Eu3+ doped Sr2CeO4 phosphors for thermometry: single-color or two-color fluorescence based temperature characterization
Lili
Shi
ab,
Hongjie
Zhang
a,
Chengyu
Li
*a and
Qiang
Su
ac
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, China. Fax: +86-431-85262005; Tel: +86-431-85262208
bGraduate School of the Chinese Academy of Sciences, Beijing, 100039, China
cState Key Laboratory of Optoelectronic Materials and Technologies, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou, Guangdong 510275, China
First published on 3rd August 2011
Abstract
Sr2CeO4:Eu3+ phosphors containing various activator contents have been systemically investigated by temperature-dependent fluorescent method. The temperature-dependent photoluminescence spectra and the integrated emission intensity of Sr2CeO4:Eu3+ at different temperatures indicate that these samples can exhibit distinct low-temperature sensing characteristics. The sensitive intensity/temperature responses of these materials, depending on tuning of the concentration of Eu3+, provide a sound foundation for their potential application in single-color or two-color fluorescence thermometry techniques.
1. Introduction
The characterization of temperature and thermal properties plays a fundamental role in all fields of science, hence the development of methods for measuring this characteristic remains in vogue.1–5 In this vast field, fluorescence thermometry appears as a tool for non-contact and non-invasive surface temperature measurements,6–8 which offers a number of advantages over conventional test methods (infrared pyrometry, thermocouples, thermistors), e.g., high accuracy, remote detection, high signal yield, cost-effective, and quantitative global temperature/heating information.9 As two models of this technique, both the single-color fluorescence thermometry method and the two-color fluorescence thermometry technique are based on a thermophosphor whose fluorescence intensity is temperature dependent.7,10Materials based on Ceria (CeO2) have been extensively investigated owing to their broad application in the fields of catalysis and optics,11–13e.g., they are employed as promoters in Three Way Catalysts, as electrolyte or electrode promoters, active supports or cocatalysts, as well as energy transfer medium in phosphors to sensitize the luminescence of Pr3+. Added with 2Sr(II), CeO2 can form a novel type of solid solution Sr2CeO4, which can emit efficient blue light when irradiated by UV light, cathode rays or X-rays.14 Its luminescence was generally considered to originate from a ligand-to-metal O2−→Ce4+ charge transfer state (CTS).15 Since it is an active center with 100% concentration, intensive studies on this phosphor have been focused on its synthesis,16 structure,17 emission mechanism18 and its potential applications, such as the employment in field emission displays.19 Besides, Sr2CeO4 as a prominent host material for the incorporation of many trivalent luminescence centers, such as Eu3+, Sm3+, Dy3+, Ho3+, Er3+, Tm3+, etc., has continued to attract a lot of attention from researchers.20–22 Effective energy transfer from Ce4+–O2− CTS to these activation centers was proposed to be responsible for their luminescence in Sr2CeO4.23 Among the many rare earth ions, Eu3+ with 4f6 electronic configuration can exhibit abundant emission colors, such as blue from 5D2, green from 5D1, red from 5D0, which have been playing important roles in modern lighting and display fields. These characteristics of Eu3+ ions motivate researchers to explore the luminescence properties of Eu3+-activated Sr2CeO4 most. When Eu3+ concentration becomes higher, emission from the Ce4+−O2− CTS will be much less until it disappears and then the emission owing to the Eu3+ transitions dominates finally. Correspondingly, emission colors of Sr2CeO4 added with different Eu3+ content changes from blue to red, in favor of their potential applications in low pressure mercury vapor (lpmv), high pressure mercury vapor (hpmv) lamps and TV tubes.24
In this research, we have investigated the temperature-dependent photoluminescence (excitation and emission) properties of Sr2CeO4 doped with different concentrations of Eu3+ ions. And then we have proposed the idea that Sr2CeO4:Eu3+ might be employed as novel thermographic phosphors to measure temperature using the single-color or two-color fluorescence-based thermometry methods, on the basis of their various emission colors and temperature-sensitive luminescence properties.
