Mechanism of proton transfer in the strong OHN intermolecular hydrogen bond
Irena
Majerz
*a and
Matthias J.
Gutmann
b
aFaculty of Chemistry, University of Wrocław, Joliot-Curie 14, PL 50-383 Wrocław, Poland. E-mail: majerz@yahoo.com
bAppleton Laboratory, ISIS Facility, Chilton Didcot, Oxfordshire OX11 0QX, United Kingdom
First published on 29th July 2011
Abstract
The structure of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine was studied by neutron diffraction at 330, 300, 270, 240, 210, 180, 150, 120, 90, 60, and 30 K. The O⋯H bond length gradually changes from 1.403(10) Å at 300 K to 1.424(4) Å at 30 K. The proton shifts in the hydrogen bridge towards the acceptor nitrogen atom. Temperature-dependent changes in the strong OHN hydrogen bond are used to discuss the proton transfer mechanism.
Introduction
For a very long time the hydrogen bond has been very intensively investigated because of its ubiquity in chemical systems,1 importance in biology2 and material sciences.3 The hydrogen bond is classified as a weak interaction but can modify the solid state motifs and packing of the molecules in a crystal4 and also alter the structure and molecular properties. Compared to other chemical bonds the hydrogen bond is very flexible and can change from very weak to very strong comparable to the covalent bond. Depending on the proton donor–proton acceptor properties of the complex components, the proton can be located near the donor or the acceptor. The parameter determining the strength of the hydrogen bond is the proton transfer degree. For the strongest hydrogen bonds it can be shifted to the centre of the hydrogen bridge. This is connected with a significant shortening of the distance between donor and acceptor. While the complexes with proton at donor or acceptor are very numerous for every type of the hydrogen bridges the strongest hydrogen bonds can be expected only in OHO, OHN and NHN systems in a very narrow range of the proton donor properties of the complex components. The difference between the weak and strong hydrogen bonds is that location of the proton between the bridge atoms in complexes with strong hydrogen bond can be modified by temperature or the complex surrounding. Investigation of the solid state structure of the complex with strong hydrogen bond at different temperature is the best method of investigation of the proton transfer process. Neutron single crystal diffraction provides the most accurate position of the proton. Despite the importance of understanding the mechanism of proton transfer there are not very many results describing migration of the proton in the hydrogen bridge.5–11,12 Only in one of these compounds the proton passes fully from the donor to the acceptor with intermediate location near the hydrogen bond centre.12 In other strong hydrogen bonds only the shifting of the proton under temperature is observed.5–11,12 The reason of the limited number of complexes exhibiting proton migration is that finding such complexes is difficult. The first reason of difficulty in predicting the hydrogen bond strength is the narrow range of the ΔpKa value in which the strong hydrogen bond exists, and the second is the precision of the pKa determination.Among the OHN complexes a group of compounds traditionally investigated as model systems with different proton transfer degree are complexes of carboxylic acids with amines. Numerous experiments13–14 showed that in this group of complexes the proton can be found at various locations: the donor, acceptor and the central location in the hydrogen bridge are all possible so these complexes can be used as model systems to investigate the mechanism of the proton transfer. It can be expected that in the strong hydrogen bond in carboxylic acid with amine complexes the proton position could shift with temperature.
In pyridinium 2,4-dinitrobenzoate (ΔpKa = 3.78) previously investigated, the O⋯N distance systematically changed with the temperature from 2.568(6) Å at 300 K to 2.565(2) Å at 30 K.15 The proton was located at the acceptor nitrogen atom and the O⋯H bond length changed from 1.403(10) Å at 300 K to 1.424(4) Å at 30 K. The proton shifted in the hydrogen bridge but did not pass through the bond center. To obtain a more complete picture of the proton behaviour the structural data of pyridinium 2,4-dinitrobenzoate can be combined with the data for another benzoic acid with pyridine.
The X-ray structure of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine (ΔpKa = 3.75) measured previously16 showed that this complex was an example of a very short O⋯N hydrogen bridge of 2.550(2) Å at room temperature and 2.529(2) Å at 80 K. The change of the O⋯N distance was connected with the migration of the proton reflected in a different occupancy of two proton sites. The ΔpKa value of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine was very similar to ΔpKa of pyridinium 2,4-dinitrobenzoate but both the O⋯N distance and the location of the proton were significantly different. Pyridinium 2,4-dinitrobenzoate is an example of a compound with the proton shifted near the acceptor. In the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine proton migrates closer to the donor atom. Combination of the results for both compounds belonging to this same group of intermolecular OHN complexes allows to follow the proton migration in the full range of the proton transfer. The neutron structures with precise determination of the proton position can be used to elucidate the mechanism of the proton transfer.
