Investigations on the spermine provoked liquid crystalline phase behavior of high molecular weight DNA in the presence of alkali and alkaline earth metal ions
Neethu
Sundaresan
a,
Thresia
Thomas
b,
T. J.
Thomas
b and
C. K. S.
Pillai
*a
aChemical Sciences & Technology Division, Regional Research Laboratory (Council of Scientific and Industrial Research), Thiruvananthapuram, 695019, India. E-mail: ckspillai@yahoo.com
bDepartments of Medicine and Environmental Community Medicine, University of Medicine and Dentistry, Robert Wood Johnson Medical School, NJ 08903, USA
First published on 5th October 2011
Abstract
The effect of alkali and alkaline earth metal ions on the spermine induced condensates of high molecular weight DNA was studied. The spermine induced DNA precipitation was facilitated by the alkali and alkaline earth metal ions, suggesting that the metal ions instead of getting into competition with spermine in DNA binding, had acted in concert to precipitate DNA from solution. Spermine-DNA–metal ion condensates exhibited mainly two different phases and at least one of them was fluid in nature, which is the cholesteric phase and a columnar hexagonal phase with a restricted fluidity where the DNA molecules are more closely packed. In the presence of alkali metal ions spermine–DNA aggregates were mainly in the cholesteric phase but at higher spermine concentrations, in the presence of Rb+ and Cs+, they adopted a cholesteric to columnar arrangement, suggesting that the increased size of Rb+ and Cs+ facilitated the cholesteric to columnar phase transition. Among the alkaline earth metal ions, the existence of fluidic cholesteric textures in the presence of Mg2+ can be thought of probably as a clue to the possible synergistic role of polyamines and the Mg2+ in the cell nucleus, in preserving the fluidity required for the biological functions of DNA within the condensates. The evolution of the columnar phase from the cholesteric phase in the presence of Ca2+ also indicates the probable role of Ca2+ in packing DNA into hexagonal arrangement in vivo. Among all the metal ions studied, the behavior of Na+ was exceptional in inducing DNA resolubilization at a very low concentration of 12 mM spermine whereas with the other metal ions DNA got resolubilized at or above 400 mM spermine concentration. Small angle X-ray diffraction peaks obtained at 2θ value between 0 and 5 also indicate the formation of the columnar hexagonal phase with Rb–DNA, Cs–DNA and Ca–DNA systems.
Introduction
In the search for an appropriate biological cation for the condensation of DNA into compact nanoparticles for potential applications to DNA packaging and gene therapy, the investigations on spermine and its chemical analogues as perspective materials for condensing DNA have given rise to encouraging results.1–10 The condensation of DNA with polyamines such as spermine has been studied extensively and they have been shown to induce DNA condensation and to stabilize compact forms of DNAin vitro under controlled conditions.11–13 Since polyamines associate with DNA by electrostatic interactions, a possible function of polyamines in the cell might involve the supramolecular organization of DNA, including LC DNA. In a series of experiments, Livolant and colleagues and Thomas and colleagues demonstrated that spermidine and spermine are capable of provoking multiple LC forms of fragmented DNA.14–21Polyamines are now believed to interact electrostatically with the charged DNA phosphates22 and it has been shown recently that spermine exhibits possibly very weak, base sequence dependence.22,23 It has been recently established that metal ions can be treated as factors modifying the character of interactions between polyamines and fragments of nucleic acids.24 Similarly polyamines interfere in the reactions of metal ions and bioligands. The stability and character of complexes with polyamines and fragments of nucleic acids depend mainly on the type of metal ion. Generally “hard” ions of alkaline earth metals interact mainly with hard oxygen atoms from phosphate groups, whereas more soft nitrogen atoms from purine and pyridine bases interact with soft ions of transition metals.24–26
In general, the affinity of a cation for a specific site on a polynucleotide is a function of its charge, hydration-free energy, coordination geometry, and coordinate bond forming capacity.27,28 Those metal ions that bind primarily to the phosphates stabilize the helix by reducing the intermolecular repulsion, which provokes LC ordering by maintaining the rigid rod like shape of the DNA molecule, which is the determinant factor of its LC ordering.
