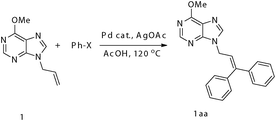
Chelation-assisted palladium-catalyzed high regioselective heck diarylation reaction of 9-allyl-9H-purine: synthesis of 9-(3,3-diaryl-allyl)-9H-purines†
Hai-Ming
Guo
*a,
Wei-Hao
Rao
a,
Hong-Ying
Niu
b,
Li-Li
Jiang
a,
Lei
Liang
a,
Yang
Zhang
a and
Gui-Rong
Qu
*a
aCollege of Chemistry and Environmental Science, Key Laboratory of Green Chemical Media and Reactions of Ministry of Education, Henan Normal University, Xinxiang, 453007, Henan, China. E-mail: guohm518@hotmail.com; quguir@sina.com; Fax: 86 373 3329276; Tel: 86 373 3329255
bSchool of Chemistry and Chemical Engineering, Henan Institute of Science and Technology, Xinxiang, 453003, China
First published on 23rd September 2011
Abstract
A Pd-catalyzed high regioselective diarylation of 9-allyl-9H-purinevia chelation-assisted Heck reaction is developed. There were two different types of β-H in the allyl substrate, while the two aryl groups are exclusively introduced to the terminal of the olefins.
The Pd-catalyzed Heck reaction of aryl or alkenyl halides or triflates with alkenes has been developed into a powerful tool for the formation of C–C bonds.1 Recently, different versions of directing groups such as organic phosphine,2tertiary amine,3pyridine,4sulfoxide5 and sulfone6 have been exploited for directing metal-catalyzed Heck reactions with varying degrees of success (eqn. (1), Scheme 1).7 Those vinyl ethers8 bearing a directing group are relatively simple since their structures bear only one type of β-H except for a few cases.4b As a consequence, challenges with elaborated olefins bearing two types of β-H lie ahead with respect to regioselectivity and catalytic efficiency. Furthermore, all those chelation-assisted Pd-catalyzed Heck reactions are based on the classical Pd(0)/Pd(II) catalyst cycle2–7 in the presence of organic ligand. As such, chelation-assisted Pd-catalyzed Heck reactionviaPd(II)/Pd(IV) catalyst cycle under an additional ligand-free condition has not been previously realized.
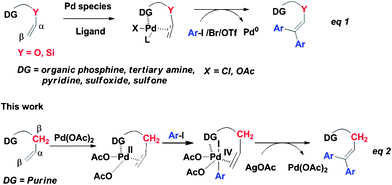 | ||
| Scheme 1 Different catalytic cycles for Pd-catalyzed diarylation Heck reaction of olefins. | ||
In the last decade, directed C–H bond activation has emerged as a versatile strategy for constructing C–heteroatom bonds with a proper choice of directing group and catalytic system.9Purine derivatives are of great importance in medicinal chemistry since they display a broad spectrum antiviral activity, antimycobacterial activity and biological activity.10Purine contains four nitrogen atoms and belongs to a special class of aromatic heterocycles. We envisioned that purine could serve as an efficient directing group for the Pd-catalyzed Heck reaction. Daugulis' group has reported a series of directed Pd-catalyzed arylation reactions with a AgOAc/AcOH system.11 Despite the reported long reaction time, we considered that this catalytic system might serve as a good partner of the purine-assisted Pd-catalyzed Heck reaction. As part of our ongoing course of study on the modification of purine analogues,12herein, a chelation-assisted Pd-catalyzed high regioselective diarylation reaction of olefinsvia a possible Pd(II)/Pd(IV) catalyst cycle is described (eqn (2), Scheme 1). In comparison to related chelation-assisted Pd-catalyzed Heck reactions,2–6 the purine directing group is more challenging for its fused ring structure and possessing multiple nitrogen atoms, and this diarylation method presumably occurs by a distinct Pd(II)/Pd(IV) mechanism, which is quite different from a classical Pd(0)/Pd(II) catalyst cycle in the presence of an organic ligand. Notably, the high regioselective diarylation and high catalytic efficiency have been achieved with the choice of purine as a directing group.