Experimental
Single crystals of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine were grown from acetonitrile solutions. Neutron diffraction data in the temperature range from 30 K–300 K were collected using the SXD instrument at the ISIS facility at Rutherford Appleton Laboratory (UK).17 To facilitate efficient data collection, two crystals were comounted and exposed simultaneously in the neutron beam, similar as was done in ref. 18. Data were treated using the locally available SXD2001 software19 and refinements carried out SHELX20 using a 1/σ2(I) weighting scheme for all data sets. To investigate properties of the electron density of the experimental structures with precisely determined proton position, for each neutron structure the wave functions were evaluated at B3LYP/6-311++G** level of theory with the Gaussian program.21 The wave functions were used as the input to the AIM200 program,22 with all the default options. The Natural Bond Orbital (NBO) analysis was performed with the ADF program.23 Details of the data collection and refinement parameters are summarized in Table 1.| Diffractometer | SXD neutron time-of-flight Laue diffractometer | ||||||||||
| Wavelength range (Å) | 0.37–8.77 | ||||||||||
| Compound | complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine, C14H13N3O6 | ||||||||||
| Molecular weight | 291.2 | ||||||||||
| Crystal size | ~100 mm3 (two crystals used simultaneously for data collection) | ||||||||||
| Unit cell | Monoriclinic, space group P21/c, Z = 4 | ||||||||||
| T (K) | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | 330 |
| a (Å) | 12.516 | 12.507 | 12.497 | 12.486 | 12.472 | 12.452 | 12.441 | 12.431 | 12.418 | 12.407 | 12.391 |
| b (Å) | 7.527 | 7.553 | 7.589 | 7.627 | 7.675 | 7.726 | 7.759 | 7.795 | 7.834 | 7.873 | 7.915 |
| c (Å) | 16.074 | 16.096 | 16.116 | 16.140 | 16.169 | 16.197 | 16.211 | 16.228 | 16.252 | 16.273 | 16.288 |
| β (°) | 112.072 | 112.091 | 112.102 | 112.136 | 112.155 | 112.186 | 112.176 | 112.201 | 112.229 | 112.237 | 112.206 |
| V (Å3) | 1403.3 | 1408.9 | 1416.3 | 1423.8 | 1433.4 | 1443.0 | 1449.1 | 1456.1 | 1463.5 | 1471.3 | 1479.1 |
| ρcalc (g cm−3) | 1.61 | 1.61 | 1.60 | 1.59 | 1.59 | 1.58 | 1.57 | 1.56 | 1.55 | 1.54 | 1.43371 |
| Refinement | SHELX, refined on F2, 1/[σ(Fo)]2 weights, isotropic extinction, all atoms treated anisotropic | ||||||||||
| Number of refls | 18120 | 16471 | 14731 | 13525 | 11304 | 10670 | 9706 | 8355 | 7372 | 5822 | |
| Unique refls I > 2σ | 6154 | 5427 | 4627 | 4099 | 3463 | 3089 | 2672 | 2350 | 2057 | 1750 | |
| N parameters | 289 | 289 | 289 | 289 | 289 | 289 | 291 | 289 | 289 | 289 | |
| R(F) | 0.085 | 0.087 | 0.090 | 0.091 | 0.090 | 0.090 | 0.088 | 0.089 | 0.086 | 0.090 | |
| wR(F2) | 0.184 | 0.180 | 0.180 | 0.178 | 0.167 | 0.167 | 0.168 | 0.166 | 0.166 | 0.164 | |
| Extinction | 0.0088 | 0.0088 | 0.0089 | 0.0092 | 0.0093 | 0.0100 | 0.0103 | 0.0111 | 0.0108 | 0.0116 | |
| Absorption coeff. | 2.770 + 0.0155λ | ||||||||||
Results and discussion
1. The parameters of the OHN hydrogen bond in the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine
In order to follow the proton location in the short hydrogen bond as a function of temperature the structure of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine was studied by single crystal neutron diffraction. The structure of the investigated compound is shown in Fig. 1. According to Table 1 temperature slightly influences the cell parameters of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine. All cell parameters increase with the temperature except the crystallographic a parameter (a = −0.0004T + 12.533, R2 = 0.9958). These changes are not connected with the hydrogen bond as the O⋯N bridge length slightly increases with temperature and the hydrogen bridge is not parallel to any cell axis so the hydrogen bond is not the primary reason for the thermal expansion of the crystal. The most temperature-sensitive parameters are the crystallographic b axis and β angle of the crystal cell changing according to the equations: b = 0.0013T + 7.477, R2 = 0.9979 and β = −0.000001x2 + 0.001033x + 112.0335, R2 = 0.9526. The temperature dependence of the cell volume displays a linear behaviour that can be described by V = 0.2577T + 1394.3, R2 = 0.9982 | ||
| Fig. 1 Crystal structure of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine from single crystal neutron diffraction. Thermal displacement ellipsoids are drawn at the 50% probability level. | ||
The parameters of the OHN hydrogen bridge as a function of temperature in the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine are collected in Table 2.
