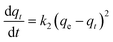DOI:
10.1039/C1RA00153A
(Paper)
RSC Adv., 2011,
1, 625-631
One-pot preparation of amine-rich magnetite/bacterial cellulose nanocomposite and its application for arsenate removal†
Received
9th May 2011
, Accepted 24th June 2011
First published on 24th August 2011
Abstract
Surface functionalization of bacterial cellulose (BC) nanofibrils with aminated magnetite nanoparticles (MH) was carried out by one-pot solvothermal reaction of 1,6-hexanediamine, FeCl3·6H2O and BC pellicle in ethylene glycol at 200 °C for 6 h. The presence of amine surface-functionalized magnetite nanoparticles on the surface of cellulose nanofibrils not only significantly enhanced the thermal and mechanical properties but also the amine content of the nanostructured bacterial cellulose pellicle. Approximately 10.5 mmol g−1 of amine was measured in the aminated magnetite-BC nanocomposite (BC@MH) which is about 14.3 fold higher than its counterpart (MH) prepared in the absence of BC pellicle. The never-dried BC@MH nanocomposite demonstrated a high adsorption capacity towards As(V) ions because of its high iron-oxide and amine content. Approximately 90 mg of As(V) can be adsorbed per gram of BC@MH.
Introduction
In recent years, nanomaterials have attracted enormous research interest due to unique optical, catalytic and structural properties. On the other hand, increasing environmental related concerns and the development of green chemistry methodologies have directed development of novel nanomaterials based on renewable resources. Cellulose is considered as a prime unlimited alternative, sustainable and renewable biopolymer resource, which holds very attractive properties such as biocompatibility, biodegradability, thermal and chemical stability.1 Particularly, lignin, hemicellulose and pectin free bacterial cellulose (BC) produced by bacteria such as Gluconacetobacter xylinum possesses superior tensile strength, crystallinity, elasticity, surface area, and water holding capacity because its nanofibrils are 200-times finer than those of plant cellulose.2,3 BC is composed of ribbon shaped porous nanofibrils of 20–30 nm width that are connected with each other by a three-dimensional network. Surface functional groups present in the cellulose have been used for many environmental applications.4
The adsorption method to remove metal ions is proven to be the most efficient method over coagulation,5precipitation,6oxidation,7 ion exchange,8 and reverse osmosis9 processes. It is also well-known that adsorbents made from natural polymers have broad applications in the food,10drug11 and medical fields.12 Among heavy metals, arsenic is considered as an element of interest in terms of worldwide water pollution and human health. Long-term or high dose consumption of arsenic dissolved in ground and surface waters is toxic and has been linked to skin, liver, kidney, lungs and bladder cancer. The World Health Organization (WHO) has already revised the maximum concentration limit for arsenic in drinking water from 50 μg L−1 to 10 μg L−1. In natural water, arsenic is available mostly as arsenite, As(III) and arsenate, As(V). Magnetite nanoparticle (MNPs) such as nano scale zero-valent iron,13,14 maghemite,15hematite, goethite,16 monodisperse Fe3O4 nanocrystals, Fe(III)-loaded resin,17iron oxide-coated sand,18iron trapped in Ca-polygalacturonate,19iron loaded cotton cellulose,4 hydrogel magnetite nanocomposite20 and iron coated pottery granules21 have been used as effective adsorbents for arsenic removal. Recently, several amine surface functionalized materials have also been demonstrated to be very effective adsorbents for arsenic removal.22,23 Thus, it can be expected that the synergistic effect on arsenic adsorption by iron oxide-based materials with surface functionalized amine groups will greatly enhance the arsenic removal performance of surface aminated magnetite nanoparticles. However, the magnetite nanoparticles tend to aggregate because of the interparticle dipolar forces. BC nanofibril networks have been used as a templates for the non-aggregated growth of magnetic particles.24 The prepared magnetite nanocomposites have synergistic benefits from organic (flexibility, strength) and inorganic (adsorption, magnetic properties) components.