We initially conducted our experiment by treating 9-allyl-6-methoxyl purine (1) with 5 mol % Pd(OAc)2 in the presence of 3.0 equiv. of PhI and 2.0 equiv. of AgOAc in acetic acid at 120 °C for 4.5 h. To our delight, the desired diarylated product 1aa was isolated in 93% yield (entry 1, Table 1). Remarkably, lowering the catalyst loading to 3 mol % could also efficiently catalyze the phenylation of 1 to afford 1aa in 88% isolated yield (entry 2) and lowering the reaction temperature did not bring about a significant reduction of 1aa (entry 3). Further studies showed that catalytic amount of other Pd(II) sources such as PdCl2, PdCl2(Ph3P)2 could also efficiently catalyze the phenylation reaction (entries 4–5). Unfortunately, PhBr was unsuitable for this transformation (entries 6 and 7), which might be due to the strong dissociation energy of the Ph–Br bond.
| Entry | X | Pd cat. | Time (h) | Yield (%)b |
|---|---|---|---|---|
| a Reaction conditions: 0.1 mmol 1 and 0.3 mmol PhI or PhBr, 2 equiv. of AgOAc, 0.25 M 1 in AcOH. b Isolated yields based on 1. c The reaction was carried out at 100 °C | ||||
| 1 | I | 5 mol % Pd(OAc) 2 | 4.5 | 93 |
| 2 | I | 3 mol % Pd(OAc)2 | 4.5 | 88 |
| 3c | I | 5 mol % Pd(OAc)2 | 4.5 | 91 |
| 4 | I | 5 mol % PdCl2 | 5 | 90 |
| 5 | I | 5 mol % PdCl2(Ph3P)2 | 5 | 88 |
| 6 | Br | 5 mol % Pd(OAc)2 | 14 | trace |
| 7 | Br | 10 mol % Pd(OAc)2 | 24 | 24 |
With the optimized conditions in hand, a variety of aryl iodides were further explored. As shown in Table 2, the non-stepwise one-pot Heck double arylation reactions proceeded smoothly despite two different aryl iodides being simultaneously present in the reaction mixture (entries 2, 3, 6 and 9). Moreover, aryl iodides bearing either an electron-withdrawing group (i.e. p-COOEt, o-COOMe) (entries 8 and 12) or an electron-donating group (i.e. p-Me, p-MeO) (entries 2–4, 7, and 9–11) could serve as good partners of the purine-assisted Heck arylation reaction. Although the E/Z selectivity was very poor in the one-pot competitive reaction, the Heck diarylation reaction proved to be mild and highly efficient. Notably, the reaction regiospecifically occurred on the terminal of the olefins even though there were two different types of β-H in the substrates.
| Entry | R | Ar1-I | Ar2-I | Product | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction conditions: 0.2 mmol 1, 0.25 M 1 in AcOH; for double arylation: 1.02 equiv. Ar1–I, 1.02 equiv. Ar2–I; for diarylation: 3.0 equiv. ArI, and the E/Z isomers ratio was determined by 1H NMR integration. b Isolated yields based on 9-allyl-6-substituted purine. c 1.5 equiv. PhI was used and 1aa was also obtained in 33% isolated yield in this reaction. d 1aa and 1dd were also obtained in respective 31% and 23% isolated yields. e (E)-monoarylated product 1f was also obtained in 20% isolated yield in this reaction. | |||||
| 1 | OMe | Ph | Ph | 1aa | 93 |
| 2 | OMe | Ph | p-Me-Ph | 1ab | 89 |
| 3c | OMe | Ph | p-MeO-Ph | 1ac | 66 |
| 4 | OMe | p-Me-Ph | p-Me-Ph | 1bb | 98 |
| 5 | Me | Ph | Ph | 2aa | 91 |
| 6d | OMe | Ph | p-EtO2C-Ph | 1ad | 22 |
| 7 | OMe | p-MeO-Ph | p-MeO-Ph | 1cc | 87 |
| 8 | OMe | p-EtO2C-Ph | p-EtO2C-Ph | 1dd | 93 |
| 9 | OMe | p-Me-Ph | p-MeO-Ph | 1bc | 68 |
| 10 | OMe | 3,5-bis(Me)-Ph | 3,5-bis(Me)-Ph | 1ee | 96 |
| 11 | Me | p-Me-Ph | p-Me-Ph | 2bb | 86 |
| 12e | OMe | o-MeO2C-Ph | o-MeO2C-Ph | 1ff | 74 |
Finally, purine as a directing group for Pd-catalyzed Heck reaction was further tested under the classical catalytic system (Scheme 2). We were pleased to find that the corresponding product 1aa was obtained in acceptable to good yields depending on different ligands. However, the use of dppp or dppf was not as good as Ph3P, which might be due to their poor steric flexibility during binding of the Pd atom.