2. Experimental
Strontium cerium oxide doped with different concentrations of europium, Sr2−xEuxCeO4 (x = 0, 0.005, 0.01, 0.05, 0.1, 0.3), were prepared by the solid-state reaction method at high temperature. The synthetic routes to these materials were similar to those reported in the literature.20 High purity SrCO3 (A.R.), CeO2 (99.99%), Eu2O3 (99.99%) employed as starting materials were blended and ground in an agate mortar. Then the mixture was heated at 1323 K in air in an alumina crucible for 10 h. After cooling to room temperature, each sample was re-ground again and the same procedure described above was adopted for them. In order to certify that the as-prepared materials belong to Sr2CeO4 structure, X-ray power diffraction spectra were collected using a Rigaku D/max-IIB X-Ray Diffractometer with Cu Kα (λ = 1.5405 Å) radiation. The photoluminescence excitation (PLE) spectra of samples, between 273 and 413 K, were measured by a FLS920-Combined Fluorescence Lifetime and Steady State Spectrometer (Edinburgh Instruments). The photoluminescence emission (PL) spectra of samples were obtained at elevated temperature ranging from 303 to 473 K, by a system which consists of a computer controlled CCD detector and a heater. During the measurements, a UV lamp with maximum wavelength of 365 nm was adopted as the excitation source.3. Results and discussion
In order to characterize the phase purity and identify the structure type, X-ray powder diffraction (XRD) measurements are performed for the as-synthesized samples at room temperature. These XRD patterns of Sr2CeO4:Eu3+ particles at different doping concentrations are plotted in Fig. 1, in good accordance with the reported powder pattern in JCPDS standard card numbered 50-0115 [Sr2CeO4], confirming the formation of single-phase crystalline products. Sr2CeO4 is indexed to an orthorhombic cell in space groupPbam.14 The structure consists of linear chains of edge-sharing CeO6 octahedra, and the terminal Ce–O distance is about 10 pm shorter than the equatorial distance.14 Therefore, both the Ce4+-site and the Sr2+-site are six-coordinated by oxygen ions in the Sr2CeO4 structure.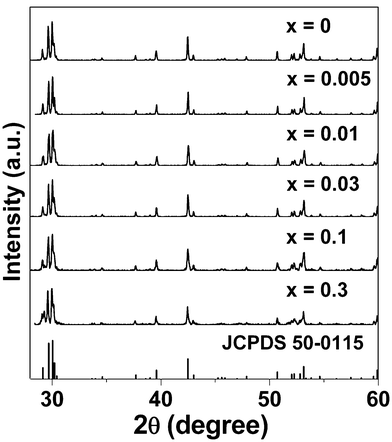 | ||
| Fig. 1 XRD patterns of Eu3+-activated Sr2CeO4 phosphors. | ||
As we know, when coordination number amounts to six, the ionic radii of cations Ce4+, Eu3+, Sr2+ are 87, 94.7 and 118 pm, respectively.25 Therefore, we believe that Eu3+ ions prefer to occupy both the Sr2+ sites and Ce4+ sites because of their similar radius and charge. Based on the similarity of ionic charges and radii between the doping ion (Eu3+) and host cations (Sr2+, Ce4+), we consider that the trivalent Eu3+ ions may non-equivalently replace divalent strontium ions or tetravalent cerium ions, acting as substitutional species, as they enter the Sr2CeO4 crystal. Furthermore, in order to keep the charge balance, four Eu3+ ions will substitute six Sr2+ ions or three Ce4+ ions (the total charge of four Eu3+ ions is equal to that of six Sr2+ ions or three Ce4+ ions).
Hence, there might exist two chemical reactions: (i) if Eu3+ ions replaced Sr2+ ions, one vacancy defect of VSr′′ with two negative charges and two positive defects of Eu•Sr would be produced by each substitution of every two Eu3+ ions in the compound: 2Eu3+ + 3Sr2+ → VSr′′ + 2Eu•Sr. (ii) If Eu3+ ions substituted Ce4+ ions, two vacancy defects of V••O with two positive charges and four negative defects of EuCe′ would be created by each substitution of every four Eu3+ ions in the compound: 4Eu3+ + 3Ce4+ → 2V••o + 4 EuCe′.
The temperature-dependent luminescent excitation spectra of Sr2−xEuxCeO4 (x = 0, 0.005, 0.3) are recorded in the temperature range T = 273 K to T = 413 K. The corresponding experimental results are shown in parts (a), (b) and (c) of Fig. 2. For x = 0, i.e. the host material, two major excitation bands are found in the excitation spectra when monitored for the Ce–O CT emission near 480 nm, as shown in Fig. 2(a). These two excitation bands are generally considered to result from the t1g-f (high-energy peak) and t1u-f (low-energy peak) charge transfer states of Ce4+, respectively.21 It is evident that Sr2CeO4 shows not only a dramatic decrease in its excitation intensity but also a significant spectral red shift in its excitation bands with the increase of temperature. At 273 K, the peak positions of the two luminescence excitation bands for Sr2CeO4 lie at about 286 and 334 nm, respectively. As temperature rises from 273 to 373 K, the first peak shifts from 286 to 305 nm, a difference of 19 nm; the second peak moves from 334 to 347 nm, difference = 13 nm.
