 95% air at 37 °C. Cells were plated on 6-well plate at 5 × 106cells per well and allowed to adhere for 12 h. Fluorescence imaging was performed with an Olympus fluorescence microscope (BX51, Olympus, Japan). Immediately before the experiments, the cells were washed with phosphate-buffered saline (PBS) and then incubated with 10 μM of Rh1 (in the culture medium) for 10 min at 37 °C. Experiments to assess Hg2+ uptake were performed in the same media supplemented with 50 μM Hg(ClO4)2 for 0.5 h.Results and discussion
Rhodamine derivatives are generally non-fluorescent and colorless, whereas when they coordinate with some specific metal ions, ring-opening of the corresponding spirolactam gives rise to a strong fluorescence emission and a pink color. Bearing this in mind, we envisaged that, if introducing proper binding sites, it would be possible to achieve a rhodamine spirolactam based chemosensor highly selective for heavy metal ions (such as Hg2+ ion) via color/fluorescence changes. Based on the theory of hard and soft acids and bases (HSAB theory), Hg2+ is a representative example of soft acid, and S2− is a soft base. Therefore, a sulfur-based functional group must be a good candidate as the S is a strong binding site for Hg2+ ions. Based on the above reasons, we designed and synthesized a fluorescent chemosensor based on rhodamine derivative Rh1 for detecting Hg2+ ions that works as a molecular keypad lock. Rh1 contains an S atom, an N atom attached to the N-bearing spiro ring and an O atom from phenolic hydroxyl of 2-hydroxy-1-naphthaldehyde, which might be a highly selective chemosensor for detecting Hg2+ ions since the S, N, O binding sites are a good choice to be parts of a selective receptor. The synthetic procedure of this material is outlined in Scheme 1. Rhodamine-6G hydrazide was prepared according to a literature method,10 and treatment of it with Lawesson's reagent in refluxing toluene gave, after flash column chromatography on silica, the corresponding rhodamine-6G thiohydrazide in a yield of ca. 16%. Rh1 was successfully synthesized with 2-hydroxy-1-naphthaldehyde and rhodamine-6G thiohydrazide as an intermediate. Both Rh1 and rhodamine-6G thiohydrazide were characterized using various analytical spectroscopic techniques (see the Experimental Section), which agreed well with the proposed structures. | ||
| Scheme 1 Synthesis procedure of Rh1. | ||
As mentioned above, we speculated the S, N, O binding sites might be sensitive towards the Hg2+ ion, thus we tested the effect of Hg2+ ions on the fluorescence behavior of Rh1. The fluorescence spectra were recorded upon excitation at 500 nm at room temperature with the gradual addition of small amounts of Hg2+ ions (0–10 equiv, 0–100 μM) into Rh1 solution, and shown in Fig. 1a. In the absence of Hg2+, as expected, the Rh1 solution exhibited very weak fluorescence in the range from 510 nm to 650 nm. Upon the addition of increasing concentrations of Hg2+, a new emission band peaking at 555 nm appeared and developed, which can be ascribed to the delocalized xanthene moiety of rhodamine group.12 The fluorescence response of Rh1 to other metal ions under the same condition was also investigated. As illustrated in Fig. 1b, no significant spectral changes of Rh1 were observed in the presence of K+, Na+, Ca2+, Mg2+, Ba2+, Zn2+, Cd2+, Cu2+ and Pb2+ (1 mM), indicating that Rh1 could recognize Hg2+ from other metal ions even those that exist in high concentrations.
