Synthesis, characterization and thermal-property measurements of ionic semi-clathrate hydrates formed with tetrabutylphosphonium chloride and tetrabutylammonium acrylate
Hiroki
Sakamoto
a,
Kai
Sato
a,
Kuniaki
Shiraiwa
a,
Satoshi
Takeya
b,
Masahiro
Nakajima
c and
Ryo
Ohmura
*a
aDepartment of Mechanical Engineering, Keio University, Yokohama 223-8522, Japan. E-mail: rohmura@mech.keio.ac.jp
bNat. Inst. Adv. Ind. Sci. Technol. (AIST), Tsukuba 305-8565, Japan
cHeat & Fluid Dynamics Department, IHI Corporation, Yokohama 235-8501, Japan
First published on 8th August 2011
Abstract
This paper reports an experimental study on the formation of the two new semi-clathrate hydrates with tetrabutylphosphonium chloride (TBPC) and tetrabutylammonium acrylate (TBAAc). The hydrate formation was demonstrated by the measurements of temperature-composition phase diagrams and dissociation heat of the hydrates, visual observations of the hydrate crystals, and single-crystal X-ray diffraction analyses. The highest equilibrium temperature for the TBPC system was 10.3 °C at wTBPC = 0.36, where wTBPC denotes the mass fraction of TBPC, (or the mole fraction of TBPC, xTBPC = 0.034). The TBAAc system was 18.2 °C at wTBAAc = 0.36, where wTBAAc is the mass fraction of TBAAc, (or the mole fraction of TBAAc, xTBAAc = 0.031). The greatest dissociation heat for TBPC system was 194 kJ kg−1 at wTBPC = 0.37 and the TBAAc system was 195 kJ kg−1 at wTBAAc = 0.33. For visual observations of the hydrate crystals, the major morphology in both systems was a columnar shape, but hexagonal plate crystals were observed at wTBPC = 0.10 in the TBPC system. It was also confirmed that the hydrate crystals grown at higher subcooling are finer than those at lower subcooling. The crystallographic structure of TBPC hydrate formed at wTBPC = 0.36 was identified to be tetragonal with 12.5 × 23.7 × 23.7 Å lattice parameters by the single-crystal X-ray diffraction analysis. Similarly, the crystallographic structure of TBAAc hydrate formed at wTBAAc = 0.36 was tetragonal with 12.2 × 33.1 × 33.1 Å lattice parameters. The above findings indicate that TBPC and TBAAc hydrates are promising for applications in hydrate based technologies, such as cool energy storage, gas storage and gas separation.
Introduction
Clathrate hydrates, or hydrates, are ice-like crystalline compounds consisting of host water molecules hydrogen-bonded to form cages that enclose different guest molecules within. There are three crystal structures that are commonly formed depending on the nature of the guest molecules: structure I with space group Pm3n, structure II with space group Fd3m, and structure H with space group P6/mmm. Clathrate hydrates have unique physical properties, such as large gas-storage capacity and large heat of formation/dissociation. These properties have been studied for applications such as storage-media for natural gas1 and hydrogen,2 ground/ocean sequestration of carbon dioxide,3–7 development of heat pump/refrigeration system8 utilizing the heat for hydrate formation/dissociation. Besides, hydrates have the physical properties to preferentially incorporate specific guest molecules in the hydrate cages from a gas mixture. This property may be applied for gas separation technology,9 which has recently attracted much attention. However, one of the greatest obstacles to realize this hydrate technology is high pressure required for the hydrate formation. The breakthrough on this problem may be the usage of semi-clathrate hydrates formed with ionic guest substances. These hydrates have the property of being formed around ambient temperature under atmospheric pressure. There are many reports that the equilibrium pressure for hydrate formation is remarkably reduced by the use of the guests that form the ionic semi-clathrate hydrates.10–12 Additionally these hydrates present no fire hazard and have no risks to the environment and to human health. Because of the above properties, the semi-clathrate hydrates have been investigated for applications in hydrate based technologies, such as cool energy storage,13 the separation and storage for hydrogen,14 CO2,15 methane,16 and H2S.17There are many studies on the guests for the semi-clathrate hydrate formation. These hydrates were first discovered by Fowler et al.18 Then, it was found by X-ray structural analysis that these hydrates were clathrate-like compounds.19 In semi-clathrate hydrates, the guests compose the part of the water host lattice and occupy cages. Among these ionic semi-clathrate hydrates, the compounds studied most thoroughly are that of tetrabutyl ammonium (Bu4N) and tetraisoamyl ammonium (iAm4N) salts.20 Especially, for Bu4N and iAm4N halides, there are many reports on the crystallographic structures and thermophysical properties.21 It was also reported by Nakayama that a number of the ionic hydrates are formed from organic-salt guest molecules, such as Bu4N carboxylates and iAm4N alkanedioates.22 In addition to the quaternary ammonium salts, quaternary phosphonium salts were known to be the guests, but the experimental information is quite limited, i.e., hydrates with Bu4P fluoride, Bu4P bromide and iAm4P bromide were exclusively reported.20
Besides the quaternary ammonium and phosphonium salts, some polymers are known to be guest molecules. Nakayama reported the first clathrate hydrates of Bu4N and iAm4N polyacrylates (PA).23 He determined the temperature-composition phase diagrams of Bu4NPA-H2O and iAm4NPA-H2O, as well as the approximate composition of the hydrates as Bu4NPA·30H2O and iAm4NPA·42H2O.23 It was found by Bogatyrev that similar hydrates could be formed inside the grains of cross-linked polyacrylic acid resin.24 Thermodynamic and X-ray studies of these hydrates were reported.25–27 The complete crystallographic structure of Bu4NPA·40H2O was determined by single-crystal X-ray diffraction measurement.28 In these studies, propionate was employed as the monomer type of the polyacrylates. However, hydrate formation with acrylate that can be viewed as another monomer type of the polyacrylates was not investigated.
As reviewed above, numbers of the semi-clathrate hydrates formed from various guest substances have been identified. Many researches for the semi-clathrate hydrates with quarternary ammonium and phosphonium salts have been done so far. However, the semi-clathrate has a rich diversity of physical properties due to their variety of crystal structure and thermal properties. Further investigation to identify new guests is necessary to develop the potential for hydrate-based technologies by checking the list of guests carefully and systematically. In this study, the formation of two new semi-clathrate hydrates with tetrabutylphosphonium chloride (TBPC) and tetrabutylammonium acrylate (TBAAc) was demonstrated by measurements of the temperature-composition phase diagrams and dissociation heat of the hydrates, visual observations of the hydrate crystals, and single-crystal X-ray diffraction analyses.
Experimental
Materials
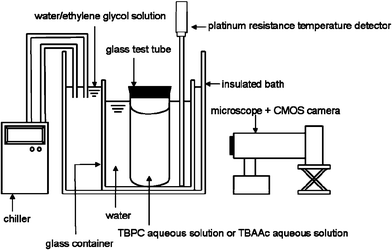
The liquid water used in the experiments was laboratory-made distilled liquid water. TBPC aqueous solution was prepared with a solid reagent of TBPC (96%, Aldrich Chemical Co.). TBAAc aqueous solution was obtained by neutralizing the tetrabutylammonium hydroxide solution (40% solution in water, Aldrich Chemical Co.) with acrylic acid (98% solution, Wako Pure Chemical Industries, Ltd.).Experimental apparatus. Fig. 1 illustrates a schematic of the apparatus used in the observation growth and dissociation of the hydrate crystals formed in TBPC aqueous solution or TBAAc aqueous solution. A glass test tube (external diameter 10 mm, bore diameter 8 mm, height 90 mm) was filled with TBPC aqueous solution or TBAAc aqueous solution. Hydrate crystals formed in the test tube were observed with a microscope. A CMOS camera was attached to the microscope and it acquired digital images of the samples. The temperature was controlled by a chiller (Tokyo Rikakikai Co., CTP-3000). The system temperature was measured by a platinum resistance temperature detector inserted in the glass container with the uncertainty of ±0.1 K.
Experimental procedures. The temperature of the system was first set at 1.0 °C. When the temperature of the system became constant, seed crystals of TBPC or TBAAc hydrate (about 5 mg), which were formed separately in advance, were dropped in each aqueous solution. This procedure artificially induced nucleation and growth of the hydrate in the test cell. The temperature of the system was kept constant for 24 h to allow for the complete growth of hydrate inside the test tube. T was then incrementally increased in steps of 0.1 °C. At each temperature step, T was maintained for several hours. By repetition of this incremental temperature increase, the hydrate was dissociated. The equilibrium temperature was determined as the one immediately before the complete dissociation of the hydrate was visually confirmed. This procedure was repeated under different compositions.
Experimental apparatus and procedures. The dissociation heat was measured using a differential scanning calorimeter (DSC-60, Shimadzu Corporation) with the uncertainty of ±5 kJ/kg.