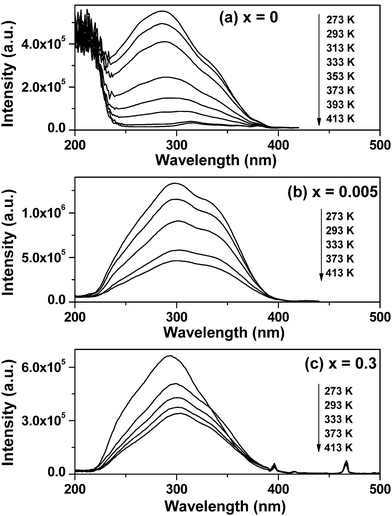 | ||
| Fig. 2 Temperature-dependent PLE spectra of Sr2CeO4:Eu3+ taken at elevated temperature showing charge-transfer bands shift. (a) x = 0, λem = 480 nm; (b) x = 0.005, λem = 480 nm; (c) x = 0.3, λem = 616 nm. | ||
For x = 0.005, the charge transfer between Ce4+ and O2− also results in two broad excitation bands, when monitored at 480 nm (the Ce–O CT luminescence emission), as displayed in Fig. 2(b). Furthermore, each excitation band decreases in intensity and distinguishably moves to longer wavelengths, behaving in a manner similar to that of x = 0. One band maximum varies from 297 to 302 nm, the other band maximum changes from 336 to 342 nm, as temperature is increased from 273 to 413 K.
Besides, by comparing the Ce–O charge transfer bands at 273 K and 373 K of x = 0.005 with those of x = 0, we note that (i) the doping of Eu3+ ions makes the two bands move to the red region of the spectrum at 273 K but shift to the blue region at 373 K, and (ii) the intensity ratio of the t1u-f CT band (low-energy peak) to t1g-f CT band (high-energy peak) increased as Eu3+ ions were added into the lattice. Perceptibly, the reasons for these changes are complicated and here we have tried to ascribe these findings to the changes in the environment around the O2− ligand, which influences the distribution of the electrons in the corresponding orbitals and finally effects the process of charge transfer from O2− to Ce4+.26
For x = 0.3, the temperature-dependent excitation spectra of the strongest red-emission line at 616 nm of Eu3+ are given in Fig. 2(c). The weak sharp lines at about 395, 415 and 466 nm are ascribed to the transitions between the 7F0 and the 5L6, 5D3, 5D2 levels of Eu3+ ions. When the sample temperature rises to 293 K (close to RT), the excitation spectrum of Sr1.7Eu0.3CeO4 can be considered as a strong broad band with a maximum at 300 nm and two shoulders at 289 and 338nm. For this broad excitation band, some authors have attributed it to the Ce4+–O2− CT band,27 however, some other authors have considered that the CT band of Ce4+ inhibits the CT band of Eu3+ in this lattice.24 In the present case, we consider this broad band as the overlap of the Ce4+–O2− CT band and the Eu3+–O2− CT band. Because the Eu3+–O2− CT band in oxides can be generally considered to be typically 0.6–0.8 eV wide and a strong band at energies between 4 and 6 eV,28 it is reasonable that we suppose the Eu3+–O2− CT band to be the middle part of this broad band, whose peak is located at 300 nm. This assumption can simplify the question: how does the sample temperature influence the Eu3+–O2− CT excitation band in our work? Based on this assumption, it is clear that there is a red-shift for the Eu3+–O2− CT band from 293 to 304 nm (difference = 11 nm), with increasing temperature from 273 to 413 K.
One notes that the temperature-dependent red-shift behavior of the charge-transfer excitation bands in Fig. 2 are more conducive to long wavelength UV absorption. And this red shift in excitation spectra is generally ascribed to the variations in the relative position of the transition from the base state to the CTS rather than the changes in the CTS itself.29 This proposed mechanism can also be used to elucidate the experimental results in Fig. 2. In the following discussion, the Ce4+–O2− and Eu3+–O2− charge transfer states will be denoted as the C-CTS and E-CTS, respectively. For the three samples (x = 0, 0.005, 0.3), upon excitation the center or system is promoted from its ground state to the C-CTS or E-CTS, and this process is called the optical absorption transition.30 At low temperature T1, the transition to the C-CTS/E-CTS starts from one vibrational level ν1 of the ground state; at higher temperature T2, the transition to the C-CTS/E-CTS does not actually start at ν1 (as it did at T1) but at the higher vibrational level ν2 of the ground state. Namely, as temperature rises up, the optical absorption transition becomes shorter and correspondingly requires less energy. If reflected in the excitation spectrum, this behavior means the transition yields a longer wavelength.