Herein, a facile one-pot solvothermal method is proposed to prepare an aminated magnetite nanoparticles impregnated BC pellicle (BC@MH). BC pellicle is solvothermally treated in the homogenous solution used for the preparation of surface amine-functionalized magnetite nanoparticles (MH). The formation of MH with much smaller sizes on the surface of BC nanofibrils will be expected because the high specific surface area of BC pellicle will provide excessive nucleation sites for the magnetite nanoparticles to grow during the solvothermal process. In this work, the effects of nanofibrous structure of BC on the size of MH formed and the amine content in the BC@MH nanocomposite were studied. The physical properties of BC@MH such as saturation magnetization, surface charge, thermal stability, and mechanical properties were characterized. Furthermore, the application of the as-prepared cost effective amine-rich BC@MH nanocomposite for arsenate adsorption was also studied.
Result and discussion
Magnetite-bacterial cellulose nanocomposite preparation and characterization
A schematic diagram of the MH@BC preparation is shown in Scheme 1. The original white colored BC pellicle turned black-brown after soaking in ethylene glycol solution containing FeCl3·6H2O, sodium acetate and 1,6-hexanediamine for 24 h, which also ensures the uniform distribution of amine and ferric source inside the BC nanofibril nanostructure. After heating in a sealed autoclave at 200 °C for 6 h, the color of the pellicle turned to black confirming the formation of magnetite nanoparticles and the intactness of the pellicle. BC@MH in pellicle form can be easily collected by applying an external magnetic field.
 |
| | Scheme 1 Schematic illustration of the preparation of amine-functionalized magnetic BC nanocomposite (BC@MH). | |
The nanostructure of BC observed by field-emission scanning electron microscopy (FE-SEM) (Fig. 1a) shows a porous 3-D network of nanofibrils with the diameter of the fibrils in the range of 20–30 nm randomly but tightly interwoven. The smooth surface of nanofibrils was decorated with aggregated nanoparticles (Fig. 1b inset and Fig. 1c) when FeCl3·6H2O was included in the reaction mixture. In addition to the nanoparticles aggregated along the surface of nanofibrils, granules of nanoparticle aggregates were observed in the inter-nanofibrils space. In comparison with magnetite nanoparticles (MH) prepared under the same conditions but without using BC pellicle as a template (Fig. 1d), the size of nanoparticles formed on the surface of nanofibrils in BC@MH are much smaller. Evidently, the very high surface area of BC nanofibrils provides a significant amount of nucleation sites for ferric ions to grow into magnetite nanoparticles. Since the transport of ferric ions through the tightly interwoven nanostructure of BC is a slow diffusion process, the continuous supply of ferric ions to the growing magnetite grafted on the surface of nanofibrils will be limited. Thus, the size of magnetite formed on the nanofibrils will be significantly reduced. Approximately 50% w/w of Fe content was detected on the surface of nanofibrils and nanoparticles aggregates in BC@MH by energy dispersive X-ray (EDX) investigation (see EDX results in the ESI, Table S1†). The theoretical Fe content in magnetite (Fe3O4) is around 72%. The lower Fe content detected by EDX may result from the presence of cellulose and other contaminants. The water-holding capacity of BC@MH was slightly reduced as the water content decreased from 98.9% w/w of the original BC to 98% w/w.