The mass of the test cell (often called the test pan) was measured by an electronic balance. Then, the aqueous solution of the guest substance (13–14 mg) was injected in the cell by a pipette. The cell containing the solution was measured again to accurately measure the mass of the aqueous solution. This cell was sealed with a round plate with a diameter of 4 mm. The test cell temperature during the measurement was controlled. First, the system was cooled to −40 °C at −10 °C min−1 to form the hydrate. Then the temperature was raised to −5.0 °C at 5.0 °C min−1. From −5.0 °C, the temperature was raised constantly by 0.5 °C min−1 until the hydrate was fully dissociated.
Experimental apparatus. For the observation of crystal morphologies, the same apparatus as the experiment of temperature-composition phase diagram was used. For single crystal structure analysis, X-ray diffraction data were collected with Mo Ka radiation (λ = 0.711 Å) using a CCD diffractometer (Bruker AXS, Model SMART APEX) at 153 K. The structure was solved by direct methods using the SHELXTL.
Experimental procedures. The temperature of the system was set at a prescribed level in the range 1.0–20 °C. When the temperature of the system became constant, a seed of the hydrate crystal (about 5 mg) that was formed separately in advance was dropped in the aqueous solution. The instance the seed crystal is dropped was set as the starting time for crystal growth (t = 0). The crystal morphology of TBPC hydrate and TBAAc hydrate was visually observed.
Results
(a) Phase diagram
The dissociation experiments for TBPC hydrate were done in the mass fraction range from 0.051 to 0.51, and the results are summarized in Table 1 and Fig. 2 in the form of a Teq–wTBPC diagram, where Teq is the equilibrium temperature of the hydrate and wTBPC is the mass fraction of TBPC in TBPC aqueous solution. xTBPC is the mole fraction of TBPC in TBPC aqueous solution. | ||
| Fig. 2 Relationship between the equilibrium temperature and the mass fraction in TBPC system. | ||
| w TBPC | x TBPC | T eq/°C |
|---|---|---|
| 0.051 | 3.3 × 10−3 | 3.4 |
| 0.10 | 6.8 × 10−3 | 5.2 |
| 0.12 | 8.3 × 10−3 | 7.0 |
| 0.17 | 0.012 | 8.4 |
| 0.19 | 0.014 | 8.8 |
| 0.22 | 0.017 | 9.3 |
| 0.26 | 0.021 | 9.9 |
| 0.30 | 0.026 | 10.2 |
| 0.34 | 0.030 | 10.2 |
| 0.35 | 0.032 | 10.2 |
| 0.36 | 0.034 | 10.3 |
| 0.37 | 0.035 | 10.2 |
| 0.40 | 0.039 | 10.0 |
| 0.45 | 0.048 | 9.7 |
| 0.51 | 0.060 | 9.5 |
At wTBPC = 0.051, the equilibrium temperature was 3.4 °C. In the mass fraction range from 0.051 to 0.26, the equilibrium temperature increased with increasing mass fraction. The equilibrium temperature at wTBPC = 0.26 was 9.9 °C. In the mass fraction range from 0.30 to 0.37, the change in the equilibrium temperature levelled off, and then reached the highest equilibrium temperature of 10.3 °C at wTBPC = 0.36. At wTBPC > 0.36, the equilibrium temperature decreased with increasing mass fraction, and the equilibrium temperature at wTBPC = 0.51 was 9.5 °C.
Similar dissociation experiments for TBAAc hydrate were done in the mass fraction range from 0.10 to 0.43, and the results are summarized in Table 2 and Fig. 3 in the form of Teq–wTBAAc diagram, where wTBAAc is the mass fraction of TBAAc in the TBAAc aqueous solution.xTBAAc is the mole fraction of TBAAc in the TBAAc aqueous solution.
 | ||
| Fig. 3 Relationship between the equilibrium temperature and the mass fraction in TBAAc system. | ||
| w TBAAc | x TBAAc | T eq/°C |
|---|---|---|
| 0.10 | 6.1 × 10−3 | 9.9 |
| 0.12 | 7.5 × 10−3 | 11.0 |
| 0.15 | 9.7 × 10−3 | 13.7 |
| 0.18 | 0.012 | 14.4 |
| 0.21 | 0.014 | 14.9 |
| 0.23 | 0.016 | 16.3 |
| 0.26 | 0.019 | 16.9 |
| 0.29 | 0.022 | 17.5 |
| 0.32 | 0.026 | 17.8 |
| 0.34 | 0.028 | 18.0 |
| 0.36 | 0.031 | 18.2 |
| 0.39 | 0.034 | 17.9 |
| 0.41 | 0.038 | 17.8 |
| 0.43 | 0.040 | 17.5 |
The obtained Teq–wTBAAc is qualitatively similar to that in the TBPC system. At wTBAAc = 0.10, the equilibrium temperature was 9.9 °C. In the mass fraction range from 0.10 to 0.29, the equilibrium temperature increased with increasing mass fraction. The equilibrium temperature at wTBAAc = 0.29 was 17.5 °C. Then, in the mass fraction range from 0.32 to 0.41, the change in the equilibrium temperature was hardly seen, and the highest equilibrium temperature was 18.2 °C at wTBAAc = 0.36. At wTBAAc > 0.36, the equilibrium temperature decreased with increasing mass fraction, and the equilibrium temperature at wTBAAc = 0.43 was 17.5 °C.