Saminathan et al. in a recent study have reported the effect of spermine, spermidine and its synthetic analogues on the LC behavior of high molecular weight DNA.14 This study showed a structural specificity effect of polyamines on LC phase transitions of DNA and suggests a possible physiological function of natural polyamines. It is believed that many metal ions in combination with ubiquitous cellular molecules such as polyamines serve critical functions across a multitude of biochemical processes, and are necessary to reach the ultimate states of condensation in the cell.27,29 Detailed investigations on the combined effects of spermine and metal ions are limited except for a few reports where the spermine induced LC organization of low molecular weight fragmented DNA was investigated in the presence of Na+, K+, Mg2+ and Ca2+ under defined ionic conditions.9–13 As the stability and character of complexes with polyamines and fragments of nucleic acids depend mainly on the type of metal ion, the present work was undertaken to investigate the spermine induced LC organization of calf thymus DNA, phase transitions and its stability in the presence of alkali metal ions such as Li+, Na+, K+, Rb+ and Cs+, and alkaline earth metal ions such as Mg2+, Ca2+, Ba2+ and Sr2+ using polarized light microscopy and small angle X-ray diffraction techniques.
Experimental
Calf thymus (CT) DNA was purchased from Worthington Biochemical Corporation, New Jersey, and was used without further purification. The weight average molecular weight of the DNA is 6 × 106. The observed A260/A280 ratio of the DNA solution is 1.88, indicating that the DNA is free of protein contamination. Preliminary experiments indicated that cacodylate buffer and phosphate buffer have interactions with various metal ions. Acetate/citrate buffer was noted to have minimal interactions. The best results were obtained when DNA was dissolved in 0.1 M NaCl (pH 7). A comparative study indicated that similar results are obtained with acetate/citrate buffer and with 0.1 M NaCl at pH 7.Spermine·4HCl was purchased from Sigma Chemical Co. (St Louis, MO). LiCl, NaCl, KCl, RbCl, CsCl, MgCl2, CaCl2, BaCl2 and SrCl2 of analytical grade were used. Autoclaved Millipore water was used as the medium in all the experiments. The concentrations of alkali and alkaline earth metals for the preparation of metal–DNA complexes were selected as 300 mM and 10 mM respectively in the present experiments, considering their abundance at the cellular level.
DNA–metal systems were prepared by dissolving 5 mg of DNA (15.01 mM), 0.3 M LiCl, 0.3 M NaCl, 0.3 M KCl, 0.3 M RbCl, 0.3 M CsCl, 0.01 M MgCl2, 0.01 M CaCl2, 0.01 M BaCl2, and 0.01 M SrCl2. In order to attain homogeneity prior to the start of the experiment DNA–metal ion solutions were vortexed for about 15–20 minutes and were allowed to attain equilibrium at room temperature. The concentration of calf thymus DNA was determined by measuring the absorbance at 260 nm and using the molar extinction coefficient of 6900 per M cm−1.
The DNA concentration of 15 mM was selected for the present series of experiments because LC phase transitions could be observed with spermine at this concentration. Spermine solutions of concentrations varying from 0.1 mM to 600 mM were prepared in autoclaved Millipore water. DNA and spermine solutions were stored at 4 °C. The solutions were homogeneous at the start of our experiments. The critical spermine concentration required for inducing DNA precipitation in the presence of each ion was determined by mixing spermine solutions of appropriate concentration with aliquots of DNA. Following the addition of salts to the solutions, the sample was incubated at room temperature (26 °C) for 15 minutes and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 5 minutes. The pellets were then sandwiched between a microscopic glass slide and cover slip and sealed using DPX (DPX is a neutral solution of polystyrene and plasticizers in xylene) to prevent dehydration of the sample.
000 × g for 5 minutes. The pellets were then sandwiched between a microscopic glass slide and cover slip and sealed using DPX (DPX is a neutral solution of polystyrene and plasticizers in xylene) to prevent dehydration of the sample.