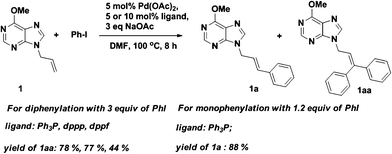 | ||
| Scheme 2 Chelation-assisted Pd-catalyzed phenylation of 1 under classical Heck reaction conditions. | ||
In summary, a Pd-catalyzed high regioselective diarylation of 9-allyl-9H-purinevia chelation-assisted Heck reaction is described. This method bears four important features: 1) purine as a novel chelate compound to direct arylation, 2) the additional ligand-free Pd(II)/Pd(IV) catalyst cycle differing from classical Heck reaction, 3) diarylation reaction does not require an inert atmosphere, and 4) high regioselective diarylation though two different types of β-H which exist in the allyl substrate. Further efforts directed to the detailed mechanism are under way in our laboratory.
Acknowledgements
We are grateful for financial support from the National Nature Science Foundation of China (Grant Nos 20802016, 21172059 and 21072047), the Program for New Century Excellent Talents in University of Ministry of Education (No. NCET-09-0122), Excellent Youth Foundation of Henan Scientific Committee (No. 114100510012), the Program for Changjiang Scholars and Innovative Research Team in University (IRT1061), the National Students Innovation Experiment Program, and the Excellent Youth Program of Henan Normal University.References
- (a) R. F. Heck, J. Am. Chem. Soc., 1968, 90, 5518 CrossRef CAS; (b) R. F. Heck and J. P. Nolley, J. Org. Chem., 1972, 37, 2320 CrossRef CAS; (c) R. F. Heck, Org. React., 1982, 27, 345 CAS; (d) R. F. Heck, in Comprehensive Organic Synthesis, Vol. 4 (Eds: B. M. Trost, I. Fleming), Pergamon Press, Oxford, 1991, pp. 833-863 Search PubMed; (e) A. de Meijere and F. E. Meyer, Angew. Chem., Int. Ed. Engl., 1994, 33, 2379 CrossRef.
- K. Badone and U. Guzzi, Tetrahedron Lett., 1993, 34, 3603 CrossRef.
- (a) P. Nilsson, M. Larhed and A. Hallberg, J. Am. Chem. Soc., 2003, 125, 3430 CrossRef CAS; (b) C.-M. Andemon, J. Larsson and A. Hallberg, J. Org. Chem., 1990, 55, 5757 CrossRef.
- (a) K. Itami, T. Nokami, Y. Ishimura, K. Mitsudo, T. Kamei and J. Yoshida, J. Am. Chem. Soc., 2001, 123, 11577 CrossRef CAS; (b) K. Itami, Y. Ushiogi, T. Nokami, Y. Ohashi and J. Yoshida, Org. Lett., 2004, 6, 3695 CrossRef CAS; (c) S. Oi, K. Sakai and Y. Inoue, Org. Lett., 2005, 7, 4009 CrossRef CAS; (d) L. Ilies, J. Okabe, N. Yoshikai and E. Nakamura, Org. Lett., 2010, 12, 2838 CrossRef CAS.
- (a) N. D. Buezo, I. Alonso and J. C. Carretero, J. Am. Chem. Soc., 1998, 120, 7129 CrossRef; (b) I. Alonso and J. C. Carretero, J. Org. Chem., 2001, 66, 4453 CrossRef CAS; (c) N. D. Buezo, J. C. de la Rosa, J. Priego, I. Alonso and J. C. Carretero, Chem.–Eur. J., 2001, 7, 3890 CrossRef CAS.
- (a) P. Mauleón, A. A. Núnez, I. Alonso and J. C. Carretero, Chem.–Eur. J., 2003, 9, 1511 CrossRef; (b) P. Mauleón, I. Alonso and J. C. Carretero, Angew. Chem., Int. Ed., 2001, 40, 1291 CrossRef; (c) T. Llamas, R. G. Arrayás and J. C. Carretero, Adv. Synth. Catal., 2004, 346, 1651 CrossRef CAS.
- For recent review on this topic, see: L. Ackermann, Top. Organomet. Chem., 2007, 24, 35 CrossRef CAS.
- (a) P. Nilsson, M. Larhed and A. Hallberg, J. Am. Chem. Soc., 2001, 123, 8217 CrossRef CAS; (b) A. Svennebring, P. Nilsson and M. Larhed, J. Org. Chem., 2004, 69, 3345 CrossRef CAS; (c) N. D. Buezo, O. G. Mancheño and J. C. Carretero, Org. Lett., 2000, 2, 1451 CrossRef CAS.
- For recent reviews on directed C-H activation, see: (a) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS; (b) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS; (c) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS; (d) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS.