In these dissociation experiments, two kinds of dissociation behavior were observed. For the TBPC system, hydrate crystals, which were formed in the mass fraction range from 0.35 to 0.37, were dissociated as soon as they reached at the equilibrium temperature. Hydrate crystals formed in the mass fraction range from 0.051 to 0.26 and the mass fraction 0.51 were gradually dissociated by increasing the temperature up to the equilibrium temperature. This result suggests that the formation and co-existence of the hydrates, each having different dissociation temperature. For TBAAc system, a similar dissociation behavior was observed.
(b) Dissociation heat
Table 3 and 4 present the results of the DSC measurements of dissociation heat of TBPC and TBAAc hydrates formed at different mass fractions, where ΔHd is the dissociation heat of hydrates. Hydration number corresponding to each mass fraction is included in these tables. For TBPC hydrate, the greatest dissociation heat was 194 kJ kg−1 at wTBPC = 0.37. For TBAAc, the greatest dissociation heat was 195 kJ kg−1 at wTBAAc = 0.33.| w TBPC | ΔHd/kJ kg−1 |
|---|---|
| 0.30 | 193 |
| 0.34 | 186 |
| 0.35 | 183 |
| 0.37 | 194 |
| w TBAAc | ΔHd/kJ kg−1 |
|---|---|
| 0.30 | 179 |
| 0.33 | 195 |
The DSC heating curve in the TBPC system at wTBPC = 0.30 is shown in Fig. 4(a), where three peaks were confirmed. Their peaks were observed, from the left, at 0 °C, 6.5 °C, and 10 °C, and the peak seen at 10 °C was the greatest. The leftmost peak should correspond to ice melting and the other two peaks are considered due to the dissociation of the hydrates. The hydrate dissociated at 6.5 °C is metastable phase and the hydrate dissociated at 10 °C is stable phase. Thus the DSC measurement in the present study indicated that TBPC hydrate has at least two types of crystal structure and it is known that tetraalkyl salt hydrates have 2–4 kinds of crystallographic structure.20 In fact, two types of crystal morphologies were observed as described in the next section. Fig. 4(b) indicates the typical DSC heating curve in TBPC system observed in the range of wTBPC = 0.34, 0.35, and 0.37. In this DSC thermograph, a single peak was observed and the existence of ice was not identified. Also for the DSC heating curve in TBAAc system at wTBAAc = 0.30 and 0.33, a single peak was observed and the existence of ice was not identified, and a typical result is shown in Fig. 5.
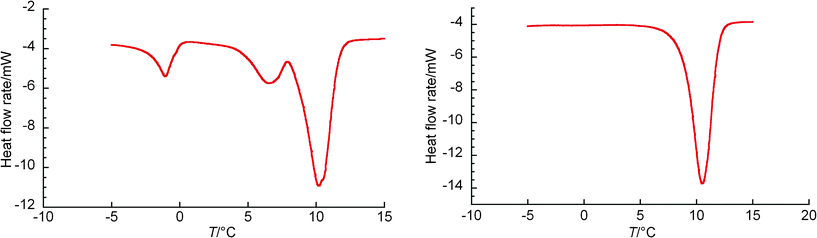 | ||
| Fig. 4 (a) The DSC heating curve in the TBPC system at wTBPC = 0.301; (b) the DSC heating curve in TBPC system at wTBPC = 0.368. | ||
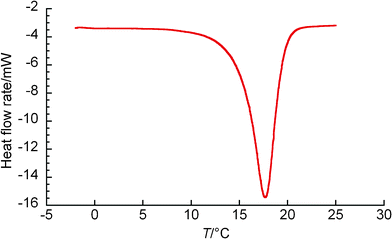 | ||
| Fig. 5 The DSC heating curve in TBAAc system at wTBAAc = 0.300. | ||
(c) Crystal morphology and structure of the semi-clathrate hydrates
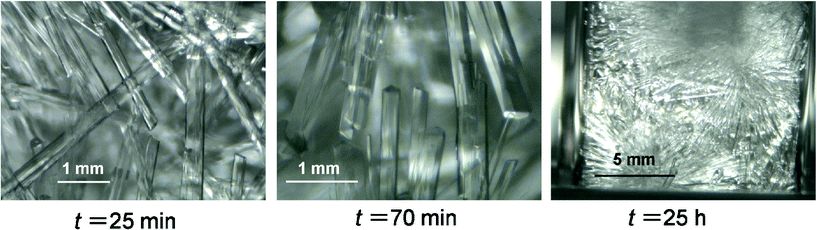