Under excitation with 365 nm ultraviolet irradiation, the temperature dependence of the PL in the Sr2−xEuxCeO4 with x = 0, 0.005, 0.01, 0.03, 0.1, 0.3 are measured and given in Fig. 3, in the temperature range 303–473 K. It is observed that the positions and shapes of emission peaks are nearly the same with an increase in temperature. However, there appears to be striking thermal quenching for the C-CTS luminescence and the 5Dj-7FJ (j = 0, 1, 2, J = 1, 2, 3) luminescence of Eu3+ in all samples, except for the 5D0-7F2 transition in the samples with low Eu concentrations.
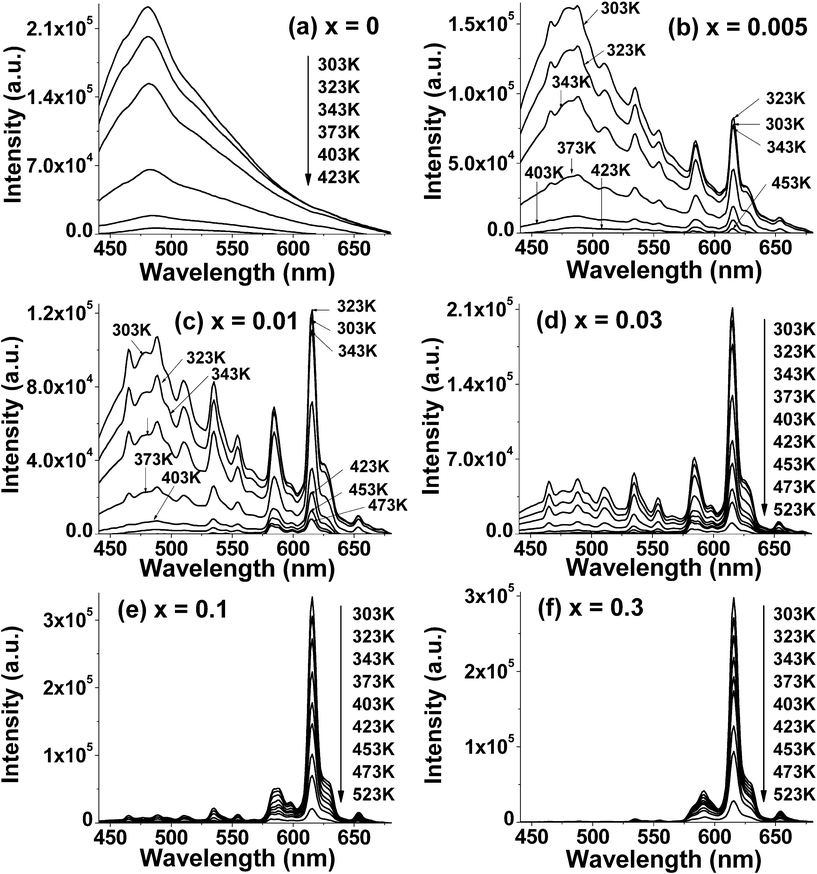 | ||
| Fig. 3 Temperature dependence of PL intensity from all samples. | ||
The specific temperature-dependent PL characteristics of as-prepared materials are listed in the following four aspects: (i) For x = 0, namely, the host material Sr2CeO4, only a broad emission band at around 480 nm is observed, which can be attributed to the charge transfer transition luminescence involving Ce4+. With an increase in sample temperature, Sr2CeO4 exhibits evident temperature quenching effect, i.e., there is a dramatic drop in its emission intensity. (ii) For, x = 0.005 and 0.01, i.e., the samples doped with low Eu3+ concentration, both the emission spectra are composed of the Ce–O CT broad band and a group of lines centered at 467, 486, 510, 535, 555, 585, 616 and 654 nm corresponding to the 5D2-7F0, 5D2-7F2, 5D2-7F3, 5D1-7F1, 5D1-7F2, 5D0-7F1, 5D0-7F2, 5D0-7F3 transitions of Eu3+, respectively. Obviously, the former dominates the emission spectra and overlaps with the latter. When sample temperature is elevated, the emission intensity of the 5D0-7F2 transition originally increases and then decreases, while that of the other emission bands and peaks decays dramatically. (iii) When the Eu3+ content reaches x = 0.03, the Ce4+–O2− CT emission band and a series of Eu3+ intra-4f6 sharp lines matches each other in the PL spectra. The emission intensity of all the bands and peaks shows a distinct trend to decrease with increasing temperature. (iv) For x = 0.1 and 0.3, the Ce4+–O2− CT emission band become such weaker that it can be neglected while the transitions from Eu3+ dominate in the given spectral region. As a result, these phosphors show distinct red emission. As the temperature increases, the emission intensity due to the characteristic transition peaks of Eu3+ becomes weaker gradually.
The temperature-dependent luminescence of Eu3+ has been explored in many systems in the past several decades. Fonger and Struck observed the thermal quenching phenomenon of Eu3+ luminescence in LaOCl, Y2O2S, and La2O2S.31,32 They interpreted the phenomenon taking into account the Franck–Condon shift between the Eu3+–O2− CT band and the 4f states and the resultant resonance crossovers between these states. That is, with increasing temperature, the excited electrons in 5D states go back to the ground state nonradiatively via the crossovers. In the present work, we think that the thermal quenching behaviors of the luminescence of Sr2CeO4 and Sr2CeO4:Eu3+ can be attributed to the similar mechanism.
Based on the above discussion, two configuration coordinate (CC) models for the mechanism of the present case are given in Fig. 4. As shown in Fig. 4(a), for the Ce4+←O2− charge transfer state (C-CTS), it intersects with the ground state of Ce4+ at a point. With an increase in sample temperature, the electrons in the excited C-CTS are thermally churned up to the intersection point and then nonradiatively go back to the ground state, leading to the thermal quenching of the Ce–O CT luminescence. As shown in Fig. 4(b), for the Eu3+←O2− charge transfer state (E-CTS), it crosses not only with the ground state of Eu3+ but also with the three excited states 5D2, 5D1, 5D0. When the sample temperature increases, the electrons in the 5D0 excited state are thermally agitated to the crossover point and then nonradiatively go back to the ground state via the E-CTS, leading to the thermal quenching of luminescence from the 5D0 state. Nevertheless, for the electrons in the 5D1,2 excited states, parts of them might go through the same path as those in the 5D0 state, while parts of them might originally cascade to the lowest 5D0 state via the two paths “5D2→E-CTS→5D0” or “5D1→E-CTS→5D0” and then go through the same path as those in the 5D0 state. This not only describes the thermal quenching processes happening to Eu3+, but also can be considered as the main reason for the abnormal thermal behavior of the 5D0-7F2 emission in the samples (x = 0.005, 0.01) where the emission from the 5D1,2 excited states and the Ce–O CTS is much stronger in the emission spectra. Another possible reason for the abnormal thermal behavior of the 5D0-7F2 emission at least in the case of low Eu3+ concentration is that the excitation spectrum shows a small red-shift at higher temperature.
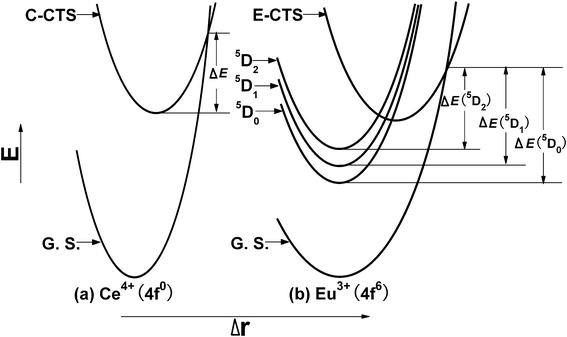 | ||
| Fig. 4 Two configuration coordinate models for the thermal quenching mechanism of Sr2CeO4:Eu3+. C-CTS and E-CTS represent the Ce4+–O2− and Eu3+–O2− charge transfer states, respectively. ΔE, ΔE (5D0), ΔE (5D1) and ΔE (5D2) refer the thermal activation energy of emissions from the Ce–O CT and the 5D0, 5D1, 5D2 excited states of Eu3+, respectively. G. S. indicates the ground state of Ce4+ or Eu3+. | ||
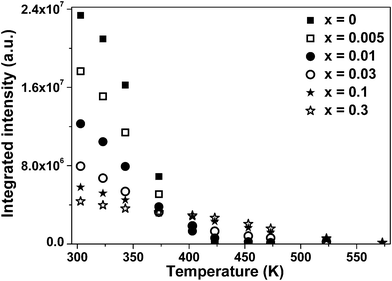
In order to further illustrate temperature response characteristics of PL intensity for these materials, their integrated emission intensity at different temperature is shown in Fig. 5. Here, the integrated intensity of all samples is defined as the area under their PL curves calculated by integrating from 440 to 680 nm. If the thermal quenching temperature (Tq) is defined as the temperature at which the integrated luminescence intensity is 50% of its original value (in this case, the original value refers to the emission intensity at around RT),33,34 the Tq value can be deduced from the intensity of emission peaks at different temperature in Fig. 3. Note that the thermal quenching temperature of samples changes with different Eu3+ doping content. For x = 0, 0.005, 0.01, they have the same Tq value, equal to about 358 K. This observation indicates that a small amount of Eu3+ ions hardly influence the thermal quenching temperature. However, for x = 0.03, 0.1, 0.3, the Tq values of them amount to 362, 399, and 446 K, respectively, which are obviously higher than those of samples with low Eu content.