 | ||
Fig. 1 (a) Emission spectra of Rh1 (10 μM) in the presence of increasing concentration of Hg2+ in buffered (NaAc-HAc, pH = 7) water/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). λex = 500 nm. (b) Normalized fluorescence response I/I0 of Rh1 (10 μM) in the presence of various metal ions (1 × 10−3 M). λex = 500 nm and λem = 555 nm. Inset shows fluorescence change upon addition of Hg2+, Zn2+, Cd2+, Cu2+ , Pb2+ and blank (5 equiv). (c) Job's plot for determining the stoichiometry of Rh1 and Hg2+ ions (the total concentration of Rh1 and Hg2+ ions was 10 μM). 1, v/v). λex = 500 nm. (b) Normalized fluorescence response I/I0 of Rh1 (10 μM) in the presence of various metal ions (1 × 10−3 M). λex = 500 nm and λem = 555 nm. Inset shows fluorescence change upon addition of Hg2+, Zn2+, Cd2+, Cu2+ , Pb2+ and blank (5 equiv). (c) Job's plot for determining the stoichiometry of Rh1 and Hg2+ ions (the total concentration of Rh1 and Hg2+ ions was 10 μM). | ||
To understand the recognition abilities of Rh1 towards Hg2+ ions, a Job's plot analysis was conducted to determine the binding stoichiometry of the Rh1-Hg2+ complex, by maintaining the total Rh1 and Hg2+ ions constant (10 μM) and changing the mole fraction of Hg2+ from 0 to 1. From the Job's plot shown in Fig. 1c, we can observe that the significant increase in fluorescence intensity of Rh1 resulted from the complexation with Hg2+. The fluorescence intensity went through a maximum at a molar fraction of about 0.5 of Hg2+, indicating that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was the most possible one for the binding mode of Hg2+ and Rh1.
1 stoichiometry was the most possible one for the binding mode of Hg2+ and Rh1.
The association constant for Rh1 binding to Hg2+ was determined from the absorption titration data. Upon gradual addition of Hg2+ into the solution, a new absorption peaking around 527 nm emerged with increasing intensity which corresponds to the appearance of a pink color, suggesting the formation of the ring-opened tautomer of Rh1 upon Hg2+ binding (Fig. 2a and b). Since Rh1 binds with Hg2+ to form a complex with a complexing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the equilibrium can thus be described as follows:
1, the equilibrium can thus be described as follows:
![(a) Absorption spectra of Rh1 (10 μM) in the presence of increasing concentration of Hg2+ in buffered (NaAc-HAc, pH = 7) water/CH3CN (1 : 1, v/v). (b) Color changes of Rh1 (10 μM) upon addition of different amounts of Hg2+. (c) Absorbance as a function of [Hg2+] calculated from eqn (3): ■, data points experimentally obtained.](/image/article/2011/RA/c1ra00488c/c1ra00488c-f2.gif) | ||
Fig. 2 (a) Absorption spectra of Rh1 (10 μM) in the presence of increasing concentration of Hg2+ in buffered (NaAc-HAc, pH = 7) water/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). (b) Color changes of Rh1 (10 μM) upon addition of different amounts of Hg2+. (c) Absorbance as a function of [Hg2+] calculated from eqn (3): ■, data points experimentally obtained. 1, v/v). (b) Color changes of Rh1 (10 μM) upon addition of different amounts of Hg2+. (c) Absorbance as a function of [Hg2+] calculated from eqn (3): ■, data points experimentally obtained. | ||

| (1) |
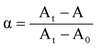
| (2) |

| (3) |
From the above discussion, it is apparent that the absorbance of Rh1 has a distinct functional relationship with the Hg2+ concentration, and the association constant Ks, which provides the basis for the detection of the Ks value. The fitted curve to incorporate experimental data for Hg2+ is presented in Fig. 2c, which gave an association constant Ks value of 8.42 × 104 M−1 for Rh1 binding to Hg2+. The strong binding ability of Rh1 towards Hg2+ could be ascribed to the introduction of the S atom.
In view of the good sensing performances of Rh1 to Hg2+ in aqueous solution, fluorescence imaging experiments were carried out in living cells on a Olympus fluorescence microscope to further demonstrate the practical applicability of Rh1 in living cells. As shown in Fig. 3b, after staining Rat Schwann cells with Rh1 (10 μM) for 10 min, they displayed no detectable fluorescence signal in living cells. Upon addition of 50 μM Hg2+ ions, the fluorescence intensity increases dramatically to show a clear red intracellular fluorescence (Fig. 3d). The results indicate that Rh1 can provide a fluorescence enhancement with excellent cell-permeability and biocompatibility for tracing the Hg2+ ion in cells, specifically and rapidly. Therefore, Rh1 could be considered for application for in vitro imaging of Hg2+ ion in living cells and potentially in vivo.
