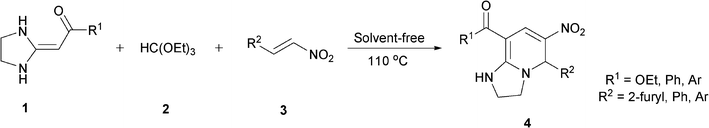
Three-component solvent-free synthesis of highly substituted tetra-hydroimidazo[1,2-a]pyridines†
Fuchao
Yu‡
,
Shengjiao
Yan‡
,
Rong
Huang
,
Yajuan
Tang
and
Jun
Lin
*
Key Laboratory of Medicinal Chemistry for Natural Resource (Yunnan University), Ministry Education, School of Chemical Science and Technology, Yunnan University, Kunming, 650091, P. R. China. E-mail: linjun@ynu.edu.cn; Fax: +86 871 5033215; Tel: +86 871 5033215
First published on 17th August 2011
Abstract
An efficient and green method has been developed for the synthesis of tetrahydroimidazo[1,2-a]pyridines by three-component reactions of heterocyclic ketene aminals (HKAs), triethoxymethane and nitroalkenes in one pot in the absence of catalyst and solvent. This protocol has advantages of environmental friendliness, high yields (73–93%), short reaction time and convenient operation.
Introduction
In recent years worldwide attention has been attracted towards environmental protection, which requires the minimization of waste and avoidance of the use of toxic organic solvents in chemical reactions.1 Thus, solvent-free and catalyst-free multi-component reactions2 (MCRs) are currently of considerable interest. A large number of compounds of a diverse variety can be prepared by one-step reactions with relatively simple starting materials via one-pot MCRs, which are usually synthetically facile, highly atom-economic and highly selective.2 These compounds can be perfect candidates for combinatorial, automated syntheses and drug discovery.3Imidazo[1,2-a]pyridine derivatives are becoming more and more important in medicinal and organic chemistry. They have displayed a broad spectrum of pharmacological and biological activities, such as antitumoral,4 antiviral,5 antiinflammatory,6 antibacterial,7 antifungal,8 antiulcer,9 antiprotozoal10 and antiretroviral activities.11 So these pyridine derivatives have found their use as glycosidase inhibitors,12inhibitors of gastric acid secretion,13cyclin-dependent kinase inhibitors,14calcium channel blockers,15 agents against herpesviruses16 and so on.17 Although various approaches to the preparation of imidazo[1,2-a]pyridine derivatives have been developed by organic or pharmaceutical chemists,18–20 environmentally benign and highly selective one-pot solvent-free preparation of these highly substituted bicyclic pyridines have rarely been reported.
In our previous paper, we developed a new type of MCR with a convenient and rapid synthetic procedure, and a series of highly substituted bicyclic pyridines containing a ring-junction nitrogen, including imidazo[1,2-a]pyridines, pyrido[1,2-a]pyrimidines, pyrido[1,2-a]diazepines, oxazolo-[3,2-a]pyridines and thiazolo-[3,2-a]pyridines, were synthesized.18a Nevertheless, this class of compounds are of such significant importance that a lot more effort is still needed to expand the scope and increase the variety of the highly substituted bicyclic pyridines possessing potential bioactivities. Therefore, we herein report the rapid construction of a library of tetrahydroimidazo[1,2-a]pyridine derivatives, which also contain a ring-junction nitrogen, starting with beta-nitrostyrenes. High yields were obtained in a short reaction time.