A TBPC aqueous solution at wTBPC = 0.36 and 0.10 and TBAAc aqueous solution at wTBAAc = 0.36 and 0.12 were used in these experiments. Here we define the subcooling temperature ΔTsub = Teq − Tex as the index of the driving force for the crystal growth, where Teq is the equilibrium temperature of the hydrate at each mass fraction and Tex is the system temperature. The results obtained with TBPC system at wTBPC = 0.36 and TBAAc system at wTBAAc = 0.36 were classified based on ΔTsub.Fig. 6 shows a sequence of images of TBPC hydrate crystals growing in the aqueous solution at wTBPC = 0.36, Tex = 5.5 °C (ΔTsub = 4.8 K). As seen in the images, hydrate crystals grow to radiate from the seed crystal. The thickness of the crystal becomes larger with elapsed time. In this system, apparently, the solution was mostly converted into the hydrate after approximately 1 h. After this time, no noticeable further hydrate crystal growth was observed. Fig. 7 shows a sequence of images of TBPC hydrate crystals growing in the aqueous solution at wTBPC = 0.10, Tex = 1.0 °C (ΔTsub = 4.2 K). In contrast with the TBPC system at wTBPC = 0.36, the majority of the crystal morphology in this system was the hexagonal plate which is 0.5 mm on a side, and the columnar-shape crystal observed in the TBPC system at wTBPC = 0.36 was hardly observed. The lateral growth the plate-like crystals was observed, but their thickening was not noticeable. It took two days or longer for the completion of the hydrate growth. These results obtained in the TBPC system at wTBPC = 0.36 and 0.10 indicate that there are at least two different types of crystal structure with different hydration numbers within this mass fraction range. The similar result was previously reported in TBAF (tetrabutylammonium fluoride) system, where it was indicated that columnar-shape and hexagonal-shape hydrate crystals were of tetragonal and cubic structures by single-crystal X-ray diffraction measurements.29 Here, in this study, single-crystal X-ray diffraction analysis showed that a tetragonal cell of 12.5 × 23.7 × 23.7 Å for columnar-shape TBPC hydrate crystal formed at wTBPC = 0.36.
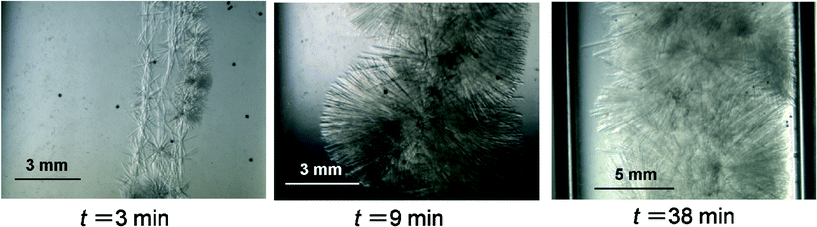 | ||
| Fig. 6 Sequential videographs of TBPC hydrate crystal growth at wTBPC = 0.363, Tex = 5.5 °C. | ||
 | ||
| Fig. 7 Sequential videographs of TBPC hydrate crystal growth at wTBPC =0.101, Tex = 1.0 °C. | ||
Fig. 8 and 9 show the sequential images of TBAAc hydrate crystals growing in the aqueous solution: wTBAAc = 0.36, Tex = 14.3 °C (ΔTsub = 3.9 K) for Fig. 8 and wTBAAc = 0.12, Tex = 6.8 °C (ΔTsub = 4.2 K) for Fig. 9. As recognized in the comparison of Fig. 8 and 9, both crystal morphologies were columnar-shape and the growth behavior was similar to that for the TBPC system at wTBPC = 0.36. However, as for the time required for the completion of the hydrate growth, it was approximately 35 min at wTBAAc = 0.36, while it was 25 h at wTBAAc = 0.12. The single-crystal X-ray diffraction analysis showed that a tetragonal cell of 12.2 × 33.1 × 33.1 Å for a columnar-shape TBAAc hydrate crystal formed at wTBAAc = 0.36, in this study. Further study is necessary for structure identification of these systems, and is in progress. On the other hand, it should be emphasized that the composition of the solution influences the hydrate crystal growth rate but may not influence the crystal structure formed. These results support the experimental results by means of DSC.