- (a) X. Chen, E. R. Kern, J. C. Drach, E. Gullen, Y.-C. Cheng and J. Zemlicka, J. Med. Chem., 2003, 46, 1531 CrossRef CAS; (b) Y.-L. Qiu, M. B. Ksebati, R. G. Ptak, B. Y. Fan, J. M. Breitenbach, J.-S. Lin, Y.-C. Cheng, E. R. Kern, J. C. Drach and J Zemlicka, J. Med. Chem., 1998, 41, 10 CrossRef CAS; (c) Y.-L. Qiu, A. Hempel, N. Camerman, A. Camerman, F. Geiser, R. G. Ptak, J. M. Breitenbach, T. Kira, L. Li, E. Gullen, Y.-C. Cheng, J. C. Drach and J. Zemlicka, J. Med. Chem., 1998, 41, 5257 CrossRef CAS; (d) R. J. Rybak, J. Zemlicka, Y.-L. Qiu, C. B. Hartline and E. R. Kern, Antiviral Res., 1999, 43, 175 CrossRef CAS; (e) S. A. Laufer, D. M. Domeyer, T. R. F. Scior, W. Albrecht and D. R. J. Hauser, J. Med. Chem., 2005, 48, 710 CrossRef CAS; (f) L.-L. Gundersen, J. Nissen-Meyer and B. Spilsberg, J. Med. Chem., 2002, 45, 1383 CrossRef CAS; (g) A. K. Bakkestuen, L.-L. Gundersen, G. Langli, F. Liu and J. M. J. Nolsøe, Bioorg. Med. Chem. Lett., 2000, 10, 1207 CrossRef CAS; (h) A. K. Pathak, V. Pathak, L. E. Seitz, W. J. Suling and R. C. Reynolds, J. Med. Chem., 2004, 47, 273 CrossRef CAS.
- (a) V. G. Zaitsev, D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2005, 127, 13154 CrossRef CAS; (b) D. Shabashov and O. Daugulis, Org. Lett., 2005, 7, 3657 CrossRef CAS; (c) A. Lazareva and O. Daugulis, Org. Lett., 2006, 8, 5211 CrossRef CAS; (d) D. Shabashov and O. Daugulis, Org. Lett., 2006, 8, 4947 CrossRef CAS.
- (a) G. R. Qu, Z. J. Mao, H. Y. Niu, D. C. Wang, C. Xia and H. M. Guo, Org. Lett., 2009, 11, 1745 CrossRef CAS; (b) H. M. Guo, Y. Y. Wu, H. Y. Niu, D. C. Wang and G. R. Qu, J. Org. Chem., 2010, 75, 3863 CrossRef CAS; (c) H. M. Guo, P. Li, H. Y. Niu, D. C. Wang and G. R. Qu, J. Org. Chem., 2010, 75, 6016 CrossRef CAS; (d) G. R. Qu, R. Xia, X. N. Yang, J. G. Li, D. C. Wang and H. M. Guo, J. Org. Chem., 2008, 73, 2416 CrossRef CAS; (e) G. R. Qu, B. Ren, H. Y. Niu, Z. J. Mao and H. M. Guo, J. Org. Chem., 2008, 73, 2450 CrossRef CAS; (f) H. M. Guo, P. Y. Xin, H. Y. Niu, D. C. Wang, Y. Jiang and G. R. Qu, Green Chem., 2010, 12, 2131 RSC; (g) G. R. Qu, J. Wu, Y. Y. Wu, F. Zhang and H. M. Guo, Green Chem., 2009, 11, 760 RSC; (h) G. R. Qu, L. Zhao, D. C. Wang, J. Wu and H. M. Guo, Green Chem., 2008, 10, 287 RSC; (i) H.-M. Guo, L.-L. Jiang, H.-Y. Niu, W.-H. Rao, L. Liang, R.-Z. Mao, D.-Y. Li and G.-R. Qu, Org. Lett., 2011, 13, 2008 CrossRef CAS; (j) H. M. Guo, W. H. Rao, H. Y. Niu, L. L. Jiang, G. Meng, J. J. Jin, X. N. Yang and G.-R. Qu, Chem. Commun., 2011, 47, 5608 RSC; (k) H. M. Guo, C. Xia, H. Y. Niu, X. T. Zhang, S. N. Kong, D. C. Wang and G. R. Qu, Adv. Synth. Catal., 2011, 353, 53 CrossRef CAS; (l) H. M. Guo, T. F. Yuan, H. Y. Niu, J. Y. Liu, R. Z. Mao, D. Y. Li and G. R. Qu, Chem.–Eur. J., 2011, 17, 4095 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, compound characterizations, and the copies of 1H NMR and 13C NMR spectra. See DOI: 10.1039/c1ra00410g/ |
| This journal is © The Royal Society of Chemistry 2011 |