 | ||
| Fig. 3 Fluorescence images of Hg2+ ions in Rat Schwann cells with Rh1 (10 μM). Bright-field transmission image (a, c) and fluorescence image (b, d) of Rat Schwann cells incubated with 0 μM and 50 μM of Hg2+ ions for 30 min, respectively (excited with green light). | ||
In the experiment of testing the selectivity of Rh1, an interesting phenomenon attracted our attentions. There was no obvious color change in the presence of interfering effect metal ions, such as K+, Na+, Ca2+, Mg2+, Ba2+, Zn2+, Cd2+, and Pb2+ as shown in Fig. 4. However, the solution of Rh1 exhibits an obvious pink color with the addition of Cu2+, thus almost no fluorescence change is observed (insert in Fig 1b). Therefore, we investigated the detailed response of Rh1 to Cu2+ ion.
 | ||
Fig. 4 Change in color of Rh1 (10 μM) in buffered (NaAc-HAc, pH = 7) water/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with metal ions: blank, K+, Na+, Ca2+, Mg2+, Ba2+, Zn2+, Cd2+, Cu2+ and Pb2+ (1 × 10−3 M, from left to right). 1, v/v) with metal ions: blank, K+, Na+, Ca2+, Mg2+, Ba2+, Zn2+, Cd2+, Cu2+ and Pb2+ (1 × 10−3 M, from left to right). | ||
Unlike the varying tendency of fluorescence emission, upon addition of small amounts of Cu2+, the absorbance was significantly enhanced with a new peak appearing at 527 nm (Fig. 5a), whereas the absorption spectra of free Rh1 exhibited only a very weak band above 500 nm, which was ascribed to the spirolactam form of Rh1. Upon the addition of 5 equiv of Cu2+, the new peak was enhanced up to log ε = 5.25, accompanied with an obvious color change from colorless to pink simultaneously (Fig. 5b). The Job's plot analysis in Fig. 5c was conducted to determine the binding stoichiometry of the Rh1-Cu2+ complex. It was observed that a maximum absorption was reached when the molar fraction of Cu2+ ions was about 0.5, confirming that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry is most possible for the binding mode of Cu2+ and Rh1. The association constant for Rh1 binding to Cu2+ was also determined from the absorption titration data. Since Rh1 bound with Cu2+ to form a complex with a complexing ratio of 1
1 stoichiometry is most possible for the binding mode of Cu2+ and Rh1. The association constant for Rh1 binding to Cu2+ was also determined from the absorption titration data. Since Rh1 bound with Cu2+ to form a complex with a complexing ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the data still can be fitted by eqn (3). Fig. 6 shows the fitted curve to incorporate the experimental data for Cu2+, which gives an association constant Ks value of 1.98 × 104 M−1 for Rh1 binding to Cu2+. Upon addition of small amounts Cu2+, the enhancement in absorbance clearly suggested the formation of the delocalized xanthane moiety of the rhodamine group, along with a distinct color change from colorless to pink. However, the fluorescence of the open-ring form of Rh1 was quenched by Cu2+, which could be explained based on the well-known paramagnetic effect of the Cu(II) d9 system.14
1, the data still can be fitted by eqn (3). Fig. 6 shows the fitted curve to incorporate the experimental data for Cu2+, which gives an association constant Ks value of 1.98 × 104 M−1 for Rh1 binding to Cu2+. Upon addition of small amounts Cu2+, the enhancement in absorbance clearly suggested the formation of the delocalized xanthane moiety of the rhodamine group, along with a distinct color change from colorless to pink. However, the fluorescence of the open-ring form of Rh1 was quenched by Cu2+, which could be explained based on the well-known paramagnetic effect of the Cu(II) d9 system.14
 | ||
