Modification of pyridine-based conjugated polymer films via Lewis acid: halochromism, characterization and macroscopic gradation patterning†
Shotaro
Hayashi
*,
Atushi
Asano
and
Toshio
Koizumi
1-10-20 Hashirimizu, Yokosuka, Kanagawa 239-9696, Japan. E-mail: shayashi@nda.ac.jp; Fax: +81 46 844 5901; Tel: +81 46 841 3810 ext 3592
First published on 6th October 2011
Abstract
Lewis acid (BF3)-modification of pyridine-based conjugated polymer films and their solid-state NMR characterization has successfully been demonstrated. We also achieved “halochromism” and macroscopic gradation patterning of the polymer films.
Because facile modification of reactive polymer films is a particularly innovative process toward tailored material preparation, developments and applications of post-functionalization of the polymer films have been expanding significantly.1 Especially, modifications of conjugated polymer films have recently become attractive due to the interest in controlling their optical and electrochemical properties (HOMO, LUMO, and band gap).1d–h Furthermore, macroscopic color patterning of a conjugated polymer film via post-functionalization is also attractive.1i Therefore, the easy functionalization of conjugated polymer films on a substrate is quite important from the viewpoint of application for semiconductor and macroscopic patterning.
Conjugated polymers containing imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) groups2 have electron-accepting properties in the π-system and show significant color changes when protonated in organic Brønsted acid solutions; therefore, they are candidates for sensor applications.3 In addition, metal complex formation of these polymers provides dramatic changes in their optical properties.4 Recently, Bazan and co-workers reported a facile band gap control in a conjugated oligomer containing benzo[c]-1,2,5-thiadiazole unit via coordination of boron-based Lewis acids in solutions.5 In contrast with these solution process, Kappaun referred to the reversible color change of a pyridine-based copolymer film by Brønsted acid (HCl) vapor treatment.6 Furthermore, Skene also reported halochlomism of a polyazomethine film using trifluoroacetic acid and triethylamine.7
N) groups2 have electron-accepting properties in the π-system and show significant color changes when protonated in organic Brønsted acid solutions; therefore, they are candidates for sensor applications.3 In addition, metal complex formation of these polymers provides dramatic changes in their optical properties.4 Recently, Bazan and co-workers reported a facile band gap control in a conjugated oligomer containing benzo[c]-1,2,5-thiadiazole unit via coordination of boron-based Lewis acids in solutions.5 In contrast with these solution process, Kappaun referred to the reversible color change of a pyridine-based copolymer film by Brønsted acid (HCl) vapor treatment.6 Furthermore, Skene also reported halochlomism of a polyazomethine film using trifluoroacetic acid and triethylamine.7
However, there are no reports, to our knowledge, of polymer film modification using Lewis acid vapor and the application for macroscopic gradation patterning.
First, we report the post-functionalization of the pyridine-based polymer films using a Lewis acid (BF3) and defunctionalization using a base (Et3N) (called halochromism). Next, we describe a characterization of the BF3-modified polymer film by the high performance solid-state 19F NMR measurement. Finally, we refer to the facile preparation of macroscopic gradation patterned materials.
The structures of fluorene-based conjugated polymer having pyridine unit, PP, or having pyridine and two thiophene units, PTP, and the photograph of the polymer films are displayed in Fig. 1. The polymer films were obtained by drop cast from 0.5 ml solutions of polymer in chloroform (10 mg ml−1) on glass plate (2 × 2 cm). To demonstrate the protonation of polymer films, PP film was exposed to HCl vapor by mounting the sample face down in a clamp stand 3 cm above the surface of a 37% hydrochloric acid bath at 20 °C (1 min). The color of PP film is changed from transparent pale-yellow to yellow-color. The emission color is also changed from blue to bluish green. Moreover, the protonated PP, PPH, was a stable and could retain the color for more than 1 month in air (r.t.). Next, the PP film was exposed