Dynamic insights into formation of honeycomb structures induced by breath figures†
Hongmin
Ma
ab,
Li
Kong
a,
Xiaohui
Guo
a and
Jingcheng
Hao
*a
aKey Laboratory of Colloid and Interface Chemistry of Ministry of Education, Shandong University, Jinan, 250100, P.R. China. E-mail: jhao@sdu.edu.cn; Fax: +86-531-88564750
bSchool of Chemistry and Chemical Engineering, University of Jinan, Jinan, 250022, China
First published on 20th September 2011
Abstract
We propose a simple approach to probe the formation dynamics of honeycomb structures induced by breath figures. A honeycomb film with different domains was obtained by interrupting the condensation of breath figures. The morphologies of different domains indicate that the condensed water droplets grow during their arrangement to ordered arrays.
Formation of ordered patterns through the condensation of water vapor on cold surfaces is a well recognized phenomenon, which has long been termed as “breath figures”.1 Besides solid substrates, breath figure patterns may also form on liquid surfaces,2 a special case of which is on the surface of a volatile droplet. In 1994, Widawski and co-workers first reported that breath figures could be used as a dynamic template to induce the formation of ordered honeycomb structures by casting a volatile droplet of polymer solution in humid conditions.3 This simple but effective method for honeycomb structures was broadly applied to a variety of materials, including polymers,4 monolayer-stabilized nanoparticles,5surfactant-encapsulated clusters,6polymer nanocomposites,7 organogelators,8 and organic/inorganic hybrids.9 The influence factors such as molecular structure,10 molecular weight,11 concentration,12solvent,13 relative humidity,14 and substrates15 were widely investigated to tune the morphology of honeycomb structures. The ordered honeycomb-like macroporous structures have shown promising properties with potential applications as templating masks,16 superhydrophobic coatings,17 tissue engineering scaffolds,18 photocatalytic materials,19etc. However, the formation mechanism of honeycomb structures by the breath figure method is still not well understood.
Several attempts have been made to investigate the dynamics of breath figure formation on liquid surfaces.20,21 Unfortunately, it is difficult to directly observe the formation process of breath figures on a volatile droplet using an optical microscope,22 because the evaporation process is very fast (within ten seconds) and the evaporation induced surface currents render it impossible to observe the surface at high magnification.23 The morphology of the honeycomb structures formed at different stages of the evaporation process may provide clues to the dynamics of breath figure formation, and thus to elucidate the formation mechanism of honeycomb structures. Recently, we have reported that highly ordered honeycomb-structured polymer nanocomposite films could be prepared by the breath figure method on both solid substrates24 and an air/water interface.25 In this work, a polymer nanocomposite film with different domains of a honeycomb structure was obtained by a very simple approach, that is, by interrupting the condensation of water droplets. The morphologies of different domains were examined to probe the formation dynamics of honeycomb structures.
Polymer nanocomposites of polystyrene (PS) and gold nanoparticles (AuNPs) were used as building blocks to prepare the honeycomb-patterned films by the breath figure method. After a nanocomposite solution is dropped on the glass substrate, the rapid evaporation of the solvent decreases the air/liquid interfacial temperature below the dew point of moist air, resulting in the condensation of micron-sized water droplets on the surface of solution. The monodisperse water droplets self-assemble into ordered hexagonal close-packed arrays and the interfacial adsorption of AuNPs prevents the coalescence of the water droplets.24 When the solvent and water droplets evaporate completely, highly ordered honeycomb structures with hexagonal arrays of pores are formed in the dried film (Fig. S1, ESI†). As the high relative humidity condition—which is indispensable for breath figure formation—is provided by a moist nitrogen gas flow, it is feasible to interrupt the condensation of water droplets during the evaporation process by removing the gas flow.
Again, because the evaporation process is very fast, it is not possible to systematically investigate the different stages of the evaporation process. However, an unfinished honeycomb structure was obtained by removing the gas flow before the solvent evaporated completely. From the optical microscope images (Fig. S2, ESI†), it can be seen that a continuous region of the honeycomb structures is formed along the margin of the film while isolated island-like regions of the honeycomb structures are located inside the continuous region. The small islands become larger and more continuous when approaching the continuous region. Moreover, no porous structures are observed at the central region of the film.
It has been shown that small islands of breath figures result from aggregation of the water droplets and rearrangements of these floating rafts gives rise to large areas of water droplet arrays.23 Maruyama and co-workers proposed that hexagonal water droplet arrays formed at the solution front with the shrinking of the three-phase contact line.26 Thus, in our case we easily attributed the formation of the continuous region to the evaporation process under the gas flow and equally, the inner regions to the evaporation process without the gas flow. The distribution of the honeycomb regions provides direct evidence that the condensed water droplets are carried to the solution front and indicates that the gas flow promotes the rearrangements of small islands to form continuous water droplet arrays.