Fig. 5 (a) Absorption spectra of Rh1 (10 μM) in the presence of increasing concentration of Cu2+ in buffered (NaAc-HAc, pH = 7) water/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). (b) Color changes of Rh1 (10 μM) upon addition of different amounts of Cu2+. (c) Job's plot for determining the stoichiometry of Rh1 and Cu2+ ions (the total concentration of Rh1 and Cu2+ ions was 10 μM). 1, v/v). (b) Color changes of Rh1 (10 μM) upon addition of different amounts of Cu2+. (c) Job's plot for determining the stoichiometry of Rh1 and Cu2+ ions (the total concentration of Rh1 and Cu2+ ions was 10 μM). | ||
![Absorbance as a function of [Cu2+] calculated from eqn (3): ■, data points experimentally obtained.](/image/article/2011/RA/c1ra00488c/c1ra00488c-f6.gif) | ||
| Fig. 6 Absorbance as a function of [Cu2+] calculated from eqn (3): ■, data points experimentally obtained. | ||
Since the Ks values of Rh1-Hg2+ and Rh1-Cu2+ are comparable, Hg2+ and Cu2+ would compete against each other to coordinate with Rh1 when they were coexist in a system, resulting in a transformation between Rh1-Hg2+ and Rh1-Cu2+ with fluorescence. Based on this, we investigated the different fluorescence state “ON and OFF” of Rh1 with changing the addition sequence, using Hg2+ (2 equiv.) and Cu2+ (10 equiv.) as inputs (Fig. 7). When the first input is Hg2+, the fluorescence emission at 555 nm significantly enhanced. Although the sequential addition of Cu2+ led to a relative decline of the fluorescence intensity, it was still in the “On” state. On reversal of the input sequence, i.e. for Cu2+ as the first input and Hg2+ as the second one, it caused the fluorescence intensity far below its initial maximum, i.e. “Off” state. The two input signals of Hg2+ and Cu2+ were defined as In 1 and In 2, respectively. These inputs can be encoded with binary digits applying positive logic conventions (off = 0, on = 1). If we regard fluorescence intensity of 75 as the threshold values, Output = 0 when its corresponding spectral value was lower than 75; Output = 1 when its corresponding spectral value was higher than 75.
 | ||
| Fig. 7 Fluorescence emission output of Rh1 with different input sequences: (1) Hg2+ as the first input followed by Cu2+ as the second one; (2) Cu2+ as the first input followed by Hg2+ as the second one. | ||
As discussed above, Fig. 8 presents the outputs in the fluorescence channel, obtained for different addition sequences. Only in input sequence of Hg2+ and Cu2+, the fluorescent intensity was higher than the threshold value (Output = 1), resulting in strong fluorescence. However, changing the sequence of the inputs, it could not initiate a strong fluorescence at 555 nm (Output = 0). Therefore, according to this sequence-dependent phenomenon, a molecular keypad lock was constructed.
 | ||
| Fig. 8 Fluorescence output resulting from different input sequences: (a) In 1 (Hg2+ first) and In 2 (Cu2+ second); b) In 2 (Cu2+ first) and In 1 (Hg2+ second). | ||
To simplify the input sequence as password of the molecular keypad lock, inputs Hg2+ and Cu2+ were designated as “K” and “E”, respectively. When the input signal “K” was added first and followed by “E”, the emission at 555 nm was in the “On” state, and it created a secret password “KEY” (Y defines On state). Inverting the addition sequence of inputs, i.e. the first input is “E” and the second input is “K”, gave an obvious fluorescence quenching (Off, designated as the character “N”). Thus, the wrong password “EKN” failed to open the lock. Therefore, only the authorized user who knows the exact password “KEY” can open the lock, which is a new approach for protecting information at the molecular scale (Fig. 9). Due to the fact that the use of numerical digits (0–9) and letters (A–Z) as PIN numbers in a two-digit password allows a total of more than 700 different combinations,5j it added to the complexity of cracking the keypad lock, and improved remarkably the security of the molecular device.