to Et3N by mounting the sample face down in a clamp stand 1 cm above the surface of pure Et3N bath at 20 °C (3 min). Restore to PP film is easily achieved. Furthermore, PTP film also shows chemically reversible change of the optical properties. To develop this modification approach, a pure Lewis acid, trifluoroborate diethylether complex (BF3-OEt2), was used. After BF3-OEt2 treatment in the similar way with HCl, transmitted PP film and yellow-colored PTP film change to yellow-colored film and red-colored film, respectively. Both BF3 coordinated PP and PTP, PPB and PTPB, also show good stability that can retain the color for more than 1 month. The PPB and PTPB also reverse PP and PTP by Et3N treatment. In previous reports, acid functionalization of pyridine-based conjugated polymers produces a red-shift in both UV-vis absorption and fluorescence spectra.3–7
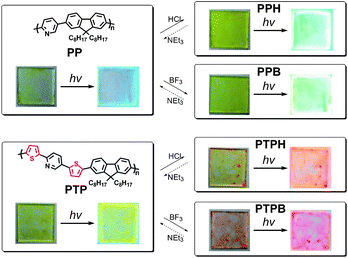 | ||
| Fig. 1 Reversible color change of polymer films, PP (top) and PTP (bottom). hv (365 nm). | ||
In Fig. 2, reversible change of their UV-vis absorption bands is performed by repeating acid–base treatment. The UV-vis absorption spectrum of PP film centered at 389 nm is shown. After exposure to HCl vapor, a red-shifted absorption maxima centered at 435 nm is observed. Et3N vapor treatment of the modified film gives a blue-shifted absorption band, which completely corresponded to the absorption band of PP film. After exposure to BF3-OEt2 vapor, a red-shifted absorption band centered at 437 nm is observed, which is completely reversible on base treatment. Moreover, perfect reversible process of PTP film is also demonstrated. After exposure to HCl and BF3 vapor, red-shifted absorption bands (λmax = 434 nm, λmax = 440 nm, respectively) are observed. The modified PTP films treated with Et3N vapor blue-shift and completely correspond to the absorption band of PTP film.
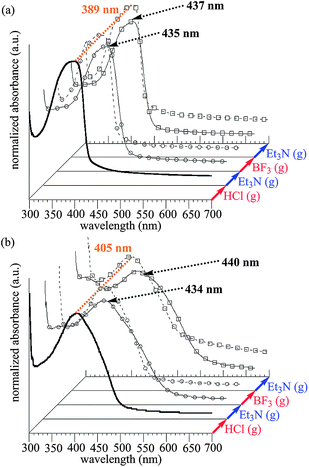 | ||
| Fig. 2 Reversible change of absorption spectra through treatment with acid and base vapors. (a) PP film. (b) PTP film. | ||
In Fig. 3, the fluorescence spectra appear to be affected by the treatment viaHCl and BF3. The emission band of PTP film (λem = 582 nm) shows longer wavelength than that of PP film (λem = 496 nm). After exposure to both HCl and BF3 vapors, the emission bands of PP film are red-shifted to 514 nm and 520 nm, respectively. Moreover, the emission bands of PTP film through both HCl and BF3-OEt2 vapors are also red-shifted to 604 nm and 623 nm, respectively. These large red-shifts of PTPB, compared with PTPH, may be caused by effective charge transfer from the strong electron-donor thiophene unit to the electron-acceptor pyridine unit enhanced by the strong Lewis acid, BF3.6a These results indicate that band gap tuning and halochromism in the pyridine-based conjugated polymer films is successfully demonstrated.
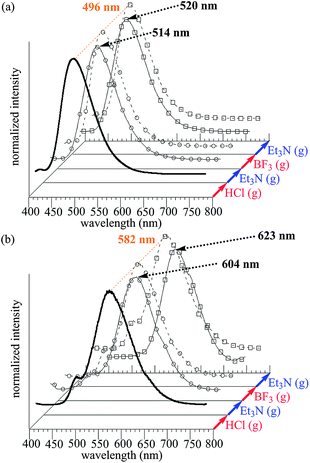 | ||
| Fig. 3 Reversible change of fluorescence spectra through treatment with acid and base vapors. (a) PP film. (b) PTP film. | ||
To investigate the details of the modified polymer films, solid-state 13C NMR spectra of PP, PPH and PPB films, which were pealed from glass plate, were measured. The observed 13C NMR signals assigned to the aromatic units of PPH and PPB films become broad and slightly shifted toward downfield after film modifications as compared with those of PP film (Fig. S1†). This phenomenon indicates that the electronic density of the pyridine unit decreases by protonation or coordination of BF3. The formation of N–B coordinating bond was confirmed by the 19F NMR measurement (Fig. 4). Fig. 4 shows the solid-state 19F magic-angle-spinning (MAS) NMR spectrum of PPB film. Two peaks are obviously observed on the 19F NMR spectrum. The main peak appears at −147 ppm and another tiny peak at −152 ppm. The tiny and relatively narrower peak at −152 ppm is ascribed to the residual BF3–OEt2. In the case of N–BF3 complexes, the 19F signals of the complexes are frequently observed at range of −145 ± 5 ppm.8 Therefore, the main component at −147 ppm is caused by the formation of complex between N atom of pyridine part of PP and BF3. One may wonder the possibility of the isotope shift of 10B and 11B. The isotope shift of 10BF3 and 11BF3 is less than 0.1 ppm,9 so that we can ignore the isotope shift for the solid state NMR measurements.