| O1⋯N1 [Å] | O1–H13 [Å] | H13⋯N1 [Å] | OHN [°] | O1–C8 [Å] | C8=O2 [Å] | C8–C9 [Å] | O2⋯H7 | |
|---|---|---|---|---|---|---|---|---|
| X-ray13 299 K | 2.550(2) | 0.90(7), 1.76(8) | 0.82(8), 1.65(7) | 177(4), 1.60(5) | 1.284(2) | 1.212(2) | 1.512(3) | 2.684(27) |
| X-ray13 80 K | 2.529(2) | 0.98(7), 1.67(11) | 0.88(10), 1.56(7) | 174(4), 163(4) | 1.295(2) | 1.223(2) | 1.514(3) | 2.628(1) |
| 330 | 2.539(6) | 1.150(12) | 1.391(12) | 176.1(10) | 1.298(7) | 1.221(6) | 1.488(6) | 2.594(12) |
| 300 | 2.544(4) | 1.181(8) | 1.365(7) | 176.1(7) | 1.288(5) | 1.213(4) | 1.507(4) | 2.593(8) |
| 270 | 2.541(4) | 1.183(7) | 1.359(7) | 175.8(6) | 1.291(4) | 1.217(3) | 1.509(4) | 2.585(7) |
| 240 | 2.537(4) | 1.192(7) | 1.347(6) | 176.4(6) | 1.294(4) | 1.218(3) | 1.507(3) | 2.573(7) |
| 210 | 2.539(3) | 1.194(6) | 1.347(6) | 175.9(5) | 1.297(4) | 1.217(3) | 1.506(3) | 2.568(7) |
| 180 | 2.536(3) | 1.222(6) | 1.317(6) | 174.9(5) | 1.289(4) | 1.215(3) | 1.510(3) | 2.585(7) |
| 150 | 2.538(3) | 1.208(5) | 1.332(5) | 175.4(4) | 1.295(3) | 1.222(2) | 1.510(3) | 2.562(5) |
| 120 | 2.537(2) | 1.213(5) | 1.326(4) | 175.4(4) | 1.293(3) | 1.225(2) | 1.513(2) | 2.556(5) |
| 90 | 2.536(2) | 1.215(5) | 1.323(4) | 175.6(4) | 1.293(3) | 1.227(2) | 1.513(2) | 2.551(5) |
| 60 | 2.534(2) | 1.222(3) | 1.314(3) | 175.4(3) | 1.293(2) | 1.230(2) | 1.513(2) | 2.544(3) |
| 30 | 2.536(2) | 1.224(3) | 1.315(3) | 174.9(3) | 1.293(2) | 1.231(2) | 1.516(2) | 2.539(3) |
The X-ray structure as well as the spectroscopic IR spectra measured previously at room and liquid nitrogen temperature15 suggested that the OHN hydrogen bond in the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine is characterized by a very short O⋯N distance. With the temperature lowered from 297 to 80 K the O⋯N bridge became shorter. Also the occupancy of two nonequivalent proton positions changed with the temperature suggesting migration of the proton in the short hydrogen bond.
The neutron measurement confirms the short O⋯N distance but only a single proton site in the hydrogen bridge is observed. Both OH and NH are very sensitive to the temperature change. In the neutron structures measured in the range of 330–30 K, the proton moves from the donor oxygen atom to the nitrogen but does not pass through the center of the hydrogen bridge. At 30 K the NH bond is 0.091 Å longer than OH what means that even at this temperature proton is located closer to donor (Table 2, Fig. 2). At this temperature the hydrogen bond is the strongest with the proton located relatively close to the hydrogen bridge centre. The linear correlations of O⋯N, OH and NH shown in Fig. 2 illustrate the sensitivity of the hydrogen bridge length to temperature which confirms the strength of the hydrogen bond of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine.
 | ||
| Fig. 2 Temperature changes in the distances in the hydrogen bridge of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine (a) O⋯N, (b) O⋯H, (c) N⋯H. | ||
The neutron structures of O⋯N hydrogen bond complexes with precisely determined proton position can be used as experimental verification of theoretical correlations linking the parameters of the hydrogen bridge. The first of them depicted in Fig. 3a links the O⋯N with the difference between OH and NH. This parameter shows how far the proton is shifted from the hydrogen bond center and can be used as a measure of the hydrogen bond strength. The experimental points derived from the neutron structures are compared with this same dependency derived theoretically.24 The theoretical curve has been calculated without taking into account the anharmonicity of the hydrogen bridge vibrations. Comparison of theoretical and experimental curves shows significant differences between them, especially in the range of the strongest hydrogen bonds for which elongation of the hydrogen bridge by ground state vibrations is significant. Including the structures of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine allows to fully reproduce the shape of the experimental curve. Comparison of experimental and theoretical curve can give the anharmonicity corrections very important in explanation of anomalous spectroscopic effects of strong hydrogen bond.
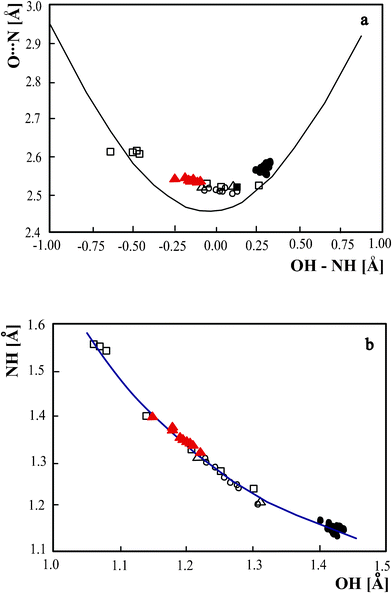 | ||
Fig. 3 Comparison of the geometric parameter of the strong hydrogen bonds. (a) Dependence of N⋯O bridge length as a function of the difference between OH and NH. The experimental neutron bridge parameters are compared with the theoretical curve calculated according to:24 p1 = exp(−(r1 − r1°)/b1), p2 = exp(−(r2 − r2°)/b2), p1 + p2 = 0, r1 = rOH, r2 = rNH, where p1 and p2 are the corresponding valence bond orders of the diatomic units, b1 = 0.371, r1° = 0.942, b2 = 0.385, r2° = 0.992 Å, (b) relation between NH and OH bond lengths. • – pyridinium 2,4-dinitrobenzoate,16 o – 4-methylpyridine–pentachlorophenol complex,12 □ – 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct of benzene-1,2,4,5-tetracarboxylic6 acid and 4,4’-bipyridyl, Δ – pyridine-3,5-dicarboxylic acid,5▾(red) – complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine. 2 adduct of benzene-1,2,4,5-tetracarboxylic6 acid and 4,4’-bipyridyl, Δ – pyridine-3,5-dicarboxylic acid,5▾(red) – complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine. | ||
Another theoretical correlation linking the bond lengths of the hydrogen bridge is the Bond Order reaction Coordinate (BORC) curve. According to Pauling’s bond order concept25 the proton moves along the theoretically estimated BORC curve along which the proton valence remains constant independent of the proton location in the transfer of the proton from donor to acceptor. The BORC curve for OHN hydrogen bonds is shown in Fig. 3b and the experimental points for the complexes with proton moving in the strong OHN hydrogen bond are marked. Despite limited range of OH and NH temperature changes which cannot reproduce the shape of the Bond Order reaction Coordinate (BORC) curve, the OH and NH bonds in the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine are accomplished along the BORC curve. All experimental points for the investigated compounds are located along the BORC curve which means that the proton moves keeping a constant valence of 1. This correlation includes not only the experimental data for benzoic acid–pyridine complexes but also the data for strong hydrogen bond in the complex of pentachlorophenol with 4-methylyridine.12 The correlation is common for the complexes of phenol and bezoic acids confirming its general character. Location of the points for the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine in the central part of the curve confirms the strength of the hydrogen bond which belongs to the group of the strongest hydrogen bridges.