 |
| | Fig. 1
FE-SEM image of (a) bacterial cellulose (b) top view of aminated magnetite-BC nanocomposites (BC@MH) (c) cross-section of BC@MH and (d) TEM image of amine-functionalized MNPs (MH) prepared in the absence of BC pellicle. | |
Ninhydrin is usually used to determine the surface amine groups quantitatively by forming a Ruhemann's purple colored molecule in solution25 (see ESI, Fig. S1†). Based on the strong color intensity developed in the reaction solution, the presence of amine groups in the MH and BC@MH are confirmed. The amine content was determined by retro-titration26 to be 0.7 mmol g−1 and 10.5 mmol g−1 for MH and BC@MH, respectively. The 14 fold higher amine content of BC@MH probably results from the smaller aminated magnetite nanoparticles formed on the surface of the nanofibrils. The surface charge of MH, BC and BC@MH were measured as the zeta potential in the range of pH 2–10. As shown in Fig. 2, the zeta potential decreases with pH. BC@MH has a zeta potential much higher than that of BC in the acidic pH range. The point of zero charge (PZC) increases from pH 5.2 for BC to 7.0 for BC@MH. In other words, BC@MH is positively charged at pH values lower than 7.0. Evidently, the higher zeta potential of BC@MH results from its cationic amine groups. When compared with MH, the effect of pH on zeta potential of BC@MH and BC is much less. MH demonstrated a much higher positive zeta potential and lower negative zeta potential than that of BC@MH. Since the size and morphology of particles will significantly affect the zeta potential measured by zeta potential analyzer, the zeta potential measured for nearly spherical MH with size about 30 nm and disrupted BC@MH nanocomposite with size in the micrometre range employed for analysis cannot be compared with each other on the same basis. The discrepant size and morphology between MH and BC@MH may also explain why BC@MH has higher amine content than that of MH but results in a lower zeta potential in the acidic pH range.
 |
| | Fig. 2 Effect of pH on zeta potential of amine-functionalized MNPs (MH), bacterial cellulose (BC) and aminated magnetite-BC nanocomposites (BC@MH). | |
XRD was also employed to investigate the nature of magnetite particles in BC@MH nanocomposite. The diffraction peaks present in Fig. 3 shows that BC has two typical diffraction peaks at a 2 θ value of 18° and 23°, which corresponds to the cellulose crystal structure.27 On the other hand, diffraction peaks with 2 θ value at 30.0°, 35.3°, 42.8°, 56.9° and 62.5° along with cellulose peak at 18° and 23° were observed for BC@MH. The positions and relative intensities of diffraction peaks confirmed the presence of face centered cubic (fcc) structure of Fe3O4 magnetite (JCPDS, 75–1610)28 in BC@MH. Thus, the MH and magnetic nanoparticles in BC@MH are mainly Fe3O4 magnetite and its crystalline structure was not affected by the presence of BC pellicle. The SQUID magnetization curve of MH and BC@MH demonstrated no hysteresis and completely reversible at room temperature. Neither coercivity nor remanence was noticed, that reflects a typical characteristic of superparamagnetic nature. It can be seen that MH possessed a higher saturation magnetization (∼70 emu g−1) than that BC@MH (18.61 emu g−1) (see ESI, Fig. S2†). The loss of saturation magnetization was expected because of the presence of an appreciable amount of nonmagnetic component in the BC@MH. However, it still guarantees the excellent response of the BC@MH towards an external magnetic field as demonstrated by its assembly onto the side of a bottle (see ESI, Video S1†).
TGA was used to examine the thermal stability of the prepared BC@MH. As shown in Fig. 4, the first phase weight loss of BC and BC@MH occurred at temperatures below 200 °C, which was associated with the evaporation of approximately 5% and 7% residual water.29 Second stage weight loss was observed in the range of 300–350 °C which resulted from the pyrolytic decomposition of cellulose subunits. The third stage of weight loss occurred around 640 °C. Since MH shows no significant weight loss during the entire heating process, the third stage weight loss is probably due to the degradation of carbonaceous materials formed under the magnetite nanoparticles coverage in BC@MH during the heating process.30 Under N2 atmosphere with heating up to 650 °C, 20% and 45% of BC and BC@MH remained. The excess amount of remains obtained for BC@MH indicates that the dried BC@MH consists of at least 25% w/w magnetite. The mechanical properties of the BC could be significantly improved by incorporating MH as shown in Fig. S3,† that the strain can be increased to 23.74% before BC@MH breaks at stress of 1100 kPa. On the other hand, BC itself is much more brittle it breaks at strain of 0.53% under 700 kPa stress. In other words, the presences of MH nanoparticles makes the BC@MH structure become more stretchable.