 | ||
| Fig. 9 Fluorescent keypad to access a secret password at 555 nm with different input sequences. | ||
Conclusions
In summary, a novel fluorescent chemosensor based on rhodamine derivative Rh1 was designed and synthesized for the detection of Hg2+ in aqueous solution and living cells. The fluorescent indicator of Rh1 can efficiently recognize Hg2+ over other metal ions by the fluorescence intensity increase, which could be ascribed to the delocalized xanthene moiety of the rhodamine group. Moreover, this “Off–On”-type fluorescent sensor could successfully mimic a molecular level keypad lock in the presence of Cu2+ ions. Only the correct password resulted in strong fluorescence emission at 555 nm which can be used to “open” this molecular keypad lock. Wrong passwords failed to open the lock, which can result in the “alarm” signal indicating the wrong password. Therefore, this molecular keypad lock has the potential for protecting information at the molecular scale, which would be used to authorize a user, to verify authentication of a product, or to initiate a higher process.Acknowledgements
The authors gratefully thank the financial supports of the National Natural Science Foundations of China (Grant No. 50872130) and the Science and Technology Developing Project of Jilin Province (Grant No. 20100533).References
- (a) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and E. T. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS; (b) B. L. Feringa, Molecular witches, Wiley-VCH, Weinheim 2001 CrossRef; (c) F. M. Raymo, Adv. Mater., 2002, 14, 401 CrossRef CAS; (d) V. Balzini, A. Credi and M. Venturi, ChemPhysChem, 2003, 4, 49 CrossRef; (e) X. Guo, D. Zhang, T. Wang and D. Zhu, Chem. Commun., 2003, 914 RSC; (f) A. P. de Silva and N. D. McClenaghan, Chem.–Eur. J., 2004, 10, 574 CrossRef; (g) D. H. Qu, Q. C. Wang and H. Tian, Angew. Chem., 2005, 117, 5430 CrossRef; (h) D. H. Qu, F. Y. Ji, Q. C. Wang and H. Tian, Adv. Mater., 2006, 18, 2035 CrossRef CAS; (i) J. Andreasson, S. D. Straight, G. Kodis, C. D. Park, M. Hambourger, M. Grevaldo, B. Albinson, T. A. Moore, A. L. Moore and D. Gust, J. Am. Chem. Soc., 2006, 128, 16259 CrossRef CAS; (j) A. Credi, Angew. Chem., Int. Ed., 2007, 46, 5472 CrossRef CAS; (k) U. Pischel, Angew. Chem., Int. Ed., 2007, 46, 4026 CrossRef CAS; (l) K. Szacilowski, Chem. Rev., 2008, 108, 3481 CrossRef CAS; (m) S. D. Straight, P. A. Liddell, Y. Terazono, T. A. Moore, A. L. Moore and D. Gust, Adv. Funct. Mater., 2007, 17, 777 CrossRef CAS; (n) R. Ballardini, P. Ceroni, A. Credi, M. T. Gandolfi, M. Maestri, M. Semararo, M. Venturi and V. Balzani, Adv. Funct. Mater., 2007, 17, 740 CrossRef CAS.
- R. Baron, A. Onopriyenko, E. Katz, O. Lioubashevski, I. Willner, S. Wang and H. Tian, Chem. Commun., 2006, 2147 RSC.
- Y. Liu, W. Jiang, H. Y. Zhang and C. J. Li, J. Phys. Chem. B, 2006, 110, 14231 CrossRef CAS.
- J. Andreasson, S. D. Straight, S. Bandyopadhyay, R. H. Mitchell, T. A. Moore, A. L. Moore and D. Gust, J. Phys. Chem. C, 2007, 111, 14274 CAS.
- (a) D. Margulies, C. E. Felder, G. Melman and A. Shanzer, J. Am. Chem. Soc., 2007, 129, 347 CrossRef CAS; (b) G. Strack, M. Ornatska, M. Pita and E. Katz, J. Am. Chem. Soc., 2008, 130, 4234 CrossRef CAS; (c) M. Kumar, R. Kumar and V. Bhalla, Chem. Commun., 2009, 7384 RSC; (d) M. Zhou, X. Zheng, J. Wang and S. Dong, Chem.–Eur. J., 2010, 16, 7719 CrossRef CAS; (e) J. Andrasson, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, Chem.–Eur. J., 2009, 15, 3936 CrossRef; (f) J. Halamek, T. K. Tam, S. Chinnapareddy, V. Bocharova and E. Katz, J. Phys. Chem. Lett., 2010, 1, 973 CrossRef CAS; (g) J. Wang and C. S. Ha, Analyst, 2010, 135, 1214 RSC; (h) J. Halamek, T. K. Tam, G. Strack, V. Bocharova, M. Pita and E. Katz, Chem. Commun., 2010, 2405 RSC; (i) S. Kumar, V. Luxami, R. Saini and D. Kaur, Chem. Commun., 2009, 3044 RSC; (j) M. Suresh, A. Ghosh and A. Das, Chem. Commun., 2008, 3906 RSC; (k) Z. Guo, W. Zhu, L. Shen and H. Tian, Angew. Chem., Int. Ed., 2007, 46, 5549 CrossRef CAS; (l) M. Kumar, A. Dhir and V. Bhalla, Org. Lett., 2009, 11, 2567 CrossRef CAS; (m) P. Singha, J. Kaura and W. Holzer, Sens. Actuators, B, 2010, 150, 50 CrossRef.