Besides the macroscopic regions, notable domains with different morphologies of the honeycomb structures (SEM images of the domain boundaries are shown in Fig. S3, ESI†) are observed at different positions along the radius of the film (as indicated in Fig. 1a). A random distribution of small pores was observed from domain I (Fig. 1b). In domain II, the pores are more ordered and hexagonal arrays are formed (Fig. 1c). The pore diameter of these two domains is too small and cannot be determined exactly by SEM (Fig. 1d). AFM measurements show that the opening diameter of these pores is about 100 nm (Fig. S4, ESI†). The pore size becomes much larger and regular arrays are formed in domains III–VI (Fig. 1e–i). Interestingly, the pores in domain III have irregular cavities (Fig. 1f), which gradually become hexagonal (Fig. S5, ESI†) and then more rounded (Fig. 1g). The shape variations of the pore cavities should be related to the growing process of the water droplet templates.
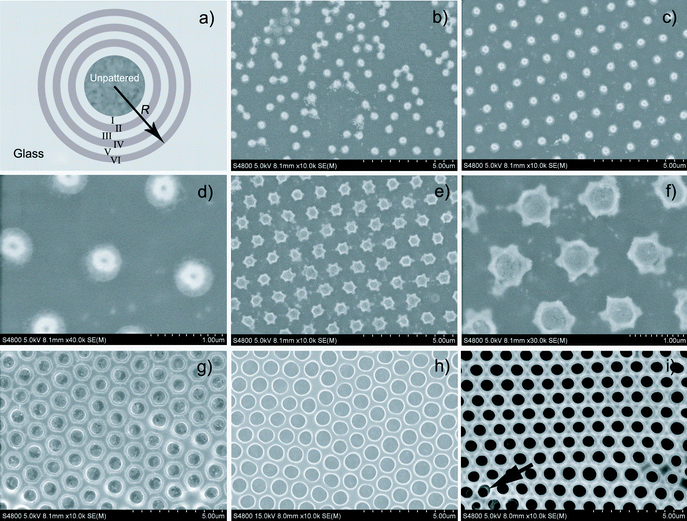 | ||
| Fig. 1 Schematic illustration of different domains formed on the cast film (a). SEM images of domain I (b), domain II (c and d), domain III (e and f), domain IV (g), domain V (h), and domain VI (i). | ||
Ordered honeycomb structures in domains II–VI have a relatively narrow size distribution of pores. The average pore diameters and separate distances between two adjacent pores are shown in Fig. 2. The pore size gradually increases and the separate distance gradually decreases with the migration from domain II to domain V. Compared with domain V, a smaller pore diameter and almost the same separation distance are observed in domain VI.
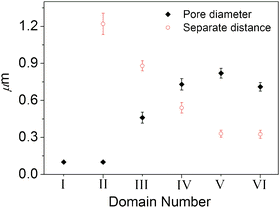 | ||
| Fig. 2 Plot of average pore diameter and separate distance against the film domains | ||
Light scattering experiments have shown that monodisperse water droplets result from fast nucleation and the subsequent slow growth of the water droplets.22 It is also shown that the coalescence of water droplets is prevented during the condensation process when the water droplets are effectively stabilized.20 In our previous work, we have given evidence that water droplets condensed on a nanocomposite solution could be stabilized by microphase separation and the interfacial adsorption of AuNPs.24 Accordingly, in the present case, the coalescence of water droplets is excluded and the variation in pore size is caused by growth of water droplets and the growth of water droplets is interrupted after the moist gas flow is removed. So the pore size in different domains should indicate the size of the water droplets in the evaporation process.
Thus, we can conclude that the water droplets grow during their arrangement to ordered arrays even if they are stabilized by AuNPs. The larger pore size of domain V compared to domain VI is probably due to the time lag in the formation of the honeycomb structures,27 that is, water droplets in domain V have more time to grow. Ordered honeycomb structures with different pore sizes in different domains indicate that monodisperse water droplets with different sizes may form hexagonal arrays, which undoubtedly enables us to obtain honeycomb structures with different morphologies by controlling the evaporation conditions.