 | ||
| Fig. 5 The integrated emission intensity of all samples as a function of temperature. | ||
The quenching temperature of emission from luminescent centers in different host lattices has been studied by G. Blasse and A. Bril.35 They have argued that there are two factors influencing on the Tq value of emission, for the luminescent centers that can be excited by charge transfer from the anion to the central cation. One is the energy of the charge transfer state (CTS); the other is Δr, the difference between the equilibrium configuration of the excited state and that of the ground state. According to their viewpoints and the CC models in Fig. 4, it can be easily summarized that the Tq value of emission exactly depends on the thermal activation energy of emission. Therefore, it can be speculated qualitatively from the Tq values that the activation energy of the Eu3+ emission might be higher than that of the Ce–O CT emission.
In addition, it is also observed that for the samples without the Ce–O CT emission, namely, x = 0.1 and x = 0.3, the Tq value of the latter is higher than that of the former. It can be unraveled by the following analysis: (i) in ref. 32, the authors have found that the 5D emissions of Eu3+ quenched sequentially in the simple order 5D2, 5D1, 5D0 with increasing temperature in host materials Y2O2S and La2O2S. For the thermal quenching trend of the 5D emissions of Eu3+ in the host Sr2CeO4, it can be derived from the Tq values of emissions from the 5D2, 5D1, 5D0 excited states in the sample x = 0.3. Therefore, the integrated emission intensity of luminescence from the 5D states of Eu3+ in x = 0.3 as a function of temperature is shown in Fig. 6. And then the Tq values of the 5D0, 5D1 and 5D2 emissions are found to be 448, 388 and 348 K, respectively. This outcome suggests that the thermal quenching order of the 5D emissions of Eu3+ in Sr2CeO4 is the same as that in Y2O2S and La2O2S. Moreover, this order accords with the decreasing trend of ΔE (5D0) > ΔE (5D1) > ΔE (5D2) observed from the CC models in Fig. 4. (ii) The integrated intensity ratio R of emission from the 5D0 state to 5D1, 5D0 to 5D2 and 5D1 to 5D2 are denoted as R0/1, R0/2, R1/2, respectively. At 303 K, i.e., the initial temperature in the present research, when the Eu3+ molar content reaches x = 0.3 from x = 0.1, the integrated intensity ratio values vary from 9.623 to 77.84 for R0/1, from 12.41 to 52.80 for R0/2 and from 0.7754 to 1.474 for R1/2, respectively. In other words, the proportion occupied by the 5D0 state becomes larger among the 5D emissions of Eu3+. As a result, the Tq value of x = 0.3 are higher than that of x = 0.1.
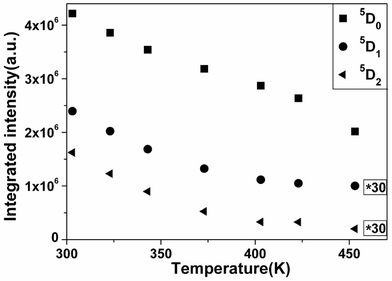 | ||
| Fig. 6 The integrated intensity of the 5Dj (j = 0, 1, 2) emissions of Eu3+ in Sr1.7Eu0.3CeO4 at different temperatures. The annotation “*30” indicates 30 times the original data. | ||
4. Conclusion
The temperature-dependent PL studies show that Eu3+-activated Sr2CeO4 with various Eu3+ concentrations have obvious thermal quenching effects. This observation suggests that these materials are not suitable as potential phosphors in lpmv, hpmv or TV tubes. However, they may be employed in fluorescence thermometry. On the one hand, the materials with x = 0, 0.1 and 0.3, radiating monochromatic light (blue or red), may be applied in the single-color fluorescence-based thermometry technique. On the other hand, the materials with x = 0.005, 0.01 and 0.03, which can emit dichromatic light (concentrated in the blue and red spectral regions), may be considered as candidates for the two-color thermographic phosphors.Acknowledgements
The financial support for this work was extended by National High-tech R&D Program of China (Grant No. 2010AA03A404) and National Natural Science Foundation of China (Grant No. 20921002).References
- W. R. Liu, C. C. Lin, Y. C. Chiu, Y. T. Yeh, S. M. Jang, R. S. Liu and B. M. Cheng, Versatile phosphors BaY2Si3O10:RE (RE = Ce3+, Tb3+, Eu3+) for light-emitting diodes, Opt. Express, 2009, 17, 18103–18109 CrossRef CAS.