- (a) A. P. de Silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399 CrossRef CAS; (b) D. Margulies, G. Melman and A. Shanzer, J. Am. Chem. Soc., 2006, 128, 4865 CrossRef CAS.
- T. Nguyen and M. B. Francis, Org. Lett., 2003, 5, 3245 CrossRef CAS.
- B. Valeur, Molecular Fluorescence: Principles and Applications, Wiley-VCH, Verlag GmbH: New York, 2001 Search PubMed.
- (a) Y. Xiang and A. Tong, Org. Lett., 2006, 8, 1549 CrossRef CAS; (b) Y. Xiang, A. Tong, P. Jin and Y. Ju, Org. Lett., 2006, 8, 2863 CrossRef CAS; (c) Y. K. Yang, K. J. Yook and J. Tae, J. Am. Chem. Soc., 2005, 127, 16760 CrossRef CAS; (d) J. Y. Kwon, Y. J. Jang, Y. J. Lee, K. M. Kim, M. S. Seo, W. Nam and J. Yoon, J. Am. Chem. Soc., 2005, 127, 10107 CrossRef CAS; (e) H. Zheng, Z. H. Qian, L. Xu, F. F. Yuan, L. D. Lan and J. G. Xu, Org. Lett., 2006, 8, 859 CrossRef CAS; (f) J. S. Wu, I. C. Hwang, K. S. Kim and J. S. Kim, Org. Lett., 2007, 9, 907 CrossRef CAS; (g) D. Wu, W. Huang, C. Duan, Z. Lin and Q. Meng, Inorg. Chem., 2007, 46, 1538 CrossRef CAS; (h) J. Chen, A. F. Zheng, A. H. Chen, Y. Gao, C. He, X. Kai, G. Wu and Y. Chen, Anal. Chim. Acta, 2007, 599, 134 CrossRef CAS; (i) K. P. Prathish, D. James, J. Jaisy and T. Prasada Rao, Anal. Chim. Acta, 2009, 647, 84 CrossRef CAS; (j) Y. Xiang, Z. Li, X. Chen and A. Tong, Talanta, 2008, 74, 1148 CrossRef CAS; (k) M. Suresh, A. Shrivastav, S. Mishra, E. Suresh and A. Das, Org. Lett., 2008, 10, 3013 CrossRef CAS; (l) Y. Zhao, X. B. Zhang, Z. X. Han, L. Qiao, C. Y. Li, L. X. Jian, G. L. Shen and R. Q. Yu, Anal. Chem., 2009, 81, 7022 CrossRef CAS; (m) V. Bhalla, R. Tejpal and M. Kumar, Sens. Actuators, B, 2010, 151, 180 CrossRef.
- (a) Y. K. Yang, K. J. Yook and J. Tae, J. Am. Chem. Soc., 2005, 127, 16760 CrossRef CAS; (b) X. F. Yang, X. Q. Guo and Y. B. Zhao, Talanta, 2002, 57, 883 CAS.
- (a) Z. X. Han, X. B. Zhang, Z. Li, Y. J. Gong, X. Y. Wu, Z. Jin, C. M. He, L. X. Jian, J. Zhang, G. L. Shen and R. Q. Yu, Anal. Chem., 2010, 82, 3108 CrossRef CAS; (b) H. Zheng, Z. H. Qian, L. Xu, F. F. Yuan, L. D. Lan and J. G. Xu, Org. Lett., 2006, 8, 859 CrossRef CAS; (c) W. Liu, L. Xu, R. Sheng, P. Wang, H. Li and S. Wu, Org. Lett., 2007, 9, 3829 CrossRef CAS.
- D. Wu, W. Huang, C. Duan, Z. Lin and Q. Meng, Inorg. Chem., 2007, 46, 1538 CrossRef CAS.
- (a) X. B. Zhang, C. C. Guo, Z. Z. Li, G. L. Shen and R. Q. Yu, Anal. Chem., 2002, 74, 821 CrossRef CAS; (b) Y. Zhao, X. B. Zhang, Z. X. Han, L. Qiao, C. Y. Li, L. X. Jian, G. L. Shen and R. Q. Yu, Anal. Chem., 2009, 81, 7022 CrossRef CAS.
- (a) J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780 CrossRef CAS; (b) M. Suresh, A. Shrivastav, S. Mishra, E. Suresh and A. Das, Org. Lett., 2008, 10, 3013 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
