A novel bath lily-like graphene sheet-wrapped nano-Si composite as a high performance anode material for Li-ion batteries†
Yu-Shi
He
a,
Pengfei
Gao
a,
Jun
Chen
b,
Xiaowei
Yang
a,
Xiao-Zhen
Liao
a,
Jun
Yang
*a and
Zi-Feng
Ma
*a
aInstitute of Electrochemical and Energy Technology, Department of Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240, China. E-mail: yangj723@sjtu.edu.cn; zfma@sjtu.edu.cn; Fax: +86-21-5474 7667; Tel: +86-21-5474 7667
bIntelligent Polymer Research Institute, ARC Centre of Excellence for Electromaterials Science, Australian Institute of Innovative Materials, Innovation Campus, University of Wollongong, Wollongong, NSW 2522, Australia
First published on 7th September 2011
Abstract
A novel bath lily-like graphene sheet-wrapped nano-Si composite synthesized via a simple spray drying process exhibits a high reversible capacity of 1525 mAh g−1 and superior cycling stability, which could be attributed to a synergistic effect between highly conductive graphene sheets and active nanoparticles in the open nano/micro-structure.
To meet the increasing demands in electric vehicles, energy storage and portable electronics, rechargeable lithium ion batteries with high energy density and long lifespan have received particular attention.1 The conventional graphite anode is still insufficient to meet the demand due to its low theoretical specific capacity (372 mAh g−1).2Silicon is one of the most promising anode materials on account of its acceptable cost, low alloy potential, and the highest known theoretical capacity of about 4200 mAh g−1 for Li storage.3 However, Si anodes show large volume changes upon lithiation and delithiation, which could cause its pulverization and loss of electrical contact between the active particles, resulting in low power capability and rapid capacity fading.4 In order to accommodate the volume change of Si during cycling, great efforts have been undertaken, such as the nanonization of Si-based materials (including nanowires,5 nanosperes,6nanotubes,7etc.), and the preparation of various Si/C composites.8
Recently, graphene as a kind of novel carbonaceous material has attracted worldwide attention since it was first reported in 2004.9 Although graphene can not substitute for graphite as an anode material for lithium ion batteries due to its large initial irreversible capacity loss, relatively high charge/discharge plateau and limited cycling stability,10 it could be an excellent substrate to accommodate active materials owing to its unique two-dimensional nanostructure with high electronic conductivity,11 superior mechanical properties,12 excellent chemical tolerance,13 and high surface area (theoretical value of 2630 m2 g−1).14 In this respect, various graphene-based composites for Li-ion batteries have been prepared, such as TiO2/graphene,15Co3O4/graphene,16SnO2/graphene,17Co(OH)2/graphene,18Mn3O4/graphene,19Li4Ti5O12/graphene,20Fe3O4/graphene,21LiFePO4/graphene,22etc. The cycling performance and rate capability of these materials are obviously improved. Graphene in the materials could not only improve the electrical conductivity of the overall electrode, but also effectively buffer the volume change of the active particles and lessen the strain caused by the volume variation during cycling. More recently, several groups reported the synthesis and electrochemical behavior of Si/graphene materials.23 Although the positive effect of graphene is revealed in these articles, some basic problems in the preparation technology and composite structures still need to be solved. For example, it was very difficult to disperse nano-Si particles in the graphene matrix and obtain a homogeneous composite only by using mortar mixing or mechanical blending.23a,b On the other hand, a high specific capacity over 2000 mAh g−1 and stable cycling performance can be achieved for carbon coated silicon–graphene granules prepared by a two-step chemical vapor deposition method.23e However, the high-cost vapor deposition procedure using extremely flammable silane as raw material may hinder its large scale production.
Herein, we make use of commercialized Si powder and a simple spray drying method to prepare a novel bath lily-like graphene sheet-wrapped nano-Si (GS-Si) composite anode material for lithium ion batteries. In our procedure, no surfactant, no filtration or washing processes and no high vacuum conditions are required. It is safe and environmentally friendly. The obtained GS-Si composite possesses an open nano/micro-structure, in which nanosized Si particles are uniformly dispersed and wrapped in the GS matrix. The GS not only constitutes a good conducting network, but also provides enough void spaces to accommodate the volume change of Si and prevent the aggregation of nano-Si particles during cycling. As a result, the GS-Si composite exhibits a reversible capacity of above 1500 mAh g−1 up to 30 cycles and good rate performance. What is far more important is that the effective structure design and simple synthesis route open a new way to massively produce useful graphene-based composite materials.