2. The change of geometry of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine upon proton transfer in the hydrogen bond
The continuous changes in the strong intermolecular hydrogen bond are connected with changes in the geometry of donor and acceptor molecule and usually the geometry of the proton donor is more sensitive to the proton transfer than the geometry of the acceptor.26 For the OHN hydrogen bond linking the benzoic acid with pyridine the bonds in the carbonyl/carboxyl group are most sensitive to proton transfer. For molecular complexes with the proton located at the donor oxygen atom, the CO bond lengths are typical for single and double bonds. When the proton is shifted to acceptor, the electron cloud is spread over the COO−group and both lengths become equal, so the difference between C–O and C=O bond length can be used as a measure of the proton transfer degree.27 The C–C bond length linking the COOH group with the aromatic ring becomes shorter when the proton moves to acceptor, which reflects its partially double character connected with the delocalization of the electron cloud in the carboxylate group.As it was shown for the pyridinium 2,4-dinitrobenzoate16 the shifting of the proton in the hydrogen bridge influences the C–O, C=O and C–C bond length. Because the changes of the bond lengths are relatively significant a question arises if they can be used as general measure of the proton transfer degree. In Fig. 4a are shown the correlation of C–O and C=O bond length with the proton transfer degree expressed as the OH–NH for both complexes of benzoic acids with pyridine. It is possible to link both sets of experimental points for 3,5-dinitro- and 2,4-dinitrobenzoic acid and obtain general correlations of C–C, C–O and C=O in a broad range of proton transfer degrees starting from molecular to proton transfer complexes. Also the OCC and OCO angles correlated with temperature in Fig. 4b and 4d are sensitive to proton transfer but it was not possible to combine the data for 3,5-dinitro- and 2,4-dinitrobenzoic acid in one correlation. Because the structure of pyridinium 2,4-dinitrobenzoate as a complex with proton shifted to the acceptor is less sensitive to proton transfer, the OCC and OCO angle changes in a more limited range of values than those for the complex for the 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine. The angle between benzoic acid and pyridine planes presented in Fig. 4c seems to be a good measure of the proton transfer degree. For both benzoic acid–pyridine complexes the changes are systematic and the values suggest that the proton transfer from donor to acceptor is connected with rearrangement from parallel to perpendicular location of benzoic acid to pyridine planes.
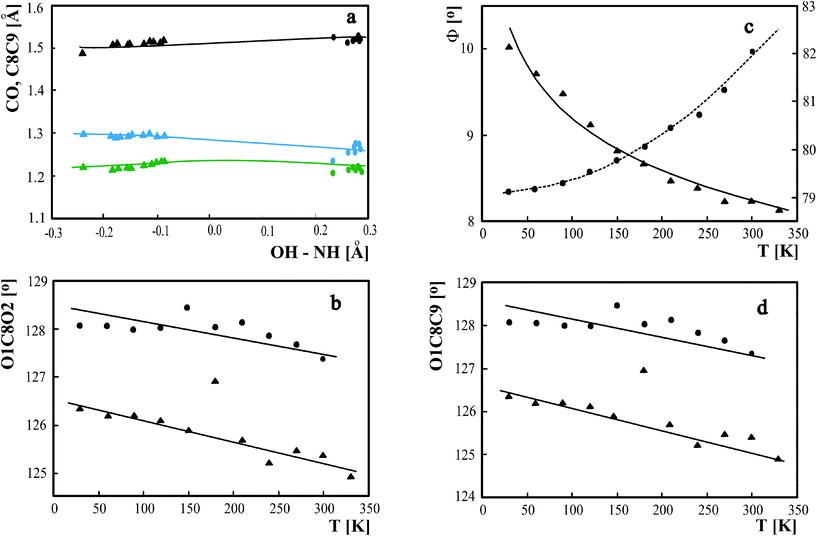 | ||
| Fig. 4 (a) C–C (black), C–O (blue) and C=O (green) distances versusOH – NH difference. Correlation of O1C6O2 (b), pyridine to benzoic acid aromatic plane (c) and O1C6C7 (d) with temperature. ▾ – complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine, • – pyridinium 2,4-dinitrobenzoate. | ||
Compared to other OHN hydrogen bonds linking benzoic acids with pyridines collected in the CCD crystal base,28 the hydrogen bond in the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine is among the shortest. In Fig. 5a the N⋯O bridge lengths for all these complexes are correlated with the difference of OH and NH distance including both X-ray and neutron measurements. For the strongest hydrogen bonds the proton is located at half the distance between the donor and acceptor atom so the OH–NH value equals zero. The values of O⋯N and OH–NH for the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine are located at the centre of the correlation and can be compared with the shortest O⋯N distance of 2.510(2) Å which is the shortest among the benzoic acid–pyridine complexes. This shortest N⋯O hydrogen bond has been found in the complex of 3,5-dinitro-4-methylbenzoic acid with trans-1,2-bis(4-pyridyl)ethane29 for which the OH–NH value is 0.07 Å. The correlation in Fig. 5a includes both the neutron and X-ray structures. For the latter the proton position is not precisely determined so the spread of the experimental points is significant and the shape of the correlation linking N⋯O with OH–NH is not determined very precisely.
 | ||
| Fig. 5 Correlations of the crystal base data (a) relation between NH and OH bond lengths, dependence of N⋯O bridge length (b) and C–O/C=O (c) with the difference between OH and NH. | ||
The correlation linking OH and NH bond lengths performed for all complexes of benzoic acid in the crystal data base in Fig. 5b is compared with the theoretical BORC curve. When the neutron data in Fig. 3b were precisely located on the BORC curve, the X-ray data are far from the theoretical curve. This difference is the most significant for molecular and ionic complexes for which the OH and NH bond distances are lower than the value of 1.1 Å. The correlations in Fig. 5a,b illustrate the importance of precisely determining the proton position in the interpretation of the mechanism of proton transfer.
Exploration of the crystal data base does not confirm that the parameters presented in Fig. 4 can be used as a measure of the proton transfer degree. Despite systematic changes of the OCC, OCO and angles between benzoic acid–pyridine plane for the neutron structures presented in Fig. 4, it is not possible to confirm their general character taking into account all structures in the crystal data base. When the correlations of the bond lengths in the hydrogen bridge are not precise because of imprecise determination of the proton position, it is not possible to reproduce the correlation of other geometrical parameters not involved in the hydrogen bridge. Among the parameters shown in Fig. 4 only the C–O and C=O bond lengths can be correlated with the proton transfer degree. For the negative OH–NH values reflecting the molecular complexes with the proton closer to the donor oxygen atom, there are two branches reflecting the C–O and C=O bonds. When the OH–NH value reaches zero, reflecting the central location of the proton, both C–O and C=O bond lengths equalize to a common value corresponding to intermediate bond length. In analogous correlation performed for the benzoic acids30 only the part of correlation with a proton located at the donor oxygen was seen so it was impossible to follow the proton transition out of the donor oxygen atom. Although the correlation is influenced by packing effects in the crystal lattice equalization of the CO bond lengths of the carbonyl/carboxyl group can be used as an indicator if the proton is located at the donor or at the acceptor. The C–C bond length linking the COOH group with the aromatic ring is less sensitive to the proton transfer although some elongation of this bond is connected with the proton transfer. For the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine the C–C bond length changes according to the equation C–C = 0.1475(OH–NH) + 1.5297, R2 = 0.806. For all intermolecular OHN hydrogen bonds in benzoic acid complexes only a general tendency of elongation of the C–C bond is seen.
It can be expected that proton transfer influences also the geometry of acceptor part of the benzoic acid – pyridine complex. Especially sensitive to shifting of the proton to nitrogen is the CNC angle in pyridine.31 As the geometry of acceptor part of the intermolecular hydrogen bond complex is less sensitive to proton transfer, it is not possible to use the experimental CNC angle in the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine as the measure of proton transfer degree. This angle decreases with the elongation of the OH bond in the very narrow range of 119.903–120.855 degrees and the spreading of the values is too high to correlate it with the proton transfer degree.
The geometric parameters sensitive to the proton transfer in the intermolecular OHN hydrogen bond can be divided into three groups. The most sensitive are the bond lengths in the hydrogen bridge and for the structures with precise determination of the proton position they undergo systematic changes. The bond lengths in the vicinity of the hydrogen bridge changes systematically with proton transfer but these changes are not very significant and can be hidden by packing effects. There are also geometrical parameters changing systematically if proton transfer takes place in a molecule as a function of the temperature but are not characteristic for all complexes.
3. Thermal ellipsoid of proton in strong hydrogen bond in the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine
Intensive continuous absorption in the fingerprint region of the IR spectrum of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine15 shows that protonic vibrations are coupled with the low frequency vibrations of the hydrogen bridge.32 It could be expected that the longest axis of the thermal ellipsoid of the proton in the hydrogen bond is directed along the hydrogen bridge. In the case of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine the longest is the y axis of the proton thermal ellipsoid (0.09811 Å at 330 K). Shorter are the x and z axes (0.07253 and 0.05891 Å respectively at 330 K). None of them is directed along the hydrogen bond and the angles of the x, y and z to OH at 330 K are 35.29°, 54.97° and 86.31° respectively (Scheme 1). Shifting of the proton toward the centre of the hydrogen bridge with decreasing temperature is connected with changes of the thermal ellipsoids of the proton. To investigate if the proton shifting toward the hydrogen bond centre is connected with the changes of the proton thermal vibration, the temperature dependence of the corresponding parameters must be extracted. Fig. 6 shows the temperature evolution of the amplitude of the proton thermal vibration. The shortest z axis of the thermal ellipsoid of the proton vibration is used as a reference to express the changes of the shape of the ellipsoid. For the proton shifted to the hydrogen bridge centre both x and y axes increase comparing to z. Except for changes of the intensity of thermal vibrations, the thermal ellipsoid of proton in the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine changes the directions of the main axes. At 30 K when the proton is closest to the hydrogen bond centre the longest y axis is tilted up to 53.38° to the OH bond. The same angle for the x is 36.96°. The z axis almost perpendicular to OH at 330 K keeps its direction within 0.7°. | ||
| Fig. 6 Temperature changes of the proton thermal ellipsoid. (a) Values of the length of the proton thermal ellipsoid axes, (b) relative values of x and y to the shortest z axis length. | ||
 | ||
| Scheme 1 Thermal ellipsoid of proton with the x,y,z directions in the hydrogen bond. | ||
All these changes of the proton thermal ellipsoid show that for the central location of the proton the vibration along the hydrogen bridge is still not preferable and the protonic vibrations are coupled with the bridge bending β vibrations rather than with the stretching hydrogen bridge νσ vibrations and this coupling results as the continuous absorption in the fingerprint region of the IR spectrum.
4. Atom in Molecule analysis of strong OHN hydrogen bonds.
From an experimental structure with precise determination of the proton parameters in short hydrogen bond much information can be extracted. An example is the electron density theoretically calculated using the experimental structure. Theoretical electron density is not only equivalent with the experimental one but additionally delivers more parameters which brings information not accessible with different methods. The Atom in Molecules (AIM) theory developed by Bader,33 not only provides a picture of the electron cloud, but also quantitative parameters describing the electron density at the critical points and in the atomic basins. Such a detailed description of electron densities allows systematization of the chemical bonds as well as weak interactions. One of the most popular subjects of AIM analysis is the theoretical investigation of the hydrogen bond. In the literature there are many papers devoted to AIM analysis which correlate the electron density with geometric parameters of the hydrogen bridge34 so the changes of electron density upon the systematic change of the hydrogen bond strength are very well known. Less often done is a systematic analysis of theoretical electron density extracted from experimental structures with systematic proton moving along the hydrogen bridge and precise determination of the proton position. In a previous paper describing proton shifting in the hydrogen bridge of pyridinium 2,4-dinitrobenzoate collected neutron structures of the complexes with proton shifted in intermolecular OHN hydrogen bond were presented. For all of them the electron density at hydrogen bond critical points was calculated and correlated with hydrogen bond strength. This correlation was not complete because it did not include the complexes with proton moving from donor to the centre of the hydrogen bridge. The neutron structures of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine allow to extend this correlation so now it covers the full range of the proton transfer degree.In Fig. 7 the electron densities at the bond critical points between the OH and NH atoms are correlated with the difference between the OH and NH distances. The correlation extended to a broader range of the proton transfer is in accordance with this performed previously. Two branches for OH and NH bonds cross at the OH–NH equal −0.0468 Å and the electron density of 0.1579 au. The strength of the hydrogen bond in the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine changes from molecular complex at 330 K with the OH–NH equal −0.241 Å to the location of the proton very close to the OH–NH value of −0.0468 Å characteristic for central location of the proton. It is characteristic that the correlation has very general character. It is common for the complexes of phenols and benzoic acids so changes of electron density at bond critical points are independent of the proton donor molecule.
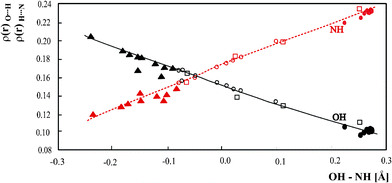 | ||
Fig. 7 (a) Relationship of ρ(r) at the OH and NH BCPs with OH – NH difference. • – pyridinium 2,4-dinitrobenzoate,16 o – 4-methylpyridine – pentachlorophenol complex,12 □ – 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct of benzene-1,2,4,5-tetracarboxylic acid6 and 4,4’-bipyridyl,5▾(red) – complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine. 2 adduct of benzene-1,2,4,5-tetracarboxylic acid6 and 4,4’-bipyridyl,5▾(red) – complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine. | ||
5. NBO analysis of the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine
A widely used model for describing the molecular electronic density is the Natural Bond Orbital (NBO) model basing on Löwdin’s concept of “natural” localized orbitals which provides the most accurate possible natural Lewis structure.35 In NBO analysis molecules are treated as being composed of atoms linked with bonds, localized electrons and nonbonding lone pairs representing the highest possible percentage of the electron density.36 This description of electron density is equivalent to the idealized Lewis structure37 in which electron density is localized on bonding orbitals and lone pairs with empty antibonding orbitals.Occupancy of the valence antibonding orbitals reflects departure from an idealized localized Lewis structure. Interactions between Lewis-type NBO (donor) and non-Lewis NBO (acceptor) is connected with transfer of charge and stabilization of the structure. Linear combination of the parent NBO with contribution of the antibonding orbital gives the Natural Localized Molecular Orbital (NLMO). The percentage of the parent NBO in NLMO gives an intrinsic measure of the accuracy of the natural Lewis structure. For typical organic compounds it is higher than 99%.
To better understand the nature of the hydrogen bond and the changes in proton donor and proton acceptor under the proton transfer, NBO analysis was carried out for the complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine as well as for the pyridinium 2,4-dinitrobenzoate. Proton donor and proton acceptor in both complexes belong to the same group of compounds so shifting of the proton causes changes in analogous molecular orbitals and combination of both complexes allows following the change of orbitals in full range of the proton transfer degree when proton moves from donor to acceptor. For both investigated compounds the proton transfer is reflected in the NLMOs localized on lone pairs of the proton donor oxygen in carboxyl group of the benzoic acid and the acceptor pyridine nitrogen. In Fig. 8 are shown the correlations of the percentage of the parent lone pairs in NLMOs. It is characteristic that delocalization of the NLMO orbitals is very significant and the percentage of parent NBO is decreased up to 80%. Among the lone pairs shown in Fig. 8 the most delocalized is the lone pair of the nitrogen (Fig. 8b) and the lone pair of the oxygen located on the direction of the hydrogen bond (Fig. 8a). Also delocalization of the orbital perpendicular to the COO plane (Fig. 8c) is very high. If the proton is shifted to the acceptor, delocalization of the lone pair of the donor oxygen decreases and delocalization of the lone pair of the nitrogen increases. It is characteristic that delocalization from the lone pair depicted in Fig. 8b takes place to the σ* of the hydrogen bonded proton and antibonding orbital of the lone pair of the pyridine N atom. Two other lone pairs of the donor carboxyl group become more delocalized under the proton transfer (Fig. 8c, d).
 | ||
| Fig. 8 % of parent NBO orbitals in delocalized NLMO. Particular orbitals are shown in the Figure. ▾ – complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine, • – pyridinium 2,4-dinitrobenzoate. | ||
Delocalization of the lone pairs is connected to the participation of the proton in the NLMO and if the molecular orbital is more delocalized the participation of the proton is more significant as it is depicted in Fig. 9. The highest participation of the proton is seen in the oxygen lone pair parallel to the hydrogen bond and in the lone pair of the pyridine nitrogen. The proton is present also in two other NLMO of the lone pairs on the oxygen. The regular changes of the proton participation in the NLMO show that also this orbitals reflect the shifting of the proton from donor to acceptor although the percentage of the proton in these orbitals is less than 1% (Fig. 9c,d).
 | ||
| Fig. 9 % of proton NBO orbital in delocalized NLMO of donor and acceptor. Particular orbitals are shown in the Figure. ▾ – complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine, • – pyridinium 2,4-dinitrobenzoate. | ||
Conclusions
Systematic changes of the hydrogen bond in complex of 3,5-dinitrobenzoaic acid with 3,5-dimethylpyridine complex as a function of the temperature combined with the changes observed in pyridinium 2,4-dinitrobenzoate presented previously allow investigation of the consequences of the proton transfer in benzoic acid–pyridine complexes which are one of the most typical systems with intermolecular hydrogen bond.Geometrical parameters sensitive to the proton transfer can be divided into three categories:
1. The geometrical parameters of the hydrogen bridge as OH, NH and O⋯N bond length which are common for all the intermolecular O⋯N hydrogen bond independent of the proton donor. The correlation linking OH and NH bond length is a result of constant valence of the proton when it moves from donor to acceptor and the balance of electron densities at the hydrogen bridge critical points.
2. C–O and C=O bond lengths in the carboxyl group equalize when proton shifts to acceptor and this change can be seen in correlation for all of benzoic acid complexes in the crystal data base.
3. Other geometrical parameters as C–C bond linking the COO−group with the aromatic ring or the COO− and angles change systematically with proton transfer but these changes are not common for all OHN hydrogen bonds formed by benzoic acids but characteristic for particular complex.
Transfer of the proton from donor to acceptor is connected with change of delocalization of the electron pairs at donor and acceptor even if they are perpendicular to the OHN plane. Also the changes of thermal ellipsoid are more complex than a simple elongation along the O⋯N direction.
Acknowledgements
The Wroclaw Center for Networking and Supercomputing is acknowledged for generous computer time. Experiments at the ISIS Neutron and Muon Source were supported by a beamtime allocation from the Science and Technology Facilities Council.References
- F. Hibbert and J. Emsley, Adv. Phys. Org. Chem., 1990, 26, 255 CrossRef CAS.
- W. W. Cleland and M. M. Kreevoy, Science, 1994, 264, 1887 CAS.
- (a) M. C. Etter, Acc. Chem. Res., 1990, 23, 120 CrossRef CAS; (b) M. C. Etter and G. M. Frankenbach, Materials, 1989, 1, 10 CAS; (c) F. Garcia-Tolledo, S. J. Geib, S. Goswani and A. D. Hamilton, J. Am. Chem. Soc., 1991, 113, 9265 CrossRef.
- G. A. Jeffrey, An Introduction to Hydrogen Bonding, Oxford University Press, 1997 Search PubMed.
- J. A. Cowan, J. A. K. Howard, G. J. McIntre, S. M.-F. Lo and J. D. Williams, Acta Cryst., 2005, B61, 724 CAS.
- J. A. Cowan, J. A. K. Howard, G. J. McIntre, S. M.-F. Lo and J. D. Williams, Acta Cryst., 2003, B59, 794 CAS.
- C. C. Wilson, Acta Cryst., 2001, B57, 435 CAS.
- C. C. Wilson, K. Shankland and N. Z. Shankland, Z. Kristallogr., 2001, 216, 303 CrossRef CAS.
- E. C. Kostanek and W. R. Busing, Acta Cryst., 1972, B28, 2454 Search PubMed.
- B. L. Rodrigues, R. Tellgreen and N. G. Fernandes, Acta Cryst., 2001, B57, 353 CAS.
- I. Olovsson, H. Ptasiewicz-Bąk, T. Gustafsson and I. Majerz, Acta Cryst., 2001, B57, 311 CAS.
- T. Steiner, I. Majerz and C. C Wilson, Angew. Chem., Int. Ed., 2001, 40, 2651 CrossRef CAS.
- M. G. Barrow, J. Am. Chem. Soc., 1956, 78, 5802 CrossRef.
- P. Huyskens and Th. Zeegers-Huyskens, J. Chim. Phys., 1964, 61, 84 Search PubMed.
- L. Sobczyk, T. Lis, Z. Olejnik and I. Majerz, J. Mol. Struct., 2000, 552, 233 CrossRef CAS.
- I. Majerz and M. J. Gutmann, J. Phys. Chem. A, 2008, 112, 9801 CrossRef CAS.
- D. A. Keen, M. J.Gutmann and C.C. Wilson, J. Appl. Crystallogr., 2006, 39, 714 CrossRef CAS.
- M. J. Gutmann, SXD2001, ISIS Facility, Rutherford Appleton Laboratory, Oxfordshire England, 2005 Search PubMed.
- C. C. Wilson, J. Appl. Crystallogr., 1997, 30, 184 CAS.
- G. M. Sheldrick, SHELXL97, University of Göttingen, Germany, 1997 Search PubMed.
- Gaussian 03, Revision A.9, Gaussian, Inc., Pittsburgh PA, 2004.
- F. Biegler-König, J. Schönbohm and D. Bayles, J. Comput. Chem., 2001, 22, 545 CrossRef.
- (a) G. te Velde, F. M. Bickelhaupt, S. J. A. van Gisbergen, C. Fonseca Guerra, E. J. Baerends, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931 CrossRef CAS; (b) C. Fonseca Guerra, J. G. Snijders, G. te Velde and E. J. Baerends, Theor. Chem. Acc., 1998, 99, 391 Search PubMed.
- H.-H. Limbach, M. Pietrzak, S. Sharif, P. M. Tolstoy, I. G. Shenderovich, S. N. Smirnov, N. S. Golubev and G. S. Denisov, Chem.–Eur. J., 2004, 10, 5195 CrossRef CAS.
- (a) L. Pauling, J. Am. Chem. Soc., 1947, 69, 542 CrossRef CAS; (b) I. D. Brown, Acta Cryst., 1992, B48, 553 CAS.
- (a) I. Majerz, Z. Malarski and L. Sobczyk, Chem. Phys. Lett., 1997, 274, 361 CrossRef CAS; (b) I. Majerz and A. Koll, Acta Cryst., 2004, B60, 406 CAS; (c) E. Kwiatkowska, I. Majerz and A. Koll, Chem. Phys. Lett., 2004, 398, 130 CrossRef CAS; (d) I. Majerz and I. Olovsson, J. Mol. Struct., 2010, 976, 11 CrossRef CAS.
- P. W. Borthwick, Acta Cryst, 1980, B36, 628 CAS.
- F. H. Allen, Acta Cryst., 2002, B58, 380 CAS.
- Sunil Varughese and V. R. Pedireddi, Tetrahedron Lett., 2005, 46, 2411 CrossRef CAS.
- C. L. Nygren, C. C. Wilson and J. F. C. Turner, J. Phys. Chem. A, 2005, 109, 2586 CrossRef CAS.
- T. Steiner, Angew. Chem., Int. Ed., 2002, 41, 48 CrossRef CAS.
- (a) G. Zundel, Ferroelectrics, 1999, 220, 221 CrossRef CAS; (b) G. Zundel, J. Mol. Struct., 2000, 552, 81 CrossRef CAS.
- R. F. W. Bader, Atoms in Molecules: A Quantum TheoryOxford University Press, New York, 1990 Search PubMed.
- (a) U. Koch and P. L. A .Popelier, J. Phys. Chem., 1995, 99, 9747 CrossRef CAS; (b) P. L. A. Popelier, J. Phys. Chem., 1998, 102, 1873 CAS; (c) I. Alkorta and J. Elguero, J. Phys. Chem. A, 1999, 103, 272 CrossRef CAS; (d) M. V. Vener, A. V. Mannaev, A. N. Egorova and V. G. Tsirelson, J. Phys. Chem. A, 2007, 111, 1155 CrossRef CAS; (e) L. F. Pacios, O. Galvez and P. C. Gomez, J. Chem. Phys., 2005, 122, 214307 CrossRef CAS; (f) S. J. Grabowski, W. A. Sokalski and J. Leszczynski, J. Phys. Chem. A, 2006, 110, 4772 CrossRef CAS; (g) E. Espinosa, I. Alkorta, J. Elguero and E. Molins, J. Chem. Phys., 2002, 117, 5529 CrossRef CAS; (h) E. Espinosa, E. Molins and C. Lecomte, Chem. Phys. Lett., 1998, 285, 170 CrossRef CAS; (i) E. Espinosa, I. Alkorta, I. Rozas, J. Elguero and E. Molins, Chem. Phys. Lett., 2001, 336, 457 CrossRef CAS; (j) E. Espinosa, C. Lecomte and E. Molins, Chem. Phys. Lett., 1999, 300, 745 CrossRef CAS; (k) E. Kwiatkowska and I. Majerz, J. Phys. Org. Chem., 2008, 21, 867 CrossRef CAS.
- P.-O. Löwdin, Phys. Rev., 1955, 97, 1471 CrossRef.
- F. Weinhold and C. R. Landis, Chem. Educ. Res. Pract. Eur., 2001, 2, 91 CAS.
- G. N. Lewis, J. Am. Chem. Soc., 1916, 38, 762 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