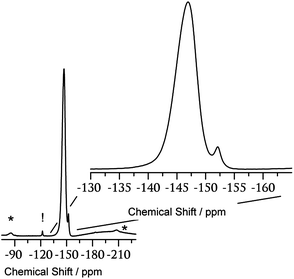 | ||
| Fig. 4 Solid-state 19F MAS NMR of PP film after BF3-treatment: symbol * represents artificially appeared at MAS NMR measurements, spinning side band (SSB) and symbol! is a background Teflon peak. | ||
In addition, the 19F spin–lattice relaxation time (T1F) measurement revealed and supported the N-BF3 complex formation. T1F value of the main peak is 1.30 ± 0.01 s, while that of the residual BF3-OEt2 is 9.1 ± 0.1 s (Fig. S2†). Usually, very high molecular motion such as a small molecule of BF3–OEt2 has a long T1F, because of the extreme narrowing condition limit. On the other hand, the molecular motion of BF3 molecules combined with PP becomes slow: BF3 moves cooperatively with PP. This indicates that the T1F value becomes short. Therefore, the current T1F measurements also prove the existence of the N-BF3 complex in PPB.
Finally, macroscopic gradation modification10 of the pyridine-based conjugated polymer films was demonstrated. The polymer films were prepared by drop casting from 0.5 ml solutions of polymer in chloroform (10 mg ml−1) on glass plate (1 × 8 cm) (Fig. 5a). Both PP and PTP films were exposed to BF3 vapor by mounting the sample edge down in a clamp stand 3 cm above the surface of a solution bath at 20 °C. After 1 min, color gradation patterned films were obtained due to the difference of the BF3-modified ratios in the place of the polymer films (Fig. 5b, c). Colors of the polymer films show more bathochromic color change when going from top to bottom. This result suggest that the BF3-modified ratio is gradually lowering from the bottom to the top of film.
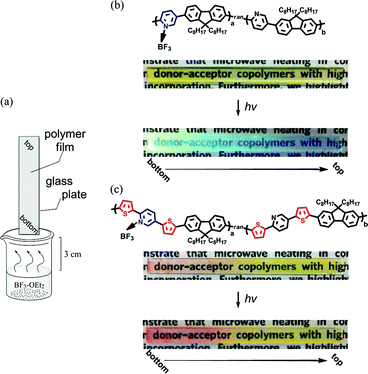 | ||
| Fig. 5 Macroscopic gradation patterning of polymer films on glass plate viaBF3-OEt2 treatment (a). Patterned PP (b) and PTP (c) films. hv (365 nm). | ||
Conclusions
We have presented here the facile Lewis acid (BF3)-modification of the pyridine-based conjugated polymer films, which was clearly characterized by high performance solid-state NMR measurements. The halochromism of the polymer films has been achieved by repeated acid–base treatment. Furthermore, the macroscopic gradation patterning of the pyridine-based conjugated polymer films was also successfully demonstrated.Notes and references
-
(a) F. A. Leibfarth, M. Kang, M. Ham, J. Kim, L. M. Campos, N. Gupta, B. Moon and C. J. A. Hawker, Nat. Chem., 2010, 2, 207–212 CrossRef CAS
; (b) T. S. Hansen, A. E. Daugaard, S. Hvilsted and N. B. Larsen, Adv. Mater., 2009, 21, 4483–4486 CrossRef CAS
; (c) J. OBouffard, M. Watanabe, H. Takaba and K. Itami, Macromolecules, 2010, 43, 1425–1429 CrossRef
; (d) S. Inagi, S. Hayashi and T. Fuchigami, Chem. Commun., 2009, 1718–1720 RSC
; (e) S. Inagi, S. Hayashi, K. Hosaka and T. Fuchigami, Macromolecules, 2009, 42, 3881–3883 CrossRef CAS
; (f) S. Inagi, K. Hosaka, S. Hayashi and T. Fuchigami, J. Electrochem. Soc., 2010, 157, E88–E91 CrossRef CAS
; (g) Y. Li, K. Kamata, S. Asaoka and T. Iyoda, Org. Biomol. Chem., 2003, 1, 1779–1782 RSC
; (h) S. Hayashi, S. Inagi and T. Fuchigami, Polym. Chem., 2011, 2, 1632–1637 RSC
; (i) S. Inagi, Y. Ishiguro, M. Atobe and T. Fuchigami, Angew. Chem., Int. Ed., 2010, 49, 10136–10139 CrossRef CAS
.
-
(a) J. Ohshita, H. Kai, K. Kimura, K.-H. Lee and A. Kunai, Polym. J., 2009, 41, 482–485 CrossRef CAS
; (b) H. Chen, Y. Lu, T.-T. Ong, X. Hu and S.-C. Ng, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2163–2171 CrossRef CAS
; (c) T. Morikita, T. Yasuda and T. Yamamoto, React. Funct. Polym., 2008, 68, 1483–1491 CrossRef CAS
.
-
(a) M. Vetrichelvan and S. Valiyaveettil, Chem.–Eur. J., 2005, 11, 5889–5898 CrossRef CAS
; (b) E. Arias-Marin, J. L. Moigne, T. Maillou, D. Guillon, I. Moggio and B. Geffroy, Macromolecules, 2003, 36, 3570–3579 CrossRef CAS
; (c) A. P. Monkman, L.-O. Palsson, R. W. T. Higgins, C. Wang, M. R. Bryce, A. S. Batsanov and J. A. K. Howard, J. Am. Chem. Soc., 2002, 124, 6049–6055 CrossRef CAS
; (d) S. Scheiner and T. Kar, J. Phys. Chem. B, 2002, 106, 534–539 CrossRef CAS
; (e) S.-C. Ng, H.-F. Lu, H. S. O. Chan, A. Fujii, T. Laga and K. Yoshino, Macromolecules, 2001, 34, 6895–6903 CrossRef CAS
; (f) A. P. Monkman, M. Halim, S. Dailey, I. D. W. Samuel and L. E. Horsburgh, Synth. Met., 1999, 101, 208–209 CrossRef CAS
; (g) H. Yun, T. K. Kwei and Y. Okamoto, Macromolecules, 1997, 30, 4633–4638 CrossRef CAS
; (h) D. K. Fu, B. Xu and T. M. Swager, Tetrahedron, 1997, 53, 15487–15494 CrossRef CAS
.
- G. L. Schulz, X. Chen, S.-A. Chen and S. Holdcroft, Macromolecules, 2006, 39, 9157–9165 CrossRef CAS
.
-
(a) G. C. Welch, R. Coffin, J. Peet and G. C. Bazan, J. Am. Chem. Soc., 2009, 131, 10802–10803 CrossRef CAS
; (b) G. C. Welch, J. Peet and G. C. Bazan, J. Am. Chem. Soc., 2011, 133, 4632–4644 CrossRef CAS
.
- S. Kappaun, S. Horner, A.-M. Kelterer, K. Waich, F. Grasse, M. Graf, L. Romaner, F. Niedermair, K. Müllen, A. C. Grimsdale, R. Saf, E. J. W. List, E. Zojer and C. Sluygovc, Macromol. Chem. Phys., 2008, 209, 2122–2134 CrossRef CAS
.
- S. Barik and W. G. Skene, Polym. Chem., 2011, 2, 1091–1097 RSC
.
-
(a) T. Chivers, Can. J. Chem., 1970, 48, 3856–3859 CrossRef CAS
; (b) A. Fratiello and R. E. Schuster, J. Org. Chem., 1972, 37, 2237–2241 CrossRef CAS
; (c) J. S. Hartman, Z. Yuan, A. Fox and A. Nguyen, Can. J. Chem., 1996, 74, 2131–2142 CrossRef CAS
.
-
(a) R. J. Gillespie and J. S. Hartman, Can. J. Chem., 1967, 45, 859–863 CrossRef CAS
; (b) V. N. Plakhotnik, L. Ernst, P. Sakhaii, N. F. Tovmash and R. Schmutzler, J. Fluorine Chem., 1999, 98, 133–135 CrossRef CAS
; (c) K. Jackowski, W. Makulski, A. Szyprowska, A. Antušek, M. Jaszuński and J. Jusélius, J. Chem. Phys., 2009, 130, 044309 CrossRef
.
-
(a) K. Tanaka, F. Ishiguro and Y. Chujo, Langmuir, 2010, 26, 10254–10258 CrossRef CAS
; (b) J. Genzer and R. R. Bhat, Langmuir, 2008, 24, 2294–2317 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthetic procedure, and figures showing solid-state 13C NMR and 19F spin–lattice relaxation time. See DOI: 10.1039/c1py00363a |
| This journal is © The Royal Society of Chemistry 2011 |