As shown in Scheme 1, the overall preparation process of the bath lily-like graphene sheet-wrapped nano-Si (GS-Si) composite involves three steps: First, nano-Si powder is sonicated with graphene oxide (GO) in deionized water. Second, the mixture is spray-dried to form graphene oxide-wrapped nano-Si (GO-Si) composite microparticles. Finally, the obtained GO-Si composite is reduced in a stream of hydrogen/argon (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) at 700 °C for 3 h to convert to the bath lily-like GS-Si composite.
80) at 700 °C for 3 h to convert to the bath lily-like GS-Si composite.
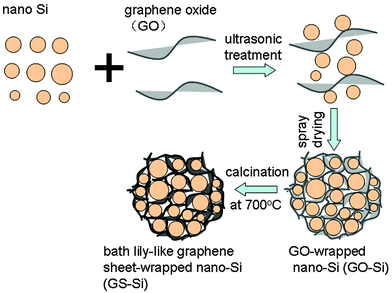 | ||
| Scheme 1 Schematic of the formation process of bath lily-like graphene sheet-wrapped nano-Si (GS-Si) composite. | ||
The XRD pattern in Fig. 1a shows a weak graphite (002) diffraction peak at about 26° for as-synthesized pristine GS. For the GS-Si composite, the major diffraction peaks are consistent with the pure Si and the broad diffraction peak between about 24° and 28° is attributed to GS. Furthermore, the peak of GO at about 11° could not been detected in the GS-Si composite,23d which indicates that GO has been reduced to GS during the thermal treatment. The Raman spectrum of the GS-Si composite in Fig. 1b exhibits a maximal peak at around 516 cm−1, which corresponds to the characteristic peak of pure Si. Another two broad peaks at about 1325 and 1597 cm−1 are respectively associated with the disorder-induced D band and the graphitic G band of the carbon structure,23e which are in accordance with the Raman peaks of pristine GS. The weight fraction of GS in the GS-Si composite was determined to be 19.1% viathermogravimetric analysis (TGA) (Fig. S1 in ESI†).
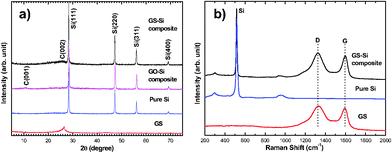 | ||
| Fig. 1 (a) X-Ray diffraction patterns for GS, Si, GO-Si composite, and GS-Si composite. (b) Raman spectra for GS, Si, and GS-Si composite. | ||
Field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) images clearly display the composite morphology (Fig. 2). The GO-Si composite obtained by spray drying consists of numerous GO-wrapped Si microparticles with the particle size ranging from 1 μm to 5 μm (Fig. S2 in ESI†). The special wrapping structure benefited from the stacking and folding of GO during the spray drying procedure. However, the inner structure of the particles can not be clearly observed due to the crinkled but close stacking of GO. After further thermal treatment, the GO-Si composite is reduced to a GS-Si composite (Fig. 2a–c). The original particle size of the GO-Si composite is retained and no more agglomeration is detected. In comparison with the GO-Si composite, the GS-Si composite presents a typical crinkled and rippled morphology of GS and the particle structure becomes more open, similar to a bath lily. Moreover, it is clearly observed from Fig. 2b that the wrapped Si nanoparticles are evenly dispersed in the composite particle. A deep insight into the GS-Si composite microstructure is actualized by TEM analysis. As shown in Fig. 2d, almost all the Si nanoparticles are encapsulated by fish net-like GS. The contents of oxygen in the obtained GS and GS-Si composite were estimated to be about 11.2 wt% and 3.5 wt%, respectively, based on the semi-quantitative energy dispersive X-ray spectroscopy (EDS) analysis. For comparison, a GS/Si mixture was also prepared by mixing nano-Si powder and pristine GS in an agate mortar with the same GS/Si ratio (19.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80.9). In the GS/Si mixture, however, the nano-Si particles demonstrate serious agglomeration and the contact between GS and Si is relatively loose (Fig. S3 in ESI†).
80.9). In the GS/Si mixture, however, the nano-Si particles demonstrate serious agglomeration and the contact between GS and Si is relatively loose (Fig. S3 in ESI†).
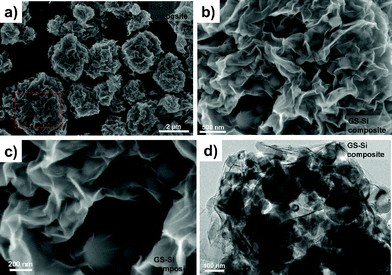 | ||
| Fig. 2 FESEM images of (a, b, c) GS-Si composite and TEM image of (d) GS-Si composite. | ||
The electrochemical performance of the prepared GS-Si composite and GS/Si mixture was evaluated and compared. The initial discharge (Li insertion) and charge (Li extraction) capacities of the GS-Si composite electrode are about 2174 and 1525 mAh g−1, with a Coulombic efficiency of 70% (Fig. S4 in ESI†). The Coulombic efficiency rises to more than 96% after 2 cycles (Fig. 3a). The charge capacity increases slightly for the initial 12 cycles, which should be related to the gradual activation of Si host. After 30 cycles, the electrode can still deliver a reversible capacity of more than 1500 mAh g−1. By contrast, the GS/Si mixture shows an initial charge capacity of only 1187 mAh g−1 and rapid capacity decay during cycling. The high reversible capacity and superior cycling performance of theGS-Si composite could be attributed to the special bath lily-like structure and the synergistic effect between the GS and the nano-silicon particles, where the crinkled structure and excellent flexibility of GS can not only provide enough void spaces to accommodate the volume change of Si particles, but also prevent the aggregation of nano-Si particles during the charging–discharging processes. In addition, highly conductive GS-wrapped active silicon can maintain a stable electrical contact during cycling. All these factors lead to the greatly improved electrochemical behavior.
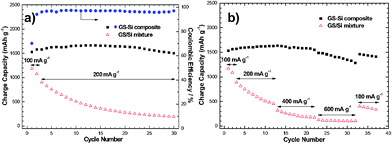 | ||
| Fig. 3 (a) Cycling performance of GS-Si composite and GS/Si mixture, and (b) rate performance of GS-Si composite and GS/Si mixture at different current densities. | ||
The electrode kinetics of both the materials is further evaluated by using different current densities. When the current density increases from 100 to 400 mA g−1, the available capacity keeps a stable value of ca.1550 mAh g−1 for the GS-Si composite (Fig. 3b). The GS-Si composite could still deliver a reversible capacity of around 1300 mAh g−1 even at a high current density of 600 mA g−1. When the current density turns back to 100 mA g−1, the capacity is recovered to 1460 mAh g−1. Nevertheless, the GS/Si mixture electrode exhibits a noticeably worse situation with the enhanced current densities.
AC electrochemical impedance spectra of the cells with GS-Si and GS/Si electrodes were taken to understand their interfacial properties. Both the cells exhibit Nyquist plots consisting of a depressed semicircle in the high–medium frequency region (Fig. 4), which is related to the charge-transfer resistance on the electrode interface.24 The much smaller semicircle of the GS-Si based cell indicates a lower electrochemical reaction resistance on the GS-Si composite electrode. This can be explained by the reliable electronic connection of the active Si particles via powerful encapsulation and adherence of highly conductive GS as shown in Fig. 2.
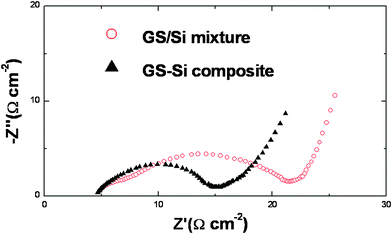 | ||
| Fig. 4 Nyquist plots of the cells with GS-Si and GS/Si electrodes at open circuit voltage (ca. 0.9 V) after two cycles. | ||
In summary, a novel bath lily-like graphene sheet-wrapped nano-Si composite was successfully synthesized by a simple spray drying route. The composite based on a commercial Si product exhibits a high reversible capacity of 1525 mAh g−1 and superior cycling stability, which could be attributed to the special open nano/micro-structure, where the advantage of a synergistic effect between highly conductive GS and active nanoparticles is fully taken. This simple but effective nano/micro-assembly technology could be applicable for the large-scale production of various graphene-based composite materials with high performance for electrochemical energy storage and conversion.
We are grateful for the financial support of this work by the National Basic Research Program of China (2007CB209700), the Natural Science Foundation of China (21006063, 21073120) and the Science and Technology Commission of Shanghai Municipality (10DZ1202702). Dr Chen would like to thank Australian DEST Inter. Linkage Aust.-China program and ARC Discovery Grant DP0877348 for continuous financial support.
References
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS.
- H. Shi, J. Barker, M. Y. Saidi, R. Koksbang and L. Morris, J. Power Sources, 1997, 68, 291 CrossRef CAS.
- B. A. Boukamp, G. C. Lesh and R. A. Huggins, J. Electrochem. Soc., 1981, 128, 725 CrossRef CAS.
- J. Yang, B. F. Wang, K. Wang, J. Y. Xie and Z. S. Wen, Electrochem. Solid-State Lett., 1999, 6, A154 CrossRef.
- C. K. Chan, H. L. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31 CrossRef CAS.
- H. Ma, F. Y. Cheng, J. Chen, J. Z. Zhao, C. S. Li, Z. L. Tao and J. Liang, Adv. Mater., 2007, 19, 4067 CrossRef CAS.
- M.-H. Park, M. G. Kim, J. Joo, K. Kim, J. Kim, S. Ahn, Y. Cui and J. Cho, Nano Lett., 2009, 9, 3844 CrossRef CAS.
- (a) P. Gao, J. Fu, J. Yang, R. Lv, J. Wang, Y. Nuli and X. Tang, Phys. Chem. Chem. Phys., 2009, 11, 11101 RSC; (b) H. Kim, B. Han, J. Choo and J. Cho, Angew. Chem., Int. Ed., 2008, 47, 10151 CrossRef CAS; (c) A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, Nat. Mater., 2010, 9, 353 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS.
- E. Yoo, J. Kim, E. Hosono, H. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S.V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197 CrossRef CAS.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457 CrossRef CAS.
- A. A. Balandin, S. Ghosh, W. Z. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902 CrossRef CAS.
- A. Peigney, C. Laurent, E. Flahaut, R. R. Bacsa and A. Rousset, Carbon, 2001, 39, 507 CrossRef CAS.
- (a) J. S. Chen, Z. Wang, X. C. Dong, P. Chen and X. W. Lou, Nanoscale, 2011, 3, 2158 RSC; (b) D. Wang, D. Choi, J. Li, Z. Yang, Z. Nie, R. Kou, D. Hu, C. Wang, L. V. Saraf, J. Zhang, I. A. Aksay and J. Liu, ACS Nano, 2009, 3, 907 CrossRef CAS.
- S. Yang, X. Feng, S. Ivanovici and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 8408 CrossRef CAS.
- S.-M. Paek, E. Yoo and I. Honma, Nano Lett., 2009, 9, 72 CrossRef CAS.
- Y.-S. He, D.-W. Bai, X. Yang, J. Chen, X.-Z. Liao and Z.-F. Ma, Electrochem. Commun., 2010, 12, 570 CrossRef CAS.
- H. Wang, L.-F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, Y. Liang, Y. Cui and H. Dai, J. Am. Chem. Soc., 2010, 132, 13978 CrossRef CAS.
- L. Shen, C. Yuan, H. Luo, X. Zhang, S. Yang and X. Lu, Nanoscale, 2011, 3, 572 RSC.
- G. Zhou, D.-W. Wang, F. Li, L. Zhang, N. Li, Z.-S. Wu, L. Wen, G. Q. Lu and H.-M. Cheng, Chem. Mater., 2010, 22, 5306 CrossRef CAS.
- X. Zhou, F. Wang, Y. Zhu and Z. Liu, J. Mater. Chem., 2011, 21, 3353 RSC.
- (a) S.-L. Chou, J.-Z. Wang, M. Choucair, H. K. Liu, J. A. Stride and S.-X. Dou, Electrochem. Commun., 2010, 12, 303 CrossRef CAS; (b) H. Xiang, K. Zhang, G. Ji, J. Y. Lee, C. Zou, X. Chen and J. Wu, Carbon, 2011, 49, 1787 CrossRef CAS; (c) J. K. Lee, K. B. Smith, C. M. Hayner and H. H. Kung, Chem. Commun., 2010, 46, 2025 RSC; (d) J.-Z. Wang, C. Zhong, S.-L. Chou and H.-K. Liu, Electrochem. Commun., 2010, 12, 1467 CrossRef CAS; (e) K. Evanoff, A. Magasinski, J. Yang and G. Yushin, Adv. Energy Mater. DOI:10.1002/aenm.201100071.
- R. Ruffo, S. S. Hong, C. K. Chan, R. A. Huggins and Y. Cui, J. Phys. Chem. C, 2009, 113, 11390 CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details and supplementary figures. See DOI: 10.1039/c1ra00429h/ |
| This journal is © The Royal Society of Chemistry 2011 |