- J. Petit, B. Viana and Ph. Goldner, Internal temperature measurement of an ytterbium doped material under laser operation, Opt. Express, 2011, 19, 1138–1146 CrossRef CAS.
- L. Salmon, G. Molnár, D. Zitouni, C. Quintero, C. Bergaud, J. C. Micheau and A. Bousseksou, A novel approach for fluorescent thermometry and thermal imaging purposes using spin crossover nanoparticles, J. Mater. Chem., 2010, 20, 5499–5503 RSC.
- P. Low, B. Kim, N. Takama and C. Bergaud, High-Spatial-Resolution Surface-Temperature Mapping Using Fluorescent Thermometry, Small, 2008, 4, 908–914 CrossRef CAS.
- W. B. Im, S. Brinkley, J. Hu, A. Mikhailovsky, S. P. DenBaars and R. Seshadri, Sr2.975−xBaxCe0.025AlO4F: a Highly Efficient Green-Emitting Oxyfluoride Phosphor for Solid State White Lighting, Chem. Mater., 2010, 22, 2842–2849 CrossRef CAS.
- M. A. R. C. Alencar, G. S. Maciel, C. B. de Araújo and A. Patra, Er3+-doped BaTiO3 nanocrystals for thermometry: Influence of nanoenvironment on the sensitivity of a fluorescence based temperature sensor, Appl. Phys. Lett., 2004, 84, 4753 CrossRef CAS.
- A. H. Khalid and K. Kontis, 2D surface thermal imaging using rise-time analysis from laser-induced luminescence phosphor thermometry, Meas. Sci. Technol., 2009, 20, 025305 CrossRef.
- S. V. Yap, R. M. Ranson, W. M. Cranton, D. C. Koutsogeorgis and G. B. Hix, Temperature dependent characteristics of La2O2S: Ln [Ln = Eu, Tb] with various Ln concentrations over 5–60 °C, J. Lumin., 2009, 129, 416–422 CrossRef CAS.
- P. F. Wang, G. Rajan, Y. Semenova and G. Farrell, Temperature dependence of a macrobending edge filter based on a high-bend loss fiber, Opt. Lett., 2008, 33, 2470–2472 CrossRef.
- M. Luong, W. Koban and C. Schulz, Novel strategies for imaging temperature distribution using Toluene LIF, J. Phys. Conf. Ser., 2006, 45, 133–139 CrossRef CAS.
- T. Montini, A. Speghini, L. D. Rogatis, B. Lorenzut, M. Bettinelli, M. Graziani and P. Fornasiero, Identification of the Structural Phases of CexZr1−xO2 by Eu(III) Luminescence Studies, J. Am. Chem. Soc., 2009, 131, 13155–13160 CrossRef CAS.
- F. Esch, S. Fabris, L. Zhou, T. Montini, C. Africh, P. Fornasiero, G. Comelli and R. Rosei, Electron Localization Determines Defect Formation on Ceria Substrates, Science, 2005, 309, 752–755 CrossRef CAS.
- B. Yan, X. W. Cai and X. Z. Xiao, Photoluminescence Enhancement Effect of CeO2 in Rare Earth Composites MM'O3/CeO2 and MM'O3/CeO2:Pr3+ (M=Ca, Sr; M'=Ti, Zr), J. Fluoresc., 2009, 19, 221–228 CrossRef CAS.
- E. Danielson, M. Devenney, D. M. Giaquinta, J. H. Golden, R. C. Haushalter, E. W. McFarland, D. M. Poojary, C. M. Reaves, W. H. Weinberg and X. D. Wu, A Rare-Earth Phosphor Containing One-Dimensional Chains Identified Through Combinatorial Methods, Science, 1998, 279, 837–839 CrossRef CAS.
- L. V. Pieterson, S. Soverna and A. Meijerink, On the nature of the luminescence of Sr2CeO4, J. Electrochem. Soc., 2000, 147(12), 4688–4691 CrossRef.
- O. A. Serra, V. P. Severino, P. S. Calefi and S. A.Cicillini, The blue phosphor Sr2CeO4 synthesized by Pechini’s method, J. Alloys Compd., 2001, 323–324, 667–669 CrossRef CAS.
- R. Ghildiyal, P. Page and K. V. R. Murthy, Synthesis and characterization of Sr2CeO4 phosphor: Positive features of sol–gel technique, J. Lumin., 2007, 124, 217–220 CrossRef CAS.
- C. H. Lu and C. T. Chen, Luminescent characteristics and microstructures of Sr2CeO4 phosphors prepared via sol–gel and solid-state reaction routes, J. Sol-Gel Sci. Technol., 2007, 43, 179–185 CrossRef CAS.
- Y. D. Jiang, F. L. Zhang, C. J. Summers and Z. L. Wang, Synthesis and properties of Sr2CeO4 blue emission powder phosphor for field emission displays, Appl. Phys. Lett., 1999, 74, 1677–1679 CrossRef CAS.
- T. Hirai and Y. Kawamura, Preparation of Sr2CeO4 Blue Phosphor Particles and Rare Earth (Eu, Ho, Tm, or Er)-Doped Sr2CeO4 Phosphor Particles, Using an Emulsion Liquid Membrane System, J. Phys. Chem. B, 2004, 108, 12763–12769 CrossRef CAS.
- A. Nag and T. R. N. Kutty, Photoluminescence of Sr2 − xLnxCeO4 + x/2 (Ln = Eu, Sm or Yb) prepared by a wet chemical method, J. Mater. Chem., 2003, 13, 370–376 RSC.
- X. Z. Xiao and B. Yan, Sr2CeO4:Eu3+ and Sr2CeO4:5 mol% Eu3+, 3 mol% Dy3+ microphosphors:Wet chemistry synthesis from hybrid precursor and photoluminescence properties, J. Phys. Chem. Solids, 2008, 69, 1665–1668 CrossRef CAS.
- O. Viagin, A. Masalov, I. Ganina and Y. Malyukin, Mechanism of energy transfer in Sr2CeO4:Eu3+ phosphor, Opt. Mater., 2009, 31, 1808–1810 CrossRef CAS.
- R. Sankar and G. V. Subba Rao, Eu3+ Luminescence, Ce4+ → Eu3+ Energy Transfer, and White-Red Light Generation in Sr2CeO4, J. Electrochem. Soc., 2000, 147, 2773–2779 CrossRef CAS.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- N. P. LL'in and V. F. Masterov, Electronic structure of the Er-O6 complex in silicon, Semiconductors, 1997, 31, 886–892 CrossRef.
- L. Li, S. H. Zhou and S. Y. Zhang, Investigation on charge transfer bands of Ce4+ in Sr2CeO4 blue phosphor, Chem. Phys. Lett., 2008, 453, 283–289 CrossRef CAS.
- P. Dorenbos, Lanthanide charge transfer energies and related luminescence, charge carrier trapping, and redox phenomena, J. Alloys Compd., 2009, 488, 568–573 CrossRef CAS.
- A. R. Bugos, S. W. Allison and M. R. Gates, “Emission Properties of Double-doped LuPO4:Eu3+,Dy3+ Thermophosphors for Application in Remote High Temperature Measurements,” in IEEE Proceedings of the Southeastcon’91, Williamsburg, VA, 1991, pp. 1148–1152 Search PubMed.
- G. Blasse, and B. C. Grabmaier, Luminesenct materials, Springer, Berlin, German, 1994, ch. 2 Search PubMed.
- W. H. Fönger and C. W. Struck, Eu+3 5D Resonance Quenching to the Charge-Transfer States in Y2O2S, La2O2S, and LaOCl, J. Chem. Phys., 1970, 52, 6364–6372 CrossRef.
- C. W. Struck and W. H. Fönger, ROLE OF THE CHARGE-TRANSFER STATES IN FEEDING AND THERMALLY EMPTYING THE 5D STATES OF Eu+3 IN YTTRIUM AND LANTHANUM OXYSULFIDES, J. Lumin., 1970, 1(2), 459–469 Search PubMed.
- C. C. Lin, R. S. Liu, Y.S. Tang and S. F. Hu, Full-Color and Thermally Stable KSrPO4:Ln (Ln = Eu, Tb, Sm) Phosphors for White-Light-Emitting Diodes, J. Electrochem. Soc., 2008, 155, J248–J251 CrossRef CAS.
- R. J. Xie, N. Hirosaki, N. Kimura, K. Sakuma and M. Mitomo, 2-phosphor-converted white light-emitting diodes using oxynitride/nitride phosphors, Appl. Phys. Lett., 2007, 90, 191101 CrossRef.
- G. Blasse, Thermal Quenching of Characteristic Fluorescence, J. Chem. Phys., 1969, 51, 3529–3530 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