 | ||
| Fig. 10 The morphology of the hydrate crystals formed in TBPC aqueous solution at wTBPC = 0.363 based on subcooling ΔTsub. | ||
Finally, Fig. 10 and 11 show the comparison of the morphology of the hydrate crystals formed in TBPC aqueous solution at wTBPC = 0.36 and TBAAc aqueous solution at wTBAAc = 0.36. These images were arranged according to the subcooling ΔTsub. Both crystal morphologies were columnar or needle shape and the diameter decreased with increasing the subcooling. In the TBPC system, at ΔTsub = 1.7 K to 2.9 K, the shape of TBPC hydrate crystals was typically columnar with the thickness between 0.1 mm to 0.3 mm. At ΔTsub = 4.8 K, needle shape crystals of the thickness less than 0.1 mm were observed. Similarly, in TBAAc system, at ΔTsub = 1.7 K to 3.2 K, the shape of TBAAc hydrate crystals was columnar with the thickness between 0.2 mm to 0.3 mm. At ΔTsub = 4.9 K, needle shape crystals of thickness less than 0.1 mm were observed. From these figures, it is evident that the morphology of the hydrate crystals significantly varies depending on the subcooling. The hydrate crystals grown at higher subcooling are finer than those at lower subcooling.
 | ||
| Fig. 11 The morphology of the hydrate crystals formed in TBAAc aqueous solution at wTBAAc = 0.362 based on subcooling ΔTsub. | ||
Discussion
For the TBPC system, we compare the effect of the anion on the hydrate formation. In Fig. 12, the equilibrium temperatures of the phosphonium hydrates are plotted against the anion radius. Here we used the value of the highest equilibrium temperature 10.3 °C for TBPC hydrate. For comparison, the results of Bu4N+ (F−, Cl−, Br−) and Bu4P+ (Br−) by Aladko et al.20 are also shown. The equilibrium temperatures of Bu4P halide systems were lower by 3 K to 5 K than those of Bu4N halide systems. The decrease in the anion radius causes the increase of the hydrate equilibrium temperature. Aladko et al. explained that the stability of the hydrate is mainly governed by the distortion effect of anion on the hydrogen-bonded water framework in the hydrate.21 By extrapolating the data for TBPC and TBPB (tetrabutylphosphonium bromide) hydrates, the equilibrium temperature of TBPF (tetrabutylammonium fluoride) hydrate is estimated to be approximately 15 °C. | ||
| Fig. 12 Equilibrium temperatures as a function of anion radius for the hydrates of Bu4N+ (F−, Cl−, Br−) (line 1) and Bu4P+ (Cl−, Br−) (line 2). | ||
For the TBAAc system, the equilibrium temperature at wTBAAc = 0.36 was the highest (18.2 °C), which is close to that of Bu4N propionate hydrate reported by Nakayama et al.22 Additionally the value is higher than that of Bu4N polyacrylate. Table 5 indicates that the equilibrium temperature of Bu4N polyacrylate hydrate depends on the degree of polymerization (that is the length of the polymer chain).
| Polymerization | T eq/°C | Ref. |
|---|---|---|
| 0 | 18.2 | This work |
| 15 | 14.3 | 28 |
| 2100 | 11.2 | 26 |
The industrial applications of TBPC hydrate and TBAAc hydrate based on the above thermal properties are discussed. One of the most promising applications may be cool energy storage technology for air-conditioning. In cool energy storage technology, ice is currently, widely used. However, the coefficient of performance (COP) of the refrigerator to form ice cannot be high because the ice formation requires temperatures as low as −4 to −10 °C or even lower. Additionally, there is a great difference between the melting temperature of ice 0 °C and the temperature required for air-conditioning. Generally, a temperature of 5 to 15 °C is sufficient for residential air-conditioning. For air-conditioning used in server rooms housing computers, even higher temperature, 15 to 20 °C, may be sufficient. Thus, TBPC hydrate is suitable as a cool energy storage medium for residential air-conditioning use because the equilibrium temperature is 10.3 °C. TBAAc hydrate is suitable as the material for server room air-conditioning use as the equilibrium temperature is 18.2 °C. We now calculate the COP of the refrigerator by supposing the inverse Carnot cycle. The COP of the inverse Carnot cycle is expressed as ε = TL / (TH − TL) where ε, TH and TL are the COP and the temperatures of the high- and low- grade heat sources. TH can be set at 27 °C, the average atmospheric temperature in the summer season. TL may be set at the value of Teq minus 10 °C, and thus 0.3 °C and 8.2 °C for TBPC and TBAAc systems. COP values are deduced to be 10.3 and 15.0 for TBPC and TBAAc systems. These values are 1.5 to 2.0 times greater than the COP for ice system.