We have also reported that three-dimensional double-layered honeycomb structures could be prepared using the breath figure method.24 In contrast, double-layered honeycomb structures only formed in the edge area, and mono-layered honeycomb structures were formed in the inner regions of the prepared film (Fig. S6, ESI†). The bottom layer of pores could also be observed from the surface of the film in domain VI (as the arrow indicated in Fig. 1i). This difference could be explained by the removal of gas flow in the evaporation process. It is proposed that convection effects could drag the water droplets into the solution to form ordered arrays at the solution front.26 The presence of the gas flow can speed up the evaporation of the solvent, and then a larger temperature gradient is achieved between the solution surface and substrate, as a result of which Marangoni convection is promoted.28 When the gas flow is removed, the convection effect is suppressed and only a mono-layered honeycomb structure is formed. The presence of gas flow should facilitate the formation of three-dimensional honeycomb structures.
In summary, we proposed a very simple approach to probe into the formation dynamics of honeycomb structures induced by breath figures. The condensed water droplets grow during their arrangement to ordered arrays and hexagonal arrays may be formed with monodisperse water droplets of different sizes. The presence of gas flow above the surface of solution promotes the formation of large areas of water droplet arrays and the formation of three-dimensional honeycomb structures. Understanding the formation mechanisms of honeycomb structures will boost the application of the breath figure method in preparing promising functional materials.
This work was financially supported by the NSFC (Grant No. 21033005) and the National Basic Research Program of China (973 Program, 2009CB930103), and NSF of Shandong Province (Z2008B01).
References
- (a) C. M. Knobler and D. Beysens, Europhys. Lett., 1988, 6, 707–712 CrossRef; (b) B. J. Briscoe and K. P. Galvin, J. Phys. D: Appl. Phys., 1990, 23, 1265–1266 CrossRef.
- (a) A. Steyer, P. Guenoun, D. Beysens and C. M. Knobler, Phys. Rev. B: Condens. Matter, 1990, 42, 1086–1089 CrossRef; (b) A. A. Nepomnyashchy, A. A. Golovin, A. E. Tikhomirova and V. A. Volpert, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 74, 021605 CrossRef CAS.
- G. Widawski, M. Rawiso and B. Francois, Nature, 1994, 369, 387–389 CrossRef CAS.
- (a) U. H. F. Bunz, Adv. Mater., 2006, 18, 973–989 CrossRef CAS; (b) M. H. Stenzel, C. Barner-Kowollik and T. P. Davis, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2363–2375 CrossRef CAS.
- (a) A. E. Saunders, P. S. Shah, M. B. Sigman Jr, T. Hanrath, H. S. Hwang, K. T. Lim, K. P. Johnston and B. A. Korgel, Nano Lett., 2004, 4, 1943–1948 CrossRef CAS; (b) H. Ma and J. Hao, Chem.–Eur. J., 2010, 16, 655–660 CrossRef CAS.
- (a) H. Sun, H. Li, W. Bu, M. Xu and L. Wu, J. Phys. Chem. B, 2006, 110, 24847–24854 CrossRef CAS; (b) P. Tang and J. Hao, Adv. Colloid Interface Sci., 2010, 161, 163–170 CrossRef CAS.
- (a) A. Böker, Y. Lin, K. Chiapperini, R. Horowitz, M. Thompson, V. Carreon, T. Xu, C. Abetz, H. Skaff and A. D. Dinsmore, Nat. Mater., 2004, 3, 302–306 CrossRef; (b) M. H. Nurmawati, P. K. Ajikumar, R. Renu and S. Valiyaveettil, Adv. Funct. Mater., 2008, 18, 3213–3218 CrossRef CAS.
- (a) J. H. Kim, M. Seo and S. Y. Kim, Adv. Mater., 2009, 21, 4130–4133 CrossRef CAS; (b) S. S. Babu, S. Mahesh, K. K. Kartha and A. Ajayaghosh, Chem.–Asian J., 2009, 4, 824–829 CrossRef CAS.
- (a) O. Karthaus, X. Cieren, N. Maruyama and M. Shimomura, Mater. Sci. Eng., C, 1999, 10, 103–106 CrossRef; (b) K. Zhang, L. Zhang and Y. Chen, Macromol. Rapid Commun., 2007, 28, 2024–2028 CrossRef CAS.
- (a) M. H. Stenzel-Rosenbaum, T. P. Davis, A. G. Fane and V. Chen, Angew. Chem., Int. Ed., 2001, 40, 3428–3432 CrossRef CAS; (b) L. A. Connal, R. Vestberg, C. J. Hawker and G. G. Qiao, Adv. Funct. Mater., 2008, 18, 3706–3714 CrossRef CAS.