Results and discussion
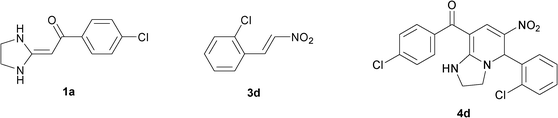
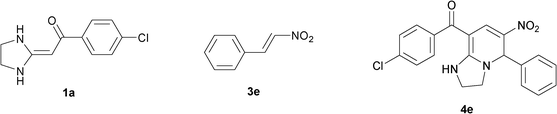
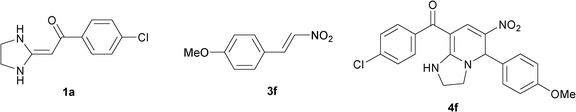
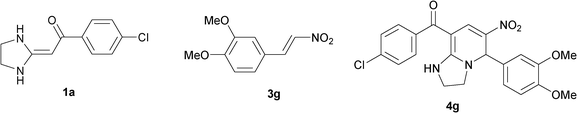
In the preceding communication, the design, synthesis and characterization of highly substituted bicyclic pyridines containing a ring-junction nitrogen were described.18a Using MCRs to synthesize a number of drug-like scaffold compounds in a single step was achieved by simply varying the reaction substrates. In this paper, we aim to utilize the novel synthetic method to afford tetrahydroimidazo[1,2-a]pyridine derivatives 4 in excellent yields by heated HKAs 1, triethoxymethane 2 and nitroalkenes 3 under solvent-free and catalyst-free conditions (Table 1).|
|
|||||
|---|---|---|---|---|---|
| Entry | 1 | 3 | 4 | Time (min) | Yield (%)ab |
| a Isolated yields after silica gel chromatography b Yield of isolated product from reaction on a 2.5 mmol scale. | |||||
| 1 |
|
33 | 82 | ||
| 2 |
|
28 | 83 | ||
| 3 |
|
26 | 85 | ||
| 4 |
|
30 | 85 | ||
| 5 |
|
27 | 89 | ||
| 6 |
|
23 | 91 | ||
| 7 |
|
24 | 93 | ||
| 8 |
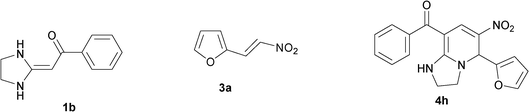
|
27 | 78 | ||
| 9 |
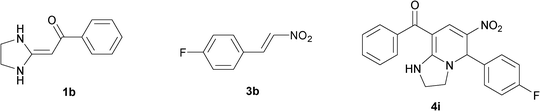
|
29 | 81 | ||
| 10 |
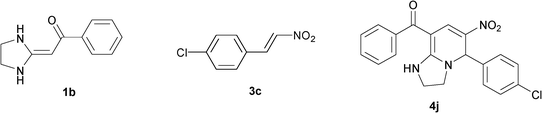
|
24 | 82 | ||
| 11 |
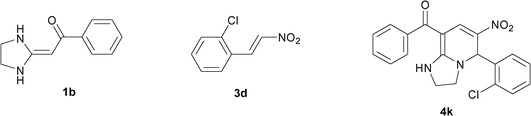
|
25 | 83 | ||
| 12 |
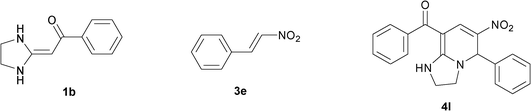
|
31 | 86 | ||
| 13 |
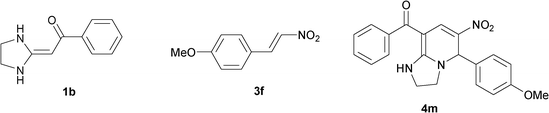
|
26 | 89 | ||
| 14 |
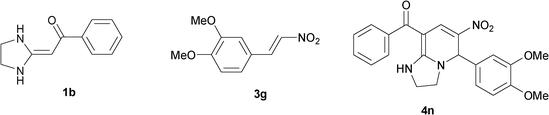
|
35 | 92 | ||
| 15 |