Moreover, from the viewpoint of the dissociation heat, which is the important thermal property in cool energy storage technology, these materials are hopeful. The greatest dissociation heat of TBPC hydrate was 194 kJ kg−1 at wTBPC = 0.37, and that of TBAAc hydrate was 195 kJ kg−1 at wTBAAc = 0.33. These values are comparable to that of TBAB hydrate that was previously studied as cool storage material of the hydrate (typeA: 193 kJ kg−1, typeB: 199 kJ kg−1).30
The above findings indicate that TBPC and TBAAc hydrates are promising cool energy storage materials. These hydrates also have the potential of other industrial applications such as the capture and storage of hydrogen, CO2, methane, and H2S. In these hydrate-based technologies, the hydrate formation at a temperature closer to ordinary room temperature is quite often favorable. In this regard, TBAAc hydrate that has a relatively high equilibrium temperature may be promising.
Conclusions
In this study, the formation of the two new semi-clathrate hydrates with TBPC and TBAAc was demonstrated by the measurements of temperature-composition phase diagrams and dissociation heat of the hydrates, visual observations of the hydrate crystals, and single-crystal X-ray diffraction analyses.In the measurements of temperature-composition phase diagrams, the highest equilibrium temperature for TBPC system was 10.3 °C at wTBPC = 0.36, (or xTBPC = 0.034) and that for TBAAc system was 18.2 °C at wTBAAc = 0.36, (or xTBAAc = 0.031). For Bu4P halide systems, the decrease in anion radius caused the increase in the equilibrium temperature of hydrate. For Bu4N polyacrylate systems, the equilibrium temperature of Bu4N polyacrylate system depended on the degree of polymerization (that is the length of the polymer chain). In the measurements of dissociation heat of the hydrates, the greatest dissociation heat for TBPC system was 194 kJ kg−1 at wTBPC = 0.37 and that for TBAAc system was 195 kJ kg−1 at wTBAAc = 0.33.
The crystallographic structure of TBPC hydrate formed at wTBPC = 0.36 was identified to be tetragonal with 12.5 × 23.7 × 23.7 Å lattice parameters by single-crystal X-ray diffraction analysis. Similarly, the crystallographic structure of TBAAc hydrate formed at wTBAAc = 0.36 was tetragonal with 12.2 × 33.1 × 33.1 Å lattice parameters.
The above findings indicate that TBPC and TBAAc hydrates are promising for the application to the hydrate based technologies, such as cool energy storage, gas storage and gas separation.
Acknowledgements
The study was subsidized by JKA through its promotion funds from KEIRIN RACE and also supported by a Grant-in-Aid for the Global Center of Excellence Program for “Center for Education and Research of Symbiotic, Safe and Secure System Design” from the Ministry of Education, Culture, Sport and Technology in Japan.References
- Y. H. Mori, J. Chem. Ind. Eng., 2003, 1–17(Suppl.), 54 Search PubMed.
- W. L. Mao and H. Mao, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 708–710 CrossRef CAS.
- P. G. Brewer, E. T. Peltzer, G. Friederich and I. Aya, Mar. Chem., 2000, 72, 83–93 CrossRef CAS.
- P. G. Brewer, E. Peltzer, I. Aya, P. Haugan, R. Bellerby, K. Yaname, R. Kojima, P. Waltz and Y. Nakajima, J. Oceanogr., 2004, 60, 751–758 CrossRef CAS.
- P. G. Brewer, C. Friederich, E. T. Peltzer and F. M. Orr, Science, 1999, 284, 943–945 CrossRef CAS.
- R. Ohmura and Y. H. Mori, Environ. Sci. Technol., 1998, 32, 1120–1127 CrossRef CAS.
- B. Tohidi, J. Yang, M. Salehabadi, R. Anderson and A. Chapoy, Environ. Sci. Technol., 2010, 44, 1509–1514 CrossRef CAS.
- T. Ogawa, T. Itoh, K. Watanabe, K. Tahara, R. Hiraoka, J. Ochiai, R. Ohmura and Y. H. Mori, Appl. Therm. Eng., 2006, 26, 2157–2167 CrossRef CAS.
- Y.-T. Seo, I. L. Moudravski, J. A. Ripmeester, J-W. Lee and H. Lee, Environ. Sci. Technol., 2005, 39, 2315–2319 CrossRef CAS.