- (a) J. Peng, Y. Han, Y. Yang and B. Li, Polymer, 2004, 45, 447–452 CrossRef CAS; (b) A. E. Saunders, J. L. Dickson, P. S. Shah, M. Y. Lee, K. T. Lim, K. P. Johnston and B. A. Korgel, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 73, 031608 CrossRef.
- (a) M. H. Stenzel, Aust. J. Chem., 2002, 55, 239–243 CrossRef CAS; (b) Y. Xu, B. Zhu and Y. Xu, Polymer, 2005, 46, 713–717 CrossRef CAS.
- (a) E. Ferrari, P. Fabbri and F. Pilati, Langmuir, 2011, 27, 1874–1881 CrossRef CAS; (b) Y. Tian, H. Ding, Q. Jiao and Y. Shi, Macromol. Chem. Phys., 2006, 207, 545–553 CrossRef CAS.
- (a) W. Liu, R. Liu, Y. Li, W. Wang, L. Ma, M. Wu and Y. Huang, Polymer, 2009, 50, 2716–2726 CrossRef CAS; (b) X. Cheng, Y. Tian, Y. Q. Shi, R. P. Tang and F. Xi, Langmuir, 2005, 21, 6576–6581 CrossRef.
- (a) C. Wang, Y. Mao, D. Wang, Q. Qu, G. Yang and X. Hu, J. Mater. Chem., 2008, 18, 683–690 RSC; (b) L. Ghannam, M. Manguian, J. François and L. Billon, Soft Matter, 2007, 3, 1492–1499 RSC.
- (a) H. Yabu and M. Shimomura, Langmuir, 2005, 21, 1709–1711 CrossRef CAS; (b) B. de Boer, U. Stalmach, H. Nijland and G. Hadziioannou, Adv. Mater., 2000, 12, 1581–1583 CrossRef CAS.
- (a) H. Yabu and M. Shimomura, Chem. Mater., 2005, 17, 5231–5234 CrossRef CAS; (b) H. Yabu, M. Takebayashi, M. Tanaka and M. Shimomura, Langmuir, 2005, 21, 3235–3237 CrossRef CAS.
- (a) J. Nishida, K. Nishikawa, S. I. Nishimura, S. Wada, T. Karino, T. Nishikawa, K. Ijiro and M. Shimomura, Polym. J., 2002, 34, 166–174 CrossRef CAS; (b) L. Li, C. Chen, J. Li, A. Zhang, X. Liu, B. Xu, S. Gao, G. Jin and Z. Ma, J. Mater. Chem., 2009, 19, 2789–2796 RSC.
- (a) H. Zhao, Y. Shen, S. Zhang and H. Zhang, Langmuir, 2009, 25, 11032–11037 CrossRef CAS; (b) K. Kon, C. N. Brauer, K. Hidaka, H.-G. Löhmannsröben and O. Karthaus, Langmuir, 2010, 26, 12173–12176 CrossRef CAS.
- O. Pitois and B. Francois, Colloid Polym. Sci., 1999, 277, 574–578 CAS.
- A. V. Limaye, R. D. Narhe, A. M. Dhote and S. B. Ogale, Phys. Rev. Lett., 1996, 76, 3762–3765 CrossRef CAS.
- O. Karthaus, N. Maruyama, X. Cieren, M. Shimomura, H. Hasegawa and T. Hashimoto, Langmuir, 2000, 16, 6071–6076 CrossRef CAS.
- O. Pitois and B. Francois, Eur. Phys. J. B, 1999, 8, 225–231 CrossRef CAS.
- H. Ma, J. Cui, J. Chen and J. Hao, Chem.–Eur. J., 2011, 17, 655–660 CrossRef CAS.
- H. Ma, J. Cui, A. Song and J. Hao, Chem. Commun., 2011, 47, 1154–1156 RSC.
- N. Maruyama, T. Koito, J. Nishida, T. Sawadaishi, X. Cieren, K. Ijiro, O. Karthaus and M. Shimomura, Thin Solid Films, 1998, 327–329, 854–856 CrossRef CAS.
- T. Nishikawa, J. Nishida, R. Ookura, S. I. Nishimura, S. Wada, T. Karino and M. Shimomura, Mater. Sci. Eng., C, 1999, 10, 141–146 CrossRef.
- H. Ma, R. Dong, J. D. Van Horn and J. Hao, Chem. Commun., 2011, 47, 2047–2049 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Optical micrographs and SEM images of the patterned polymer nanocomposite film. See DOI: 10.1039/c1ra00367d |
| This journal is © The Royal Society of Chemistry 2011 |