Polarizing microscopy
DNA was precipitated on addition of the spermine solution either directly [10 μl of the metal ion DNA solution (5 mg ml−1) was mixed with the corresponding spermine solution hence the concentration of DNA in the precipitate i.e.DNA rich region was ∼47.5 μg and in the DNA poor region i.e. supernatant was∼2.5 μg, which was highly insufficient to show LC behaviour] on the glass slide or in an Eppendorf tube and centrifuged to sediment the precipitate for observation. The glass slides were soaked in chromic acid, cleaned with distilled water, rinsed with ethanol and dried prior to sample preparation. The DNA precipitate spread over the glass slides with a cover slip and sealed with DPX as described above was observed under polarizing light or phase contrast light in a Nikon Optiphot microscope. After observing the initial phase appearance at room temperature, the preparations were incubated at 37 °C for extended time periods to observe the phase changes until crystallization or complete darkening (isotropization) occurred.14 The preparations were examined periodically for phase changes and photographs were taken when the phases became prominent and distinct. The phases and granular boundaries were clear and sharp when the sample was incubated at 37 °C. A triplicate of each sample was made to ensure the reproducibility of the phase changes. The results were reproducible in three sets of separate experiments. The following parameters were noted: (a) Csp—critical spermine concentration required to exhibit anisotropy, (b) Tiso—time required for the phases to darken and disappear i.e. isotropization to occur.SAXS measurements
Small angle X-ray diffractionsof DNA samples were recorded by a Philips Analytical diffractometer using CuK-α emission. The spectra were recorded in the range of 2θ = 0–40 and analyzed using X′ Pert software. We prepared the slides simultaneously for both microscopic and XRD studies which were kept for ∼4 to 5 hours for phase stabilisation at 37 °C. Phase transitions were slow enough to record XRD profiles which were done once the phases became prominent and distinct. The samples were exposed for 5 minutes. Observation of LC textures on a polarizing Nikon Optiphot Microscope was also made in parallel with X-ray measurements.Results and discussion
General observations
Previous studies of LC phase transitions of calf thymus DNA in the presence of polyamines were carried out with low molecular weight fragments, prepared by either sonication or micrococcal nuclease digestion.17,18,30 The first study, as stated earlier, using high molecular weight DNA and spermine and its synthetic analogues was reported by Saminathan et al.14 In another study on the effects of various metal ions on the LC phases of high molecular weight DNA, we have observed that several parameters such as size, charge, mode of binding, hydration and time were found to influence the induction and stabilization of the LC phases.31 Use of high molecular weight DNA in these studies would be beneficial as they may probably provide information on the supramolecular organization of DNA in the cell. In the present study, LC DNA could be obtained at concentrations (5 mg ml−1) that are far less than that necessary for low molecular weight (150 mg ml−1) DNA and the mesophases are comparable with those obtained with low molecular weight DNA.The in vitroprecipitation/condensation of DNA by spermine has been investigated by a number of researchers and it was found that the DNA precipitation efficacy of spermine is highly dependent on the other counter ions bound to DNA.11–17 The spermine induced DNA pellets/aggregates appeared as transparent colloid-like mass to white opaque gels, which exhibited multiple macroscopic polymorphic behavior depending on the concentration of both spermine and the counter ions. They are either dense or partially fluid in nature flowing spontaneously under the microscope. The fluidity of the ordered phase suggests that spermine binds along the strands, which would allow the strands to slide on each other. When deposited between the glass slide and the cover slip these aggregates show multiple textures (Fig. 3–5), which depend on the nature of the phase and also on the thickness of the preparation. Two main phases, cholesteric and columnar hexagonal, are found in our study, either separately or in coexistence, with variations depending on local conditions. A cholesteric phase is frequently obtained in the samples, but its characteristic fingerprint pattern could not be observed usually. Instead, birefringent domains are usually seen with homogeneous illumination. But the presence of tear-shaped defects attest to the formation of the cholesteric phase. Numerous isotropic droplets of various sizes are also seen trapped in the birefringent cholesteric phase. Sometimes nematic textures were also observed with two or four branch brush defects. These textures probably correspond to the previously observed cholesteric phase, and are formed rarely where anchoring effects on the glass slides prevent the twist. They are slightly distorted into squares in very thin preparation.