- M. Arjmandi, A. Chapoy and B. Tohidi, J. Chem. Eng. Data, 2007, 52, 2153–2158 CrossRef CAS.
- N. Mayoufi, D. Dalmazzone, W. Furst, A. Delahaye and L. Fournaison, J. Chem. Eng. Data, 2010, 55, 1271–1275 CrossRef CAS.
- S. Li, S. Fan, J. Wang, X. Lang and Y. Wang, J. Chem. Eng. Data, 2010, 55, 3212–3215 CrossRef CAS.
- M. Darbouret, M. Cournil and J. M. Herri, Int. J. Refrig., 2005, 28, 663–671 CrossRef CAS.
- A. Chapoy, R. Anderson and B. Tohidi, J. Am. Chem. Soc., 2007, 129, 746–747 CrossRef CAS.
- S. Fan, S. Li, J. Wang, X. Lang and Y. Wang, Energy Fuels, 2009, 23, 4202–4208 CrossRef CAS.
- W. Wang, B. O. Carter, C. L. Bray, A. Steiner, J. Bacsa, J. T. A. Jones, C. Cropper, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Chem. Mater., 2009, 21, 3810–3815 CrossRef CAS.
- Y. Kamata, Y. Yamakoshi, T. Ebinuma, H. Oyama, W. Shimada and H. Narita, Energy Fuels, 2005, 19, 1717–1722 CrossRef CAS.
- D. L. Fowler, W. V. Loebenstein, D. B. Pall and C. A. Kraus, J. Am. Chem. Soc., 1940, 62 Search PubMed.
- G. A. Jeffrey and R. K. McMullan, Progress in Inorganic Chemistry, John Wiley, New York, 1967, vol. 8, pp. 43–108 Search PubMed.
- (a) Yu. A. Dyadin and K. A. Udachin, J. Inclusion Phenom., 1984, 2, 61–72 CrossRef CAS; (b) Y. A. Dyadin and K. A. Udachin, J. Struct. Chem., 1987, 28, 394–432 CrossRef.
- L. S. Aladko, Yu.A. Dyadin, T. V. Rodionova and I. S. Terekhova, J. Mol. Liq., 2003, 106, 229–238 CrossRef CAS.
- (a) H. Nakayama and K. Watanabe, Bull. Chem. Soc. Jpn., 1978, 51, 2518–2522 CrossRef CAS; (b) H. Nakayama and S. Torigata, Bull. Chem. Soc. Jpn., 1984, 57, 171–174 CrossRef CAS; (c) H. Nakayama and H. Usui, Bull. Chem. Soc. Jpn., 1986, 59, 833–837 CrossRef CAS; (d) H. Nakayama, K. Nakamura, Y. Haga and Y. Sugiura, Bull. Chem. Soc. Jpn., 1991, 64, 358–365 CrossRef CAS.
- H. Nakayama, Bull. Chem. Soc. Jpn., 1987, 60, 2319–2326 CrossRef CAS.
- V. L. Bogatyryov, Yu. A. Dyadin, A. V. Pirozhov, G. A. Maksakova, S. M. Zemskova, N. K. Moroz, F. V. Zhurko, V. I. Skobeleva and G. V. Villevald, Izu. Akad. Nauk SSSR, Ser. Khim., 1986, 9, 2152 Search PubMed.
- I. S. Terekhova, A. Yu. Manakov, D. V. Soldatov, K. Suwinska, S. S. Skiba, Y. G. Stenin, G. V. Villevald, T. D. Karpova and A. S. Yunoshev, J. Phys. Chem. B, 2009, 113, 5760–5768 CrossRef CAS.
- V. L. Bogatyryov, I. S. Terekhova and S. V. Vasilieva, J. Struct. Chem., 1998, 39, 615–618 CrossRef.
- D. V. Soldatov, K. Suwinska, I. S. Terekhova and A. Yu. Manakov, J. Struct. Chem., 2008, 49, 712–718 CrossRef CAS.
- K. A. Udachin and J. A. Ripmeester, Angew. Chem., Int. Ed., 1999, 38, 1983–1984 CrossRef CAS.
- T. V. Rodionova, A. Yu. Manakov, Yu. G. Stenin, G. V. Villevald and T. D. Karpova, J. Inclusion Phenom. Macrocyclic Chem., 2008, 61, 107–111 CrossRef CAS.
- H. Oyama, W. Shimada, T. Ebinuma, Y. Kamata, S. Takeya, T. Uchida, J. Nagao and H. Narita, Fluid Phase Equilib., 2005, 234, 131–135 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |