Altering the polarity of self-assembled carbon nanotubes stationary phasevia covalent functionalization
Chaudhery Mustansar
Hussain
a,
Chutarat
Saridara
ab and
Somenath
Mitra
*a
aDepartment of Chemistry and Environmental Science, New Jersey Institute of Technology, Newark, NJ 07102, USA. E-mail: mitra@njit.edu; Fax: (+01) 973-596-3586; Tel: (+01) 973-596-5611
bDepartment of Chemistry, Faculty of Science and Technology, Rajamangala University of Technology, Thanyaburi, Thailand
First published on 25th August 2011
Abstract
We present for the first time that self assembled carbon nanotubes (CNTs) can be functionalized to alter their polarity and chromatographic behavior. The nanotube phase was synthesized viaethanol chemical vapor deposition (CVD) and functionalized by acid oxidation. Compared to an equivalent CNT column, the functionalized nanotubes (f-CNTs) showed strong retention and enhanced separation for polar organics such as alcohols, where the capacity factor increased by more than 100%, and the number of plates per metre increased by as much as 60%. The f-CNTs phase showed classical chromatographic behavior and good reproducibility. This is an important first step toward the development of diverse functionalized CNT columns.
1. Introduction
Carbon nanotubes (CNTs) have some extraordinary sorption properties which make them excellent sorbents for a variety of analytical applications.1–3 Studies have shown that CNTs are highly effective gas and aqueous phase sorbents and can trap a wide range of molecules from H2, and methane to large semi-volatiles.4–8 Therefore, they demonstrate tremendous versatility as far as analytical sorbents are concerned. Several reviews have been published on their applications in solid-phase extraction (SPE), solid-phase microextraction (SPME), μ-SPE and microconcentrators.9–12CNTs have been used as chromatography stationary phases. Their high capacity, π–π stacking interactions with aromatic and unsaturated compounds, combined with high thermal stability, make them attractive as gas solid chromatography (GSC) stationary phases.13–20 The superior sorption properties of CNTs have also been utilized in liquid chromatography and capillary electrophoresis to provide better resolution and enhanced signal-to-noise ratio.21–23
A major advantage of CNTs when applied to chromatographic separation is that they can be self-assembled on the walls of a tube viachemical vapor deposition leading to the formation of an open tubular column, which is associated with larger number of plates per metre.16,17,20,24 Both single and multi-walled carbon nanotubes have been self-assembled on the tube surface and different precursors have been used providing different advantages.16,17 While CNT stationary phases have shown excellent performance, they are non polar in nature and it is anticipated that the alternation of their polarity would greatly enhance their performance.
Functionalization of CNTs has been effectively used to alter dispersibility in different solvents, their compatibility with other materials, such as polymers, and even for developing substrates for cell growth.25–29 Functionalization of CNTs also offers the unique opportunity of tailor making sorbent properties and has been used in air sampling30 and in packed GC columns.31 The objective of this paper is to study the enhancement of polar compound retentionvia appropriate covalent functionalization of multi-walled carbon nanotubes (MWNTs) in a GC column fabricated viachemical vapor deposition self-assembly of CNTs.
2. Experimental
All chemicals were of analytical grade and purchased from Sigma Aldrich Inc (St. Louis, MO, USA) and were used without further purification. All gases were zero grade, and obtained from Matheson Tri-Gas (Montgomeryville, PA, USA). Silico Steel 304 (Alltech, Deerfield, IL) metal capillary tubings (Restek, Bellefonte, PA, USA) were used for the self-assembly of CNTs.The CVD system was used for the fabrication of GC columns and has been described before24 and is shown in Fig. 1. Ethanol was used as the carbon source. The GC columns were fabricated on 1–2 m long, 0.53 mm i.d. 304 stainless steel tubing, which was coiled and placed inside the tube furnace. The tubes were washed with acetone to remove any particles/impurities, dried, and the steel surface was prepared at 550° C with air flow of 10 mL min−1 for 30 min. Typical CVD time was between 15–60 min at 700 °C under the argon flow rate of 10 mL min−1. Check valves (R. S. Crum & Co., Mountainside, NJ, USA) were placed on both lines to restrict backflow. Experiments were carried out in a fume hood and necessary safety precautions were taken as ethanol was used at high temperature. After the CNT deposition, the columns were heated at 200 °C in air for 1 h to oxidize amorphous carbon and other impurities generated during the process. Later, the column was heated in argon at 425 °C for 1 h to anneal and remove any low-boiling impurities.
 | ||
| Fig. 1 Schematic diagram of CVD system. | ||
2.1. Functionalization of the self-assembled CNT-stationary phase
After oxidative purification of the CNT-column, functionalization of the CNT-stationary phase was carried out as follows. The CNT-column was filled with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture of relatively dilute HNO3 and H2SO4 and the column was placed in beaker containing same acids, so that it was totally submerged in the acids. This beaker was heated for 10–15 min to 150 °C. The column was removed, washed with water and placed in a flow of Ar and annealed at 250 °C for 30 min. In order to ensure that the CNT coating was uniform, 1 cm-long segments were cut at different equidistant locations and analyzed using a Leo 1530 VP (Carl Zeiss SMT AG Company, Oberkochen, Germany) field emission scanning electron microscope.
50 mixture of relatively dilute HNO3 and H2SO4 and the column was placed in beaker containing same acids, so that it was totally submerged in the acids. This beaker was heated for 10–15 min to 150 °C. The column was removed, washed with water and placed in a flow of Ar and annealed at 250 °C for 30 min. In order to ensure that the CNT coating was uniform, 1 cm-long segments were cut at different equidistant locations and analyzed using a Leo 1530 VP (Carl Zeiss SMT AG Company, Oberkochen, Germany) field emission scanning electron microscope.
2.2. Gas chromatography
A gas chromatograph (model Varian 3400, Varian Inc. Palo Alto, CA, USA) equipped with a conventional flame ionization (FID) was used in this study. A Perkin Elmer Laboratory Computing Integrator Model LCI-100 (Waltham, MA, USA) was used for data acquisition. Injections were made using a 10 μL Hamilton model 701 microlitre syringe (Hamilton Co., Reno, NV, USA) through a split-splitless injection port. Injector and detector temperatures were set at 250 °C, and a typical injection volume was 1.0 μL unless otherwise specified. All injections were performed in the split mode with the split ratios varying between 1 to 20. N2 was used as a carrier gas with a flow rate of 3 mL min−1. The FID gases comprised of air flow at 300 mL min−1 and hydrogen at 30 mL min−1. All GC measurements were repeated at least three times.3. Results and discussion
Mechanism of CNT growthviaethanol CVD has been described before and is not discussed here for brevity.24 The steel tubing was made catalytically active by air oxidation. SEM images of steel tubing surface before and after air oxidation are shown in Fig. 2 (a, b) respectively. Similarly, SEM images of CNTs after ethanol CVD and functionalization carbon nanotubes (f-CNT) are shown in Fig. 2 (c, d) respectively. Multiple GC columns were made and analyzed to check the reproducibility of the process. | ||
| Fig. 2 SEM images (a) original steel tubing surface, (b) surface after air oxidation at 550 °C, (c) CNT-coating after ethanol CVD, (d) CNT-coating after functionalization | ||
To incorporate functional groups into the CNT stationary phase uniformly in the capillary column, it was necessary to evenly introduction of acidic solution to CNTs. Several acid oxidative methods have been developed to functionalize the CNTs to generate carboxylic groups on the surface; these include sonication and microwave treatment.32–38 However, functionalization of self-assembled CNTs on a steel surface is complicated by the fact that the metal leaches out of the steel tubing. SEM images of f-CNT-coatings in Fig. 2 d reveal that indeed some of the components were leached from the metal and some discrete nano scale particles are deposited on the CNTs’ surface. However, the CNT is not completely coated and the surface is available for sorption of analytes. Other images at different spots along the column are not presented here for brevity, but they showed similar morphology.
3.1. Chromatography on f-CNT
The influence of functionalization on the separation efficiency of the f-CNT column was evaluated with selected polar (alcohol) and non polar (aromatic) organic compounds, whose boiling points ranged from 64.7 to 117 °C (Table 1). Chromatograms on as prepared and functionalized columns are presented in Fig. 3 (a, b). Although both columns were able to separate polar analytes, under the same chromatographic conditions, the retention time on the f-CNT column was significantly higher (nearly twice), therefore it was clear that these analytes were more strongly retained. On the other hand, when the nonpolar compounds namely benzene, toluene and xylene were injected into both columns, the separation was excellent on the CNT column (Fig. 4a) and in line with previous reports,16,24 but the f-CNT column showed no separation (Fig. 4b). This implied that the CNT surface had a high density of carboxylic groups, and the polarity was quite high. | ||
| Fig. 3 Chromatographic separations of methanol, (1), ethanol, (2), propanol, (3) and 1-butanol (4). Temperature program was as follows, 50 °C for 0.5 min, ramp at 20 °C min−1 to 100 °C at a carrier gas flow rate of 2.5 mL min−1 and injection volume was 5 μL. (a) CNT-coating column (b) f-CNT-coating column. | ||
 | ||
| Fig. 4 (a) Chromatogram of benzene (1), toluene (2), and m-xylene (3) on: (a) CNT column, (b) chromatogram on f-CNT-column. The temperature program was as follows, 100 °C for 1 min, ramp at 10 °C min−1 to 160 °C at a carrier gas flow rate of 3.0 mL min−1 and injection volume was 5 μL. | ||
| Compounds | Boiling point (°C) | k | N | |||
|---|---|---|---|---|---|---|
| CNT-Column | f-CNT-Column | CNT-Column | f-CNT-Column | |||
| Polar | Methanol | 64.7 | 1.02 | 1.98 | 1213 | 1820 |
| Ethanol | 78 | 2.05 | 3.76 | 1280 | 1920 | |
| Propanol | 97 | 2.62 | 5.89 | 1122 | 1684 | |
| 1-Butanol | 117 | 3.21 | 7.12 | 1116 | 1674 | |
| Non-polar | Benzene | 80 | 1.59 | — | 1133 | — |
| Toluene | 111 | 2.59 | — | 966 | — | |
| Xylene | 139 | 5.02 | — | 1125 | — | |
Comparison between chromatographic parameters of the pristine and carboxylated CNT for selected compounds are as listed in Table 1. These were obtained under isothermal conditions and at an optimum flow rate of 3 mL min−1. The number of theoretical plates (N) per metre for the polar compounds varied between 1117 to 1280 on CNT column, whereas the range on f-CNT was between 1675 to 1920. This was the equivalent of 50% increase in the number of plates per metre, and this was attributed to strong interactions of polar analytes with the functionalized surface.
The Van’t Hoff plots of log k as a function of 1/T for ethanol on both columns are shown in Fig. 5. The linear plot (with correlation coefficients of 0.99) on both columns suggests that the separation followed classical chromatographic behavior and well in accordance of previous report.24Fig. 6 shows the Van Deemter plot for the column with ethanol. The minimum height equivalent theoretical plate (HETP) was 0.089 cm for CNT and 0.059 cm for f-CNT column. The reduction of 0.03 cm represents an significant enhancement.
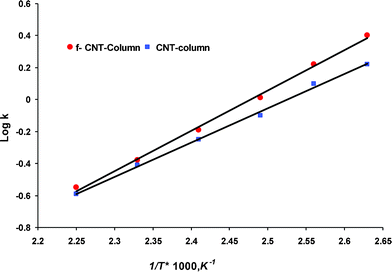 | ||
| Fig. 5 Van’t Hoff plot of variation in capacity factor with temperature for ethanol on (a) CNT and (b) f-CNT column. | ||
 | ||
| Fig. 6 Van Deemter plot for propanol; CNT-column (Hmin: 0.089 cm) and f-CNT-column (Hmin: 0.059 cm). | ||
The reproducibility and stability of the f-CNT-column was also calculated. Table 2, presents the run-to-run (n=5) and day-to-day (n=10) RSD values obtained for the retention times and peak width at half height. The RSD in retention time was less than 1% for polar analytes on the f-CNTs column, which indicated that the stationary phase was quite stable. Three CNT columns were functionalized under the same conditions, and the capacity factors were obtained for selected polar analytes. The low RSD values (0.94–1.04) show that with acidic functionalization process was a consistent and reproducible.
4. Conclusions
In summary, it was possible to functionalize self-assembled CNTs by conventional acid treatment. The functionalization altered separation behavior of the CNTs phase quite dramatically. The increase in capacity factor and plates per metre was quite significant for the polar compounds studied here. The oxidized phase showed excellent structural and functional integrity, number of plates per metre were as high as 1920 and RSDs were less than 1. The functionalized phase was relatively ineffective for nonpolar compounds. The advantage of this approach is that self-assembled CNTs can be altered to change selectivity for different classes of analytes.Acknowledgements
We wish to acknowledge financial support from NCE-EHWM, Chulalongkorn University, Thailand, Department of Chemistry, Faculty of Science & Technology, RMUTT, Thanyaburi, Thailand and the US Department of Energy.References
- M. Valcárcel, S. Cárdenas and B. M. Simonet, Anal. Chem., 2007, 79, 4788–4797 CrossRef.
- G. P. Rao, C. Lu and F. Su, Sep. Purif. Technol., 2007, 58, 224–231 CrossRef CAS.
- D. E. Raynie, Anal. Chem., 2010, 82, 4911–4916 CrossRef CAS.
- M. Valcárcel, S. Cárdenas, B. M. Simonet, Y. Moliner-Martínez and R. Lucena, TrAC, Trends Anal. Chem., 2008, 27, 34–43 CrossRef.
- M. Valcárcel, B. Simonet and S. Cárdenas, Anal. Bioanal. Chem., 2008, 391, 1881–1887 CrossRef.
- C. M. Hussain, C. Saridara and S. Mitra, Analyst, 2008, 133, 1076–1082 RSC.
- C. M. Hussain, C. Saridara and S. Mitra, J. Chromatogr., A, 2008, 1185, 161–166 CrossRef CAS.
- C. Saridara, S. Ragunath, Y. Pu and S. Mitra, Anal. Chim. Acta, 2010, 677, 50–54 CrossRef CAS.
- O. Sae-Khow and S. Mitra, J. Chromatogr., A, 2009, 1216, 2270–2274 CrossRef CAS.
- L. M. Ravelo-Pérez, A. V. Herrera-Herrera, J. Hernández-Borges and M. Á. Rodríguez-Delgado, J. Chromatogr., A, 2010, 1217, 2618–2641 CrossRef.
- F. Augusto, E. Carasek, R. G. C. Silva, S. R. Rivellino, A. D. Batista and E. Martendal, J. Chromatogr., A, 2010, 1217, 2533–2542 CrossRef CAS.
- C. Hussain and S. Mitra, Anal. Bioanal. Chem., 2011, 399, 75–89 CrossRef CAS.
- B. Pan and B. Xing, Environ. Sci. Technol., 2008, 42, 9005–9013 CrossRef CAS.
- C. Lu, H. Bai, B. Wu, F. Su and J. F. Hwang, Energy Fuels, 2008, 22, 3050–3056 CrossRef CAS.
- D. Lin and B. Xing, Environ. Sci. Technol., 2008, 42, 7254–7259 CrossRef CAS.
- C. Saridara and S. Mitra, Anal. Chem., 2005, 77, 7094–7097 CrossRef CAS.
- M. Karwa and S. Mitra, Anal. Chem., 2006, 78, 2064–2070 CrossRef CAS.
- M. Stadermann, A. D. McBrady, B. Dick, V. R. Reid, A. Noy, R. E. Synovec and O. Bakajin, Anal. Chem., 2006, 78, 5639–5644 CrossRef CAS.
- L.-M. Yuan, C.-X. Ren, L. Li, P. Ai, Z.-H. Yan, M. Zi and Z.-Y. Li, Anal. Chem., 2006, 78, 6384–6390 CrossRef CAS.
- M. Karwa, Z. Iqbal and S. Mitra, J. Mater. Chem., 2006, 16, 2890–2895 RSC.
- Y. Li, Y. Chen, R. Xiang, D. Ciuparu, L. D. Pfefferle, C. Horváth and J. A. Wilkins, Anal. Chem., 2005, 77, 1398–1406 CrossRef CAS.
- A. Fonverne, F. Ricoul, C. Demesmay, C. Delattre, A. Fournier, J. Dijon and F. Vinet, Sens. Actuators, B, 2008, 129, 510–517 CrossRef.
- J.-L. Chen, J. Chromatogr., A, 2010, 1217, 715–721 CrossRef CAS.
- C. M. Hussain, C. Saridara and S. Mitra, Anal. Chem., 2010, 82, 5184–5188 CrossRef CAS.
- M. Foldvari and M. Bagonluri, Nanomed.: Nanotechnol., Biol. Med., 2008, 4, 183–200 CrossRef CAS.
- Y. Chen and S. Mitra, in M. Umeno, P. R. Somani, (Editors), Carbon Nanotubes: Synthesis, Properties and Applications, Applied Science Innovations Press, Maharashtra, India, 2009 Search PubMed.
- L. Meng, C. Fu and Q. Lu, Prog. Nat. Sci., 2009, 19, 801–810 CrossRef CAS.
- P.-C. Ma, N. A. Siddiqui, G. Marom and J.-K. Kim, Composites, Part A, 2010, 41, 1345–1367 CrossRef.
- C.N.R. Rao, A. Ghosh, A. Gomathi, in L.A. David, D.S. Gregory, P.W. Gary (Editors), Comprehensive Nanoscience and Technology, Academic Press, Amsterdam, 2011, p. 445 Search PubMed.
- C. M. Hussain, C. Saridara and S. Mitra, Analyst, 2009, 134, 1928–1933 RSC.
- D. Merli, A. Speltini, D. Ravelli, E. Quartarone, L. Costa and A. Profumo, J. Chromatogr., A, 2010, 1217, 7275–7281 CrossRef CAS.
- W. Huang, Y. Lin, S. Taylor, J. Gaillard, A. M. Rao and Y.-P. Sun, Nano Lett., 2002, 2, 231–234 CrossRef CAS.
- Y. Wang, Z. Iqbal and S. Mitra, Carbon, 2005, 43, 1015–1020 CrossRef CAS.
- Y.-L. Hsin, J.-Y. Lai, K. C. Hwang, S.-C. Lo, F.-R. Chen and J. J. Kai, Carbon, 2006, 44, 3328–3335 CrossRef CAS.
- L. Qu, K. M. Lee, L. Dai and D. Liming, in Carbon Nanotechnology, Elsevier, Amsterdam, Editon edn, 2006, pp. pp. 191–234. Search PubMed.
- F. Barroso-Bujans, J. L. G. Fierro, S. Rojas, S. Sánchez-Cortes, M. Arroyo and M. A. López-Manchado, Carbon, 2007, 45, 1669–1678 CrossRef CAS.
- A. G. Osorio, I. C. L. Silveira, V. L. Bueno and C. P. Bergmann, Appl. Surf. Sci., 2008, 255, 2485–2489 CrossRef CAS.
- D. Vairavapandian, P. Vichchulada and M. D. Lay, Anal. Chim. Acta, 2008, 626, 119–129 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