It should be pointed out here that blue phases commonly known as precholesteric phases32 which are a transition from the isotropic to the cholesteric phase, are not observed in the present case. However, the higher ordered columnar hexagonal phases were observed when the concentration of spermine is above 200 mM, but only with metal ions of higher sizes. These domains are much more viscous than the cholesteric phase. In the columnar hexagonal phase, the DNA molecules are unidirectionally aligned with a lateral hexagonal order.16 Undulations typical of the hexagonally ordered columnar phase were also noticed. The fluidity and order required for a LC state are observed here. It is important to note here that the time dependent changes in LC textures of DNA occurred under conditions in which solvent evaporation was prevented by sealing the glass slides with a neutral solution of polystyrene and plasticizers in toluene. Therefore, the observed changes are a consequence of the reorganization of polyamines and metal ions on the DNA strands.
Fig. 1 shows the Csp values of spermine concentration required for the onset of DNA precipitation in the presence of metal ions differing in their charge, size and hydration energy. Two distinct plateaus can be seen from Fig. 1. Plateau 1 for the alkali metal–DNA systems occur around a concentration of 0.75 mM. It should be noted that spermine alone precipitates DNA at a concentration of 0.85 mM. In the presence of alkaline earth metal ions, spermine precipitates DNA at a concentration of 0.275 mM. In short, Csp (spermine alone) > Cspalkali metal ions > Cspalkaline earth metal ions. This suggests that alkali and alkaline earth metal ions might have acted in concert with spermine which facilitated the precipitation range of spermine (Fig. 1). Fig. 1 also reveals that charge and size of the ion are playing important roles in the precipitation of DNA in the presence of metal ions.
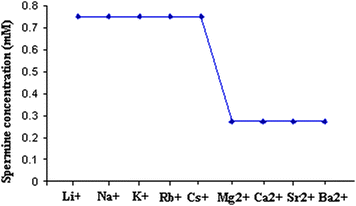 | ||
| Fig. 1 Critical spermine concentration (Csp) required for the onset of DNA precipitation with alkali and alkaline earth metal ions. | ||
Fig. 2 gives the overall LC phase behavior of DNA in the presence of the metal ion studied with respect to the change in spermine concentration. It can be seen from Fig. 2 that at or near 400 mM of spermine concentration, the precipitated DNA resolubilizes due possibly to the screening of short-range electrostatic interactions between the polyamine molecules and the DNA strands and goes to the isotropic state. Another important finding is that of the sodium ion, which resolubilizes the DNA at extremely low spermine concentration. Any increase in spermine concentration after the precipitation of DNA would naturally increase the osmotic stress of the medium. The increase in number of the spermine molecules around DNA would screen the intermolecular interaction between DNA strands thereby reducing the order parameter and finally to the isotropic state.
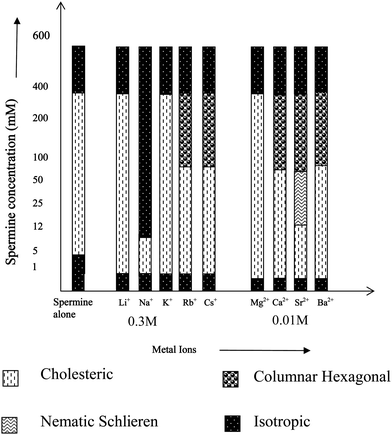 | ||
| Fig. 2 LC behavior of spermine induced DNA condensates in the presence of metal ions of varying size and charge. | ||
Effect of alkali metal ions on the spermine induced LC DNA
Among the alkali metal ions, Na+ and K+ are widely distributed in most of the biological systems and are involved in a variety of cellular functions.In the presence of Li+, Na+ and K+, spermine-DNA aggregates were mainly in the cholesteric phase (Fig. 3a–c) that flows spontaneously under the microscope (Fig. 4a–c) and no transition to the columnar phase has been observed. In our previous study, both cholesteric and columnar phases were observed when high molecular weight DNA was condensed with spermine,14 but these experiments were carried out in the absence of any counter ions.