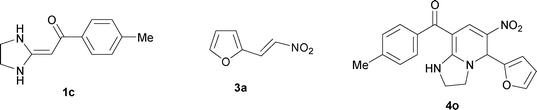
|
28 | 75 | ||
| 16 |
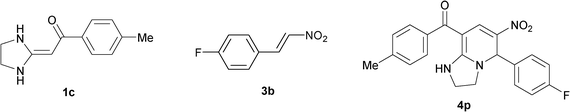
|
20 | 78 | ||
| 17 |
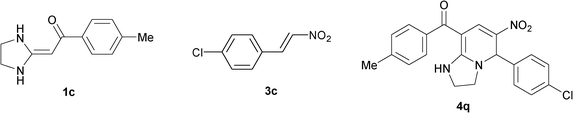
|
25 | 79 | ||
| 18 |
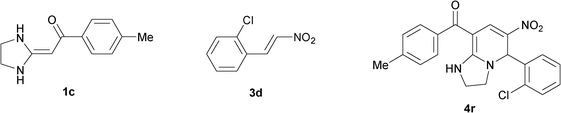
|
22 | 80 | ||
| 19 |
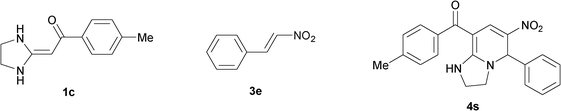
|
33 | 84 | ||
| 20 |
|
26 | 86 | ||
| 21 |
|
35 | 90 | ||
| 22 |
|
26 | 73 | ||
| 23 |
|
23 | 75 | ||
| 24 |
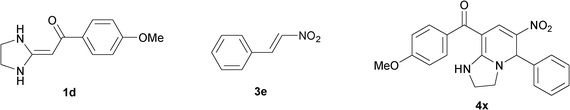
|
21 | 84 | ||
| 25 |
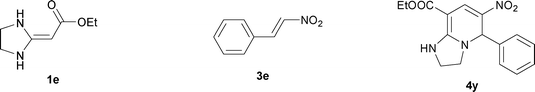
|
38 | 74 | ||
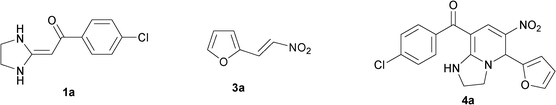
In the initial stage, we continued to use the strategy of reacting HKA 1a with triethoxymethane 2 and nitroalkenes3a, instead of active methylene compounds, in one pot under solvent-free and catalyst-free conditions at 110 °C for 33 min. The tetrahydro-imidazo[1,2-a]pyridine 4a was successfully produced in a good yield (82%) (Table 1, entry 1).
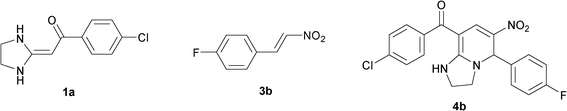
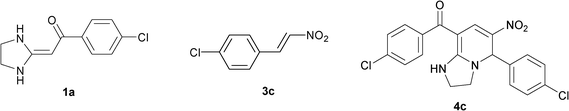
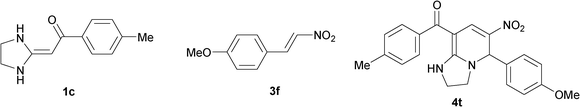
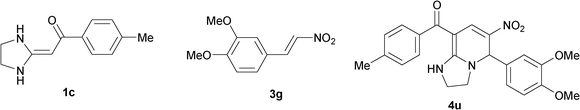
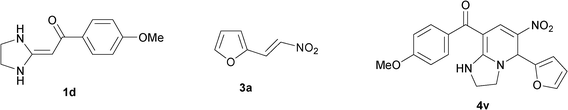
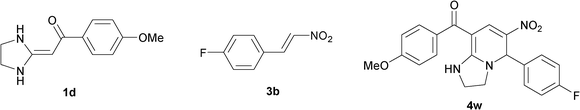
To explore the scope of the method, HKAs 1a–e and nitroalkenes 3a–g were used as substrates to react with triethoxymethane 2 (Table 1). The results demonstrated that HKAs with various substituents were all good substrates for the cyclocondensation reaction (Table 1, entries 1–25). The reactions usually took 20–38 min at 110 °C to reach completion and afforded the products with good to excellent yields.
The structure of HKAs 1 and nitroalkene 3 had an influence on the yield. It was observed that the electron-rich group on the aromatic ring of nitroalkene 3 can contribute to the yield of the reaction. Specifically, under the experimental conditions, the order of the contribution to the yield was 3g > 3f > 3e > 3d > 3c >3b > 3a (Table 1, entries 1–7, 8–14, 15–21 and 22–24). It was also demonstrated that different substituents of HKA 1 contribute to the yield when the same nitroalkene 3 was used. The electron-deficient group on the aromatic ring of HKA 1 can also contribute to the yield of the reaction (Table 1, entries 1, 8, 15, 22; 2, 9, 16, 23; 3, 10, 17; 4, 11, 18; 5, 12, 19, 24, 25; 6, 13, 20 and 7, 14, 21). Under the experimental conditions, the order of contribution to the yield was 1a > 1b > 1c > 1d > 1e.
To verify the structure of the bicyclic pyridine product, 4f was selected as a representative compound and was characterized by X-ray crystallography as shown in Fig. 1 (CCDC 818211†).21
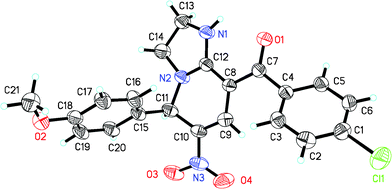 | ||
| Fig. 1 ORTEP diagram of 4f; ellipsoids are drawn at the 30% probability level. | ||
A proposed mechanism for the three-component reaction is depicted in Scheme 1. HKAs 1 reacted with triethoxymethane 2 to form intermediate 5, which was different from what we have previously described.18a Then, 5 reacted with nitroalkenes 3 possibly via an Aza–Michael type reaction mechanism to afford 6. Finally, EtOH was removed from compound 6 to give the final product 4.
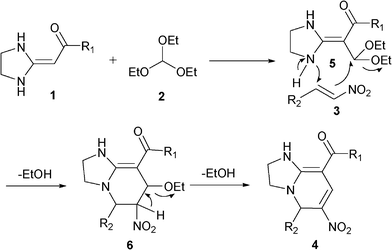 | ||
| Scheme 1 Proposed mechanism for the three-component reaction. | ||
Conclusions
To summarize, compared with our previous report, we have expanded the scope of a one-pot three-component, solvent-free and catalyst-free reaction to synthesize another type of highly functional bicyclic pyridine containing a ring-junction nitrogen. These pyridines are substituted by nitro, aryl and hetero-aryl. Using HKAs, triethoxymethane and nitroalkenes as building blocks, a novel library of highly substituted tetrahydroimidazo-[1,2-a]pyridine derivatives was constructed. It demonstrated that this facile and environmentally friendly process has great potential to be applied to parallel synthesis in drug discovery.Experimental
General Procedure
HKA derivative 1 (2.5 mmol), triethoxy-methane 2 (3 mmol) and nitroalkene derivatives 3 (3 mmol) were charged into a 25 mL round-bottom flask and the mixture was heated to 110 °C. The resulting solution was stirred for 20–38 min until the HKA derivative 1 was completely consumed. The mixture was diluted with EtOAc (50 mL × 2) and quenched with water (50 mL). The organic layer was dried by Na2SO4, concentrated, and purified by flash column chromatography (Petro/AcOEt = 1/1) to afford product 4 with 73–93% yield. The products were further identified by FTIR, NMR and HRMS, being in good agreement with the assigned structures (ESI†).(4-Chlorophenyl)(5-(furan-2-yl)-1,2,3,5-tetrahydro-6-nitro-imidazo[1,2-a]pyridin-8-yl)methanone (4a)
Yellow solid; Mp 257–258 °C; IR (KBr): 3288, 2900, 1594, 1408, 1292, 1228, 1163, 1075, 769 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 3.18–3.23 (m, 1H, NCH2), 3.75–3.80 (m, 3H, NCH2CH2N), 6.02 (s, 1H, NCH), 6.48 (s, 1H, ArH), 6.54 (s, 1H, ArH), 7.55 (d, J = 8.1 Hz, 2H, ArH), 7.60 (d, J = 8.1 Hz, 2H, ArH), 7.67 (s, 1H, ArH), 7.99 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 9.37 (br, 1H, NH); 13C NMR (125 MHz, DMSO-d6): δ = 43.7, 45.9, 51.6, 91.4, 109.7, 111.1, 123.1, 128.9, 130.2, 135.6, 137.3, 138.4, 143.8, 150.2, 159.4, 188.3; HRMS (TOF ES+): m/z calcd for C18H15ClN3O4 [(M+H)+], 372.0746; found, 372.0753.
), 9.37 (br, 1H, NH); 13C NMR (125 MHz, DMSO-d6): δ = 43.7, 45.9, 51.6, 91.4, 109.7, 111.1, 123.1, 128.9, 130.2, 135.6, 137.3, 138.4, 143.8, 150.2, 159.4, 188.3; HRMS (TOF ES+): m/z calcd for C18H15ClN3O4 [(M+H)+], 372.0746; found, 372.0753.
Acknowledgements
We gratefully acknowledge the financial support from the Natural Science Foundation of China (grant numbers 30860342 and 20762013) and the Natural Science Foundation of Yunnan Province (2009CC017, 2008CD063).References
- (a) Special issue in Green Chemistry, see: Chem. Rev., 2007, 107, 2167 Search PubMed; (b) D. J. Adams, P. J. Dyson and S. J. Tavener, Chemistry in Alternative Reaction Media, John Wiley & Sons, Chichester, UK, 2004 Search PubMed; (c) N. R. Candeias, L. C. Branco, P. M. P. Gois, C. A. M. Afonso and A. F. Trindade, Chem. Rev., 2009, 109, 2703 CrossRef CAS.
- (a) For reviews see: Multicomponent Reactions; J. P. Zhu and H. Bienayme, ed.; Wiley-VCH: Weinheim, Germany, 2005; (b) B. Ganem, Acc. Chem. Res., 2009, 42, 463 Search PubMed.
- (a) W.-B. Liu, H.-F. Jiang and C.-L. Qiao, Tetrahedron, 2009, 65, 2110 Search PubMed; (b) D. Tejedor and F. García-Tellado, Chem. Soc. Rev., 2007, 36, 484 Search PubMed.
- (a) M. Hranjec, M. Kralj, I. Piantanida, M. Sedić, L. Sÿuman, K. Pavelić and G. Karminski-Zamola, J. Med. Chem., 2007, 50, 5696 Search PubMed; (b) A. Kamal, J. S. Reddy, M. J. Ramaiah, D. Dastagiri, E. V. Bharathi, M. V. P. Sagar, S. N. C. V. L. Pushpavalli, P. Ray and M. Pal-Bhadra, Med. Chem. Commun., 2010, 1, 355 Search PubMed; (c) M. A. Martínez-Urbina, A. Zentella, M. A. Vilchis-Reyes, A. Guzmán, O. Vargas, M. T. R. Apan, J. L. V. Gallegos and E. Díaz, Eur. J. Med. Chem., 2010, 45, 1211 Search PubMed; (d) M. A. Vilchis-Reyes, A. Zentella, M. A. Martínez-Urbina, A. Guzmán, O. Vargas, M. T. R. Apan, J. L. V. Gallegos and E. Díaz, Eur. J. Med. Chem., 2010, 45, 379 Search PubMed.
- (a) A. Gueiffier, M. Lhassani, A. Elhakmaoui, R. Snoeck, G. Andrei, O. Chavignon, J. C. Teulade, A. Kerbal, E. M. Essassi, J. C. Debouzy, M. Witvrouw, Y. Blache, J. Balzarini, E. D. Clercq and J. P. Chapat, J. Med. Chem., 1996, 39, 2856 Search PubMed; (b) J.-M. Chezal, J. Paeshuyse, V. Gaumet, D. Canitrot, A. Maisonial, C. Lartigue, A. Gueiffier, E. Moreau, J.-C. Teulade, O. Chavignon and J. Neyts, Eur. J. Med. Chem., 2010, 45, 2044 Search PubMed; (c) S. Mavel, J. L. Renou, C. Galtier, H. Allouchi, R. Snoeck, G. Andrei, E. D. Clercq, J. Balzarinic and A. Gueiffiera, Bioorg. Med. Chem., 2002, 10, 941 Search PubMed; (d) K. S. Gudmundsson, J. D. Williams, J. C. Drach and L. B. Townsend, J. Med. Chem., 2003, 46, 1449 Search PubMed.
- (a) A. D. Chiacchioa, M. G. Rimolia, L. Avallonea, F. Arenaa, E. Abignentea, W. Filippellib, A. Filippellib and G. Falconeb, Arch. Pharm., 1998, 331, 273 Search PubMed; (b) A. K. Saxena and K.-J. Schaper, QSAR Comb. Sci., 2006, 25, 590 Search PubMed.
- P. J. Beswick , I. B. Campbell and A. Naylor, WO 9,631,509, 1996 Search PubMed.
- A. Özdemir, G. Turan-Zitouni, Z. A. Kaplancıkli, G. Işcan, S. Khan and F. Demirci, Eur. J. Med. Chem., 2010, 45, 2080 Search PubMed.
- J. J. Kaminski and A. M. Doweyko, J. Med. Chem., 1997, 40, 427 Search PubMed.
- (a) M. A. Ismail, R. Brun, T. Wenzler, F. A. Tanious, W. D. Wilson and D. W. Boykin, J. Med. Chem., 2004, 47, 3658 Search PubMed; (b) M. Bollini, J. J. Casal, D. E. Alvarez, L. Boiani, M. González, H. Cerecetto and A. M. Bruno, Bioorg. Med. Chem., 2009, 17, 1437 Search PubMed; (c) W.-W. Zhang, Y.-B. Chen, W.-D. Chem, Z.-W. Liu and Z. Li, J. Agric. Food Chem., 2010, 58, 6296 Search PubMed.
- A. Chaouni-Benabdallaha, C. Galtiera, H. Allouchic, A. Kherbecheb, J. C. Debouzyd, J. C. Teuladee, O. Chavignone, M. Witvrouwf, C. Pannecouquef, J. Balzarinif, E. de Clercqf, C. Engueharda and A. Gueiffiera, Arch. Pharm., 2001, 334, 224 Search PubMed.
- T. A. Engler, J. R. Henry, S. Malhotra, B. Cunningham, K. Furness, J. Brozinick, T. P. Burkholder, M. P. Clay, J. Clayton, C. Diefenbacher, E. Hawkins, P. W. Iversen, Y.-H. Li, T. D. Lindstrom, A. L. Marquart, J. McLean, D. Mendel, E. Misener, D. Briere, J. C. ÓToole, W. J. Porter, S. Queener, J. K. Reel, R. A. Owens, R. A. Brier, T. E. Eessalu, J. R. Wagner, R. M. Campbell and R. Vaughn, J. Med. Chem., 2004, 47, 3934 Search PubMed.
- A. M. Palmer, G. Münch, C. Brehm, P. J. Zimmermann, W. Buhr, M. P. Feth and W. A. Simon, Bioorg. Med. Chem., 2008, 16, 1511 Search PubMed.
- (a) C. Jaramillo, J. E. de Diego, C. Hamdouchi, E. Collins, H. Keyser, C. Sánchez-Martínez, M. del Prado, B. Norman, H. B. Brooks, S. A. Watkins, C. D. Spencer, J. A. Dempsey, B. D. Anderson, R. M. Campbell, T. Leggett, B. Patel, R. M. Schultz, J. Espinosa, M. Vieth, F.-M. Zhang and D. E. Timm, Bioorg. Med. Chem. Lett., 2004, 14, 6095 Search PubMed; (b) J. Subramanian, S. Sharma and C. B-Rao, J. Med. Chem., 2006, 49, 5434 Search PubMed; (c) M. Anderson, J. F. Beattie, G. A. Breault, J. Breed, K. F. Byth, J. D. Culshaw, R. P. A. Ellston, S. Green, C. A. Minshull, R. A. Norman, R. A. Pauptit, J. Stanway, A. P. Thomas and P. J. Jewsbury, Bioorg. Med. Chem. Lett., 2003, 13, 3021 Search PubMed.
- P. J. Sanfilippo, M. Urbanski, J. B. Press, B. Dubinsky and J. B. Moore, Jr., J. Med. Chem., 1988, 31, 2221 Search PubMed.
- (a) K. S. Gudmundsson and B. A. Johns, Org. Lett., 2003, 5, 1369 Search PubMed; (b) K. S. Gudmundsson and B. A. Johns, Bioorg. Med. Chem. Lett., 2007, 17, 2735 Search PubMed.
- (a) Z.-P. Zhuang, M.-P. Kung, A. Wilson, C. W. Lee, K. Plössl, C. Hou, D. M. Holtzman and H. F. Kung, J. Med. Chem., 2003, 46, 237 Search PubMed; (b) S. C. Goodacre, L. J. Street, D. J. Hallett, J. M. Crawforth, S. Kelly, A. P. Owens, W. P. Blackaby, R. T. Lewis, J. Stanley, A. J. Smith, P. Ferris, B. Sohal, S. M. Cook, A. Pike, N. Brown, K. A. Wafford, G. Marshall, J. L. Castro and J. R. Atack, J. Med. Chem., 2006, 49, 35 Search PubMed; (c) C. Hamdouchi, J. d. Blas, M. d. Prado, J. Gruber, B. A. Heinz and L. Vance, J. Med. Chem., 1999, 42, 50 Search PubMed; (d) Y. Abe, H. Kayakiri, S. Satoh, T. Inoue, Y. Sawada, N. Inamura, M. Asano, C. Hatori, H. Sawai, T. Oku and H. Tanaka, J. Med. Chem., 1998, 41, 4053 Search PubMed; (e) G. Trapani, M. Franco, L. Ricciardi, A.. Latrofa, G. Genchi, E. Sanna, F. Tuveri, E. Cagetti, G. Biggio and G. Liso, J. Med. Chem., 1997, 40, 3109 Search PubMed; (f) N. Margiotta, R. Ostuni, R. Ranaldo, N. Denora, V. Laquintana, G. Trapani, G. Liso and G. Natile, J. Med. Chem., 2007, 50, 1019 Search PubMed; (g) D. P. Becker, D. L. Flynn, A. E. Moormann, R. Nosal, C. I. Villamil, R. Loeffler, G. W. Gullikson, C. Moummi and D.-C. Yang, J. Med. Chem., 2006, 49, 1125 Search PubMed.
- (a) S.-J. Yan, Y.-L. Chen, L. Liu, N.-Q. He and J. Lin, Green Chem., 2010, 12, 2043 Search PubMed; (b) S.-J. Yan, C. Huang, C.-X. Su, Y.-F. Ni and J. Lin, J. Comb. Chem., 2010, 12, 91 Search PubMed; (c) C. Huang, S.-J. Yan, X.-H. Zeng, X.-Y. Dai, Y. Zhang, C. Qing and J. Lin, Eur. J. Med. Chem., 2011, 46, 1172 Search PubMed; (d) S.-J. Yan and J. Lin, Chin. J. Org. Chem., 2010, 30, 465 Search PubMed.
- (a) S.-J. Yan, Y.-F. Niu, R. Huang and J. Lin, Synlett., 2009, 17, 2821 Search PubMed; (b) For reviews, see: Z.-T. Huang and M.-X. Wang, Heterocycles, 1994, 37, 1233 Search PubMed; (c) Z.-T. Huang and M.-X. Wang, Prog. Natural Sci., 1999, 11, 971 Search PubMed.
- (a) A.-M. Zhang, X.-L. Zheng, J.-F. Fan and W. Shen, Tetrahedron Lett., 2010, 51, 828 Search PubMed; (b) P. Tremmel and A. Geyer, Eur. J. Org. Chem., 2005, 3475 Search PubMed; (c) C. Bengtsson and F. Almqvist, J. Org. Chem., 2010, 75, 972 Search PubMed; (d) M. Sellstedt and F. Almqvist, Org. Lett., 2009, 11, 5470 Search PubMed.
- CCDC 818211 contain the supplementary crystallographic data for compound 4f. These data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary information. CCDC reference number 818211. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1ra00242b |
| ‡ These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2011 |