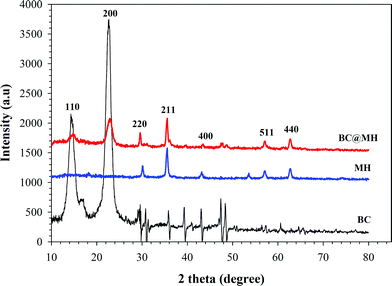 |
| | Fig. 3
Powder X-ray diffractograms of amine-functionalized magnetite particles (MH), bacterial cellulose (BC) and aminated magnetite-BC nanocomposites (BC@MH). | |
 |
| | Fig. 4
TGA curves of bacterial cellulose (BC), amine-functionalized magnetite (MH) and aminated magnetite-BC nanocomposites (BC@MH). | |
| |  | (1) |
where qe and qt are the amount of As(V) adsorbed per unit mass of the adsorbent (mg g−1) at equilibrium and time t, respectively, and k1 is the rate constant of adsorption (min−1). The pseudo-second order model31 can be expressed as: | | 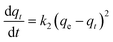 | (2) |
where qe is the maximum adsorption capacity (mg g−1); k2 is rate constant of the pseudo-second order equation (g mg−1 min−1); qt is the amount of As(V) adsorbed per unit mass of the adsorbent (mg g−1). The equilibrium value of qe and rate constant of these two kinetic models were obtained by using non-linear regression and shown in Table 1. It seems that the pseudo-second order kinetic model fitted the data well because of the higher correlation coefficients (r2).32–34
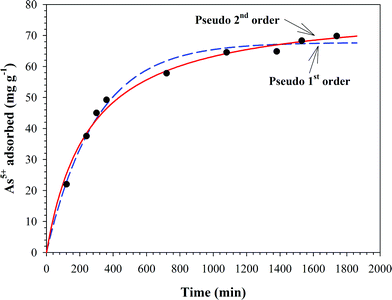 |
| | Fig. 5 Time course of As(V) adsorption onto BC@MH at 25 °C, initial As(V) concentration of 7 mg L−1, pH 4, and shaking rate of 150 rpm. | |
Table 1 Kinetic parameters of As(V) adsorption onto the BC@MH at 25 °C
| Kinetic model |
Parameter constant |
Value |
| Pseudo-first order |
q
e (mg g−1) |
67.759 |
|
k
1 (min−1) |
0.0034 |
|
r
2
|
0.9921 |
| Pseudo-second order |
q
e (mg g−1) |
79.415 |
|
k
2(g mg−1 min−1) |
4.921 × 10−5 |
|
r
2
|
0.9940 |
Effect of pH on As(V) removal
The arsenate adsorption behaviors in different pH environments were studied. Fig. 6 shows that the uptake capacity of BC@MH at 25 °C for 30 h and initial As(V) concentration of 7 mg L−1 decreased from 85 mg g−1 at pH 3 to 30 mg g−1 at pH 9. The higher uptake capacity obtained at pH lower than the PZC of BC@MH (pH 7.0) is probably due to the electrostatic interaction between the positively charged BC@MH and negative arsenate species because arsenate exists as H3AsO4, H2AsO4−, HAsO42−, and AsO43− species in aqueous solution. The H2AsO4− form is the main species in solution at pH 3 to 6, whereas HAsO42− and AsO43− appear at pH above 8.23,35 Therefore, at pH < pHPZC the positively charged BC@MH is favorable for arsenate adsorption. By increasing pH, the amine groups in BC@MH becomes less protonated thus decreases the net positive charge of BC@MH that leads to a decreased arsenate uptake.
 |
| | Fig. 6 Effect of initial pH on the sorption of As(V) adsorption onto BC@MH at 25 °C for 30 h, initial As(V) concentration of 7 mg L−1 and shaking rate of 150 rpm. | |
| |  | (3) |
In eqn (3)qe is the amount of arsenate ion adsorbed at equilibrium (mg g−1), Q0 is the maximal adsorption capacity (mg g−1), Ce is the equilibrium concentration (mg L−1) and b is the adsorption constant (L mg−1). Fig. 7 shows the adsorption isotherms at pH 3, 5, 7 and 9. The isotherms can be well-fitted with the Langmuir model as demonstrated by the square of correlation coefficients close to unity (r2 > 0.96) in Table 2. The maximal adsorption capacity decreases from 90 mg g−1 at pH 3.0 to 31 mg g−1 at pH 9.0. The higher adsorption capacity at lower pH is due to the fact that the amine groups in BC@MH were protonated at low pH to become more positively charged which will favorably interacted with arsenate species as shown in eqn (4):22| | | (BC@MH)–NH3+ + H2AsO4− ⇔ (BC@MH)–NH3·H2AsO4 | (4) |
 |
| | Fig. 7 Adsorption isotherm of As(V) onto BC@MH and MH at 25 °C for 30 h and shaking rate of 150 rpm. | |
Table 2 Effect of pH on parameter of adsorption isotherm of As(V) onto BC@MH at 25 °C
| Sample |
Isotherm constant |
pH 3 |
pH 5 |
pH 7 |
pH 9 |
|
BC@MH
|
Q
0 (mg g−1) |
90.403 |
63.005 |
48.298 |
30.988 |
|
b (L mg−1) |
0.247 |
0.832 |
0.442 |
1.243 |
|
r
2
|
0.998 |
0.972 |
0.976 |
0.963 |
In comparison with MH, BC@MH shows the maximum adsorption capacity of 48.29 mg g−1 at pH 7.0 which is approximately 12.6 fold higher than that of MH (3.83 mg g−1). The higher adsorption capacity of BC@MH is presumably due to its higher amine content and much smaller size of aminated magnetite formed in BC@MH nanostructure.
The adsorption capacity of BC@MH towards As(V) was compared with that reported by other investigators using various adsorbents in Table 3. Under the same equilibrium arsenate concentration, the adsorption capacity of BC@MH is approximately 2.4 fold higher than iron oxide-based adsorbents and 1.2 fold higher than aminated nano-fibers. The repeated use of BC@MH for As(V) adsorption has also been carried out by regenerating As(V) loaded BC@MH in 0.1 M NaOH for 24 h.37 After 5 repeated uses, no significant As(V) adsorption capacity decrease was observed for 7 ppm initial concentration of As(V) solutions at 25 °C, pH 3 (see ESI, Fig. S4†).
Table 3 Comparison of As(V) adsorption capacity with previously reported adsorbents
| Adsorbent |
As(V) (mg g−1)a |
Reference |
|
Equilibrium concentration of As(V) at 6 ppm, pH 7.
|
|
BC@MH
|
36.49 |
this study |
|
Magnetite reduced graphene oxide (M-RGO) |
4.20 |
36
|
| Mechanochemical maghemite |
15.0 |
16
|
| Commercial maghemite |
5.80 |
16
|
| Sol–gel maghemite |
7.00 |
16
|
| Aminated fiber |
31.0 |
24
|
|
Iron coated pottery granules (ICPG) |
3.6 |
22
|
Experimental
Materials
Bacterial cellulose pellicle was obtained by statically culturing Acetobacter xylinum (BCRC12334) in Hestrin–Schramm (HS) medium at 27 °C for 7 days. The harvested BC pellicle was soaked in 1 N NaOH and heated to 90 °C for 20 min followed by washing thoroughly with deionized (DI) water 3 times and washing until neutral pH was achieved. The rest of chemicals were analytical grade and used without any further treatment.
Surface amine-functionalized MNPs were synthesized according to the procedure used by Wang et al.28 Briefly, anhydrous sodium acetate (1.6 g) and FeCl3·6H2O (0.8 g) were dissolved in ethylene glycol (24 mL) with vigorous stirring at 50 °C to give an orange solution. When 1,6-hexanediamine (7 mL) was added, the solution turned dark-orange. Then magnetite nanoparticle composites BC@MH were synthesized by soaking 3.0 g never-dried BC pellicle into the above solution for 24 h before solvothermal reaction was carried out at 200 °C for 6 h. After cooling to room temperature, the BC magnetite composites were collected from the solution by employing a magnet and was rinsed with DI water followed by ethanol each for 3 times to remove the remaining chemicals. The obtained magnetite bacterial cellulose nanocomposites was designated as BC@MH and kept in DI water for future use.
The BC@MH was tested for the adsorption of As(V). Typically, 20 mg (approximately 0.2 × 0.2 cm) of BC@MH was added to 5 mL of arsenate solution (Na2HAsO4·7H2O) in different concentrations (2, 4, 6, 8, 10 mg L−1) and the pH was adjusted by 0.1 N HCl or NaOH solutions. The adsorption was carried out whilst shaking at room temperature for 30 h. The supernatant of the suspension obtained by filtering through a 0.45 μm membrane filter was immediately analyzed by inductively coupled plasma spectrophotometer to determine the remaining ion concentration. To regenerate BC@MH for repeated use, As(V) was desorbed from BC@MH (1% w/v) by shaking in 0.1 M NaOH for 24 h. After washing thoroughly with DI water, the regenerated BC@MH was loaded for the next cycle of As(V) adsorption.
Characterization
Transmission electron microscopy (TEM) images were taken by using a Hitachi H-800 transmission electron microscope (Japan). Field emission scanning electron microscopy (FE-SEM) images of the samples were obtained by scanning electron microscope (JEOL, JSM-6500 LV). Critical point drying (Samdri PVT-3D) was employed to prepare the BC and BC@MH for FE-SEM observation. The present of amine in MH and BC@MH were determined by using Ninhydrin reagent.25 The amine content in the sample was determined by the retro-titration method. Briefly, to 25 mL solution of 0.01 M HCl, 50 mg sample was added. The mixture was shaken for 2 h at room temperature, after separation, 5 mL supernatant was titrated with 0.01 N NaOH. The concentration of amine group was calculated by: | |  | (5) |
where CHCl is the concentration of HCl solution (mmol L−1), CNaOH is the concentration of NaOH solution (mmol L−1), VHCl is the volume of HCl solution (L), VNaOH is the volume of NaOH used in the titration of non-reacted acid's excess (L) and m is the sample mass (g). The surface charge BC, MH, and BC@MH were determined by zeta potential analyzer (Zetasizer 2000, Malvern Instrument, Malvern, UK). The X-ray diffraction (XRD) measurement was performed on Rigaku D/MAX-B X-ray diffractometer by using Copper K-alpha (Cu-Kα) radiation. The operation voltage and current were kept at 40 kV and 100 mA, respectively. The magnetic property of magnetic nanoparticles (MNPs) was studied by a superconducting quantum interference device (SQUID, LakeShore 7307) magnetometer. TGA was carried out with thermogravimetry-differential thermogravimetry (TG-DTG) analyzer: Perkin Elmer, Diamond TG/DTA equipped with a platinum cell. Samples (6–8 mg, dry weight) were heated from ambient temperature to 700 °C under nitrogen flow (20 mL min−1) at a constant rate of 10 °C min−1. The BC content in the BC@MH was estimated by the percentage of weight loss from the corresponding TGA curves. Mechanical testing was performed on a testometric test machine (M500-25AT) using a load cell of 10 kg operating at a deformation rate of 100 mm s−1 for BC and BC@MH, and was performed under ambient conditions. Five samples were tested for each datum. Tensile-strain and elongation to break were calculated using the Win test software. Inductively coupled plasma spectrophotometer (Horiba Jobin Yvon, ICP-AES JY2000) was used for metal ion concentration determination.
Conclusions
The amine-functionalized magnetite nanoparticles deposited onto nanofibrils of BC pellicle can be easily prepared via one-pot thermal synthesis in solution phase at temperatures around 200 °C. The presence of cellulose nanofibers facilitates the magnetite nanoparticles formation and deposition along the cellulose nanofibrils. In comparison with the magnetite particles prepared without using BC as the template, the amine-rich BC@MH nanocomposite can be used as an efficient and convenient recyclable absorbent for arsenate removal. In addition, the high surface area, good chemical and mechanical stability, and the rich surface amine groups of BC@MH nanocomposites will also find other potential applications such as in the fields of catalysis, drug delivery, and enzyme immobilization.
Acknowledgements
The authors are grateful for the financial support from the Directorate General of Higher Education, Ministry of National Education and Republic of Indonesia which provides a scholarship for Iryanti. F. Nata (academic staff of Lambung Mangkurat University, Indonesia) during her study at the National Taiwan University of Science and Technology (NTUST), Taiwan.
References
- M. Seifert, S. Hesse, V. Kabrelian and D. Klemm, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 463–470 CrossRef CAS.
- W. K. Czaja, D. J. Young, M. Kawecki and R. M. Brown, Biomacromolecules, 2006, 8, 1–12 Search PubMed.
- E. J. Vandamme and W. Soetaert, FEMS Microbiol. Rev., 1995, 16, 163–186 Search PubMed.
- Y. Zhao, M. Huang, W. Wu and W. Jin, Desalination, 2009, 249, 1006–1011 Search PubMed.
- T. Yuan, Q. Luo, J. Hu, S. Ong and W. Ng, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2003, 38, 1731–1744 Search PubMed.
- Y. Jia, L. Xu, Z. Fang and G. P. Demopoulos, Environ. Sci. Technol., 2006, 40, 3248–3253 CrossRef CAS.
- M. Zaw and M. T. Emett, Toxicol. Lett., 2002, 133, 113–118 CrossRef CAS.
- J. Kim and M. M. Benjamin, Water Res., 2004, 38, 2053–2062 CrossRef CAS.
- R. Y. Ning, Desalination, 2002, 143, 237–241 CrossRef CAS.
- A. Kurosumi, C. Sasaki, Y. Yamashita and Y. Nakamura, Carbohydr. Polym., 2009, 76, 333–335 Search PubMed.
- A. Stoica-Guzun, M. Stroescu, F. Tache, T. Zaharescu and E. Grosu, Nucl. Instrum. Methods Phys. Res., Sect. B, 2007, 265, 434–438 Search PubMed.
- C. J. Grande, F. G. Torres, C. M. Gomez and M. Carmen Bañó, Acta Biomater., 2009, 5, 1605–1615 CrossRef CAS.
- S. R. Kanel, J.-M. Grenèche and H. Choi, Environ. Sci. Technol., 2006, 40, 2045–2050 CrossRef CAS.
- G. Jegadeesan, K. Mondal and S. B. Lalvani, Environ. Prog., 2005, 24, 289–256 Search PubMed.
- T. Tuutijärvi, J. Lub, M. Sillanpää and G. Chen, J. Hazard. Mater., 2009, 166, 1415–1420 CrossRef CAS.
- J. Giménez, M. Martínez, J. D. Pablo, M. Rovira and L. Duroc, J. Hazard. Mater., 2007, 141, 575–580 CrossRef CAS.
- T. Matsunaga, Trends Biotechnol., 1991, 9, 91–95 Search PubMed.
- O. S. Thirunavukkarasu, T. Viraraghavan and K. S. Subramanian, Water, Air, Soil Pollut., 2003, 142, 95–111 Search PubMed.
- S. Deiana, L. Deiana, A. Premoli and C. Senette, Plant Physiol. Biochem., 2009, 47, 615–622 Search PubMed.
- K. S. Sivudu and K. Y. Rhee, Colloids Surf., A, 2009, 349, 29–34 Search PubMed.
- L. Donga, P. V. Zininb, J. P. Cowenb and L. C. Mingb, J. Hazard. Mater., 2009, 168, 626–632 CrossRef CAS.
- K. C. M. Kwok, V. K. C. Lee, C. Gerente and G. McKay, Chem. Eng. J., 2009, 151, 122–133 Search PubMed.
- S. Deng, G. Yu, S. Xie, Q. Yu, J. Huang, Y. Kuwaki and M. Iseki, Langmuir, 2008, 24, 10961–10967 Search PubMed.
- R. T. Olsson, M. A. S. A. Samir, G. Salazar-Alvarez, L. Belova, V. Ström, L. A. Berglund, O. Ikkala, J. Nogués and U. W. Gedde, Nat. Nanotechnol., 2010, 5, 584–588 CrossRef CAS.
- H. Rosen, Arch. Biochem. Biophys., 1957, 67, 10–15 CAS.
- J. O. Karnitz, L. V. A. Gurgel, J. C. P. de Melo, V. R. Botaro, T. M. S. Melo, R. P. de Freitas Gil and L. F. Gil, Bioresour. Technol., 2007, 98, 1291–1297 CrossRef.
- K.-Y. Lee, J. J. Blaker and A. Bismarck, Compos. Sci. Technol., 2009, 69, 2724–2733 CrossRef CAS.
- L. Wang, J. Bao, L. Wang, F. Zhang and Y. Li, Chem.–Eur. J., 2006, 12, 6341–6347 CrossRef CAS.
- H. S. Barud, A. M. de Araújo Júnior, D. B. Santos, R. M. N. de Assunção, C. S. Meireles, D. A. Cerqueira, G. Rodrigues Filho, C. A. Ribeiro, Y. Messaddeq and S. J. L. Ribeiro, Thermochim. Acta, 2008, 471, 61–69 Search PubMed.
- L. Zhao, Z. Bacsik, N. Hedin, W. Wei, Y. Sun, M. Antonietti and M.-M. Titirici, ChemSusChem, 2010, 3, 840–845 CrossRef CAS.
- Y. S. Ho and G. McKay, Water Res., 2000, 34, 735–742 CrossRef CAS.
- S. Zhang, H. Niu, Y. Cai, X. Zhao and Y. Shi, Chem. Eng. J., 2010, 158, 599–607 CrossRef CAS.
- Y. Kim, C. Kim, I. Choi, S. Rengaraj and J. Yi, Environ. Sci. Technol., 2004, 38, 924–931 Search PubMed.
- M. Jang, E. W. Shin, J. K. Park and S. I. Choi, Environ. Sci. Technol., 2003, 37, 5062–5070 CrossRef CAS.
- L. Dambies, E. Guibal and A. Roze, Colloids Surf., A, 2000, 170, 19–31 Search PubMed.
- V. Chandra, J. Park, Y. Chun, J. W. Lee, I.-C. Hwang and K. S. Kim, ACS Nano, 2010, 4, 3979–3986 CrossRef CAS.
- R. K. Xu, G. Yu, L. M. Kozak and P. M. Huang, Geoderma, 2008, 148, 55–62 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Video V1 Response of aminated magnetite-BC nanocomposites (BC@MH) towards an external magnetic field, Table T1 Energy dispersive X-ray (EDX) results of BC and BC@MH, Fig. S1 Ninhydrin test for amine group assay (a) amine-functionalized MNPs (MH), (b) aminated magnetite-BC nanocomposites (BC@MH) for ten times dilution, Fig. S2 Room temperature magnetization curves of amine-functionalized MNPs (MH) and aminated magnetite-BC nanocomposites (BC@MH). Fig. S3 Stress-strain curve of bacterial cellulose (BC) aminated magnetite-BC nanocomposites (BC@MH) and insert: dry BC and dry BC@MH. Fig. S4. The adsorption performance of BC@MH during 5 repeated used for adsorbing 7 ppm As(V) solutions at 25 °C for 30 h at pH 3. See DOI: 10.1039/c1ra00153a |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here.