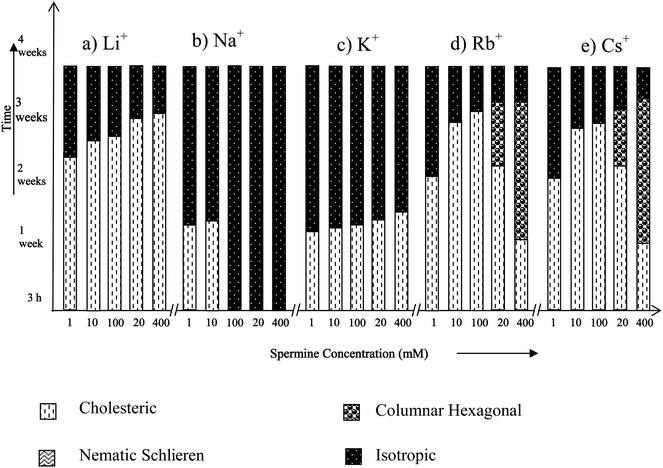 | ||
| Fig. 3 Time dependent phase transitions of spermine induced DNA condensates in the presence of alkali metal ions. | ||
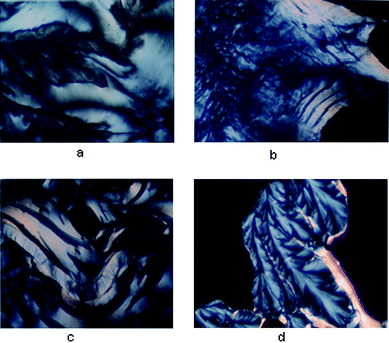 | ||
| Fig. 4 (a) Li–DNA was treated with 200 mM spermine and incubated in a glass slide at 37 °C—a fluid cholesteric phase was obtained after 3 h. (b) Cholesteric phase obtained after 3 h. with Na–DNA when treated with 200 mM spermine and incubated in a glass slide at 37 °C. (c) Cholesteric phase with tear shaped defects obtained after 3 h. with K–DNA when treated with 200 mM spermine and incubated in a glass slide at 37 °C. (d) Dendrimeric growth obtained after 1 week with Rb–DNA when treated with 200 mM spermine and incubated in a glass slide at 37 °C. | ||
Pelta et al. and Saminathan et al. have studied the precipitation of short DNA fragments by the polycations spermidine and spermine in the presence of Na+, and have shown that resolubilization of DNA aggregate is essentially dependent on the Na+ concentration present in the DNA solution.13,27 This is comparable to the present observation with high molecular weight DNA molecules also. Na+ appears to be exceptional among alkali metal ions in resolubilising precipitated DNA (see Fig. 3), which suggests that spermine and Na+ concentrations would be balanced in the cell nucleus to maintain DNA in the condensed states. Na+ and K+ are the major extracellular and intracellular cations respectively in the body fluids of animals including human beings. Even though, Na+ and K+ are abundant in biological systems, Na+ concentration is always maintained low inside the cell (high K+ inside the cell) by a Na+–K+ pump with the help of an enzyme Na–K ATPase present in the cell membrane through the active ion transport mechanism.33 Each operation of the pump pulls out a larger number of sodium ions from the cell than the number of K+ ions it pumps into the cell. The maintenance of low Na+ concentration inside the cell could also be due to the fact that higher Na+ concentration will not favor the existence of condensed states of DNA essential for its biological functions such as replication, etc. Below 12 mM spermine, DNA exhibited an iridescent fluidic cholesteric phase in the presence of sodium, which remained stable for two weeks. The influence of Na+ in the presence of a polyamine in controlling the supramolecular order of DNA will have many biological implications.
Fig. 3 shows that at higher spermine concentrations (200 and 400 mM), in the presence of Rb+ and Cs+, spermine–DNA condensates adopted a cholesteric to columnar arrangement, showing broken-fan shaped textures (Fig. 4d) but at lower spermine concentrations transition from the cholesteric to columnar phase was not observed, and the cholesteric phase directly underwent isotropization. It appears that the size of the ion exerts additional influence on the induction and stabilization of the LC phases of DNA.
The X-ray diffraction patterns of few spermine–metal ion–DNA condensates are presented in Fig. 7. In general, a sharp and strong peak at a low angle (1° < 2θ < 4°) in the small angle X-ray scattering (SAXS) curve and a broad peak associated with lateral packing in the wide angle X-ray diffraction region (WAXD) can be observed for the columnar structure. For the cholesteric structure no patterns appear in SAXS, however a broad peak occurs at 2θ = 16–18. The diffraction angles of the cholesteric structure at wide angle are obviously less than that of columnar and nematic structures and this is a very important character to judge the cholesteric structure.34,35Small angle X-ray diffraction patterns obtained at 2θ value between 0 and 5 also indicate the formation of a columnar hexagonal phase (Fig. 7). The broad peak obtained at the wide angle region could be of the cholesteric phase. It appears that higher spermine concentration and the increased size of Rb+ and Cs+ presumably facilitated the cholesteric to columnar transition. In the presence of Li+, an unusual stability of DNA mesophase was reported in our recent work.36 However, in the presence of spermine lithium did not impart the unusual stability. The hydrated structure of Li+ ions37,38 which is responsible for the stability factor might have been disturbed in the presence of spermine. The stability of the phases was in the following order: TisoCs+ = TisoRb+ > TisoLi+ > TisoK+ > TisoNa+. In the case of all the alkali metal ions, except sodium, higher spermine concentrations rendered more stability to the LC phases.
Effect of alkaline earth metal ions on the spermine induced LC DNA
Magnesium and calcium ions are the most abundant alkaline earth metal ions in the biological systems. They serve a number of critical functions across a multitude of biochemical processes and are also necessary to reach the ultimate states of condensation.28,30 They are reported to be essential in folding and winding of the polynucleic acids and to assume compact arrangements in vivo.39,40 A previous study on the influence of Mg2+/Ca2+ on low molecular weight DNA fragments has reported the formation of LC phases, but the phases were not identified.41Fig. 5 shows that except Mg2+, the alkaline earth metal ions exhibit both cholesteric and columnar hexagonal phases as a time dependent phenomenon above a spermine concentration of 200 mM. It shows that the cholesteric to columnar transitions depend on the size of the ion.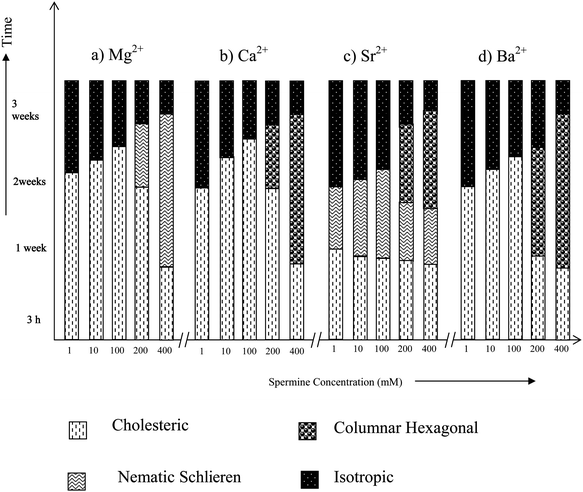 | ||
| Fig. 5 Time dependent phase transitions of spermine induced DNA condensates in the presence of alkaline earth metal ions. | ||
In the presence of Mg2+, below 400 mM spermine concentrations cholesteric with nematic thread-like fluidy structures (Fig. 6a and c) were formed initially which got darkened eventually without transforming into the higher ordered columnar phase (Fig. 5a). The existence of fluidic textures gives a clue to the possible physiological role of polyamines and the Mg2+ in the cell nucleus, where chromatin is condensed, still maintaining the mobility of the double strands required for the biological functions of DNA within the condensates. A cholesteric organization of DNA was reported in dinoflagellate chromosomes42–44 and in certain bacterial nucleoids.45 It was also shown that the condensed DNA in the presence of polyamines can stimulate the functional properties of DNA such as the efficiency of replication and transcription of DNA.18
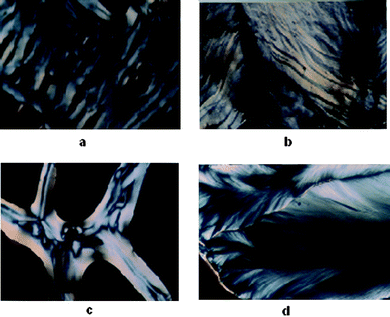 | ||
| Fig. 6 (a) Fluid cholesteric phase obtained after 3 h when Mg–DNA was treated with 100 mM spermine and incubated in a glass slide at 37 °C. (b) Cholesteric phase with striped pattern obtained after 12 h with Ca–DNA when treated with 200 mM spermine and incubated in a glass slide at 37 °C. (c) Cholesteric phase with nematic threaded structures obtained after 3 h with Sr–DNA when treated with 200 mM spermine and incubated in a glass slide at 37 °C. (d) Fan shaped textures of columnar phase obtained after 1 week with Ca–DNA when treated with 200 mM spermine and incubated in a glass slide at 37 °C. | ||
A cholesteric with nematic thread like fluidy structure (Fig. 6c) was observed with Sr2+ also, the phase instead of darkening transformed itself into the higher ordered columnar phase. This transition from cholesteric to columnar hexagonal phase could be considered mainly as a size effect.
In the presence of calcium and barium below 400 mM spermine concentrations, the cholesteric phase was formed initially which developed a striped appearance followed by broken fan shaped textures typical of the columnar phase (Fig. 6b and d) and became darkened subsequently (Fig. 5b and d). Small angle X-ray diffraction patterns obtained at 2θ value between 0 and 5 also indicate the formation of a columnar hexagonal phase (Fig. 7). Our earlier work with spermine alone did not give a well-defined hexagonal order, instead some antiparallel arrangement adopting a hexagonal order was observed.
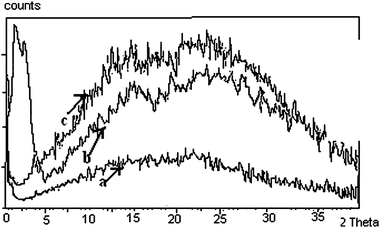 | ||
| Fig. 7 XRD patterns obtained with the biphasic sample obtained on precipitation with 200 mM spermine. The low angle peak corresponds to the columnar packing of DNA molecules and the wide-angle broad peak is related to the cholesteric phase of the sample. (a) Rb–DNA and (b) Cs–DNA (c) Ca–DNA system. The arrows indicate the curves of the corresponding metal–DNA condensates. Rb–DNA (a) and Cs–DNA (b) being biphasic have peaks both at the small and wide angle region, whereas Ca–DNA ‘c’ has only one peak at the wide angle region. | ||
The formation of well defined, broken fan shaped textures typical of the columnar phase suggests the importance of the synergistic effect of calcium ions and spermine in inducing the most efficient columnar packing of DNA molecules in vivo. A hexagonal packing of DNA was observed in bacteriophages and certain sperm heads.46 As alternate condensation and decondensation of DNA are believed to occur when DNA functions in the cell nucleus, both cholesteric and columnar phases may exist according to the local requirement.
Earlier studies with the induction and stabilization of the LC phases with metal ions of varying sizes indicated that the transition from the cholesteric to columnar hexagonal phase was facilitated by the size of the concerned ion.31Fig. 2 also indicates a similar effect. It can also be noted for Fig. 5 that as the spermine concentration increases the stability of the phases also increases. Thus at 400 mM, TisoMg2+ = TisoCa2+ = TisoSr2+ = TisoBa2+. Thus <400 mM, TisoMg2+ > TisoCa2+ = TisoSr2+ > TisoBa2+.
Conclusions
The effect of spermine on the induction and stabilization of liquid crystalline phases of high molecular weight DNA in the presence of alkali and alkaline earth metal ions was investigated. The spermine induced DNA pellets/aggregates exhibited multiple macroscopic polymorphic behavior depending on the concentration of both spermine and the charge and size of the metal ions and time. The LC phases of high molecular weight DNA were observed at concentrations (5 mg ml−1) that are far less than that necessary for low molecular weight (150 mg ml−1) DNA. The concentration of spermine to precipitate DNA in the presence of the metal ions followed an order (spermine alone > Cspalkali metal ions > Cspalkaline earth metal ions) based both on the charge and size of the ion. However, the higher ordered columnar hexagonal phases were observed when the concentration of spermine is above 200 mM and with metal ions of higher sizes. The alkaline earth metal ions with the exception of Mg2+, exhibited a more mobile cholesteric phase that got transformed into broken fan shaped columnar hexagonal phases as a time dependent phenomenon above a spermine concentration of 200 mM. The transformation of the cholesteric phase into a stable higher ordered columnar hexagonal phase in the case of Ca2+ might suggest a probable role of Ca2+ in packing DNAin vivo into hexagonal arrangement. The existence of a stable fluid cholesteric phase induced by spermine in the presence of Mg2+ can be thought of possibly as a clue to the role of Mg2+ in condensing DNA synergistically with spermine in the cell nucleus and preserving the mobility required for the biological functions in the condensed state due to its (Mg2+) higher hydration energy.Among all the metal ions studied, the behavior of Na+ was exceptional in inducing resolubilization at a very low spermine (12 mM) concentration whereas with the other metal ions DNA got resolubilized at or above 400 mM concentration of spermine. Small angle X-ray diffraction peaks obtained at 2θ value between 0 and 5 also indicate the formation of a columnar hexagonal phase with Rb–DNA, Cs–DNA and Ca–DNA systems. It appears that the alkali and alkaline earth metal ions might have been involved in a condensation process in concert with spermine, which facilitated the precipitation of DNA.
Acknowledgements
Financial support of CSIR to NS is highly acknowledged.References
- S. B. Zimmerman and L. D. Murphy, FEBS Lett., 1996, 390, 245–248 CrossRef CAS.
- C. W. Tabor and H. Tabor, Annu. Rev. Biochem., 1976, 45, 285–306 CrossRef CAS.
- E. S. Canellakis, D. Viceps-Madore, D. A. Kyriakidis and J. S. Heller, Curr. Top. Cell. Regul., 1979, 15, 155–202 CAS.
- T. Thomas and T. J. Thomas, Cell. Mol. Life Sci., 2001, 58, 244–258 CrossRef CAS.
- L. V. Dam, N. Korolev and L. Nordenskiöld, Nucleic Acids Res., 2002, 30(2), 419–428 CrossRef.
- L. M. Santhakumaran, A. Chen, C. K. S. Pillai, T. Thomas, H. X. He and T. J. Thomas, Nanotechnology in Non-Viral Gene Delivery, Book Chapter in Nanofabrication for Biomedical Applications, Wiley-VCH, 2004 Search PubMed.
- V. Vijayanathan, T. Thomas and T. J. Thomas, Biochemistry, 2002, 41, 14085–14094 CrossRef CAS.
- V. Vijayanathan, T. Thomas, L. H. Sigal and T. J. Thomas, Antisense Nucleic Acid Drug Dev., 2002, 12, 225–233 CrossRef CAS.
- V. Vijayanathan, T. Thomas, A. Shirahata and T. J. Thomas, Biochemistry, 2001, 40, 9387–9395 CrossRef.
- V. B. Morris, N. Sundaresan, T. E. Abraham, C. P. Sharma and C. K. S. Pillai, J. Biomed. Mater. Res., Part B, 2009, 89(2), 282–292 CrossRef.
- V. A. Bloomfield, Biopolymers, 1991, 31, 1471–1481 CrossRef CAS.
- L. C. Gosule and J. A. Schellman, Nature, 1976, 259, 333–335 CrossRef CAS.
- E. Raspaud, I. Chaperon, A. Leforestier and F. Livolant, Biophys. J., 1999, 1547–1555 CrossRef CAS.
- M. Saminathan, T. J. Thomas, T. Thresia, A. Shirahata, C. K. S. Pillai and T. J. Thomas, Nucleic Acids Res., 2002, 30, 3722–3731 CrossRef CAS.
- J. Pelta, F. Livolant and J. L. Sikorav, J. Biol. Chem., 1996, 271, 5656–5662 CrossRef CAS.
- F. Livolant and A. Leforestier, Prog. Polym. Sci., 1996, 21, 1115–1164 CrossRef CAS.
- A. Leforestier and F. Livolant, Biophys. J., 1993, 65, 56–72 CrossRef CAS.
- J. L. Sikorav, J. Pelta and F. Livolant, Biophys. J., 1994, 67, 1387–1392 CrossRef CAS.
- A. Leforestier, S. Fudaley and F. Livolant, J. Mol. Biol., 1999, 290, 481–494 CrossRef CAS.
- E. Raspaud, M. Olvera de la Cruz, J. L. Sikorav and F. Livolant, Biophys. J., 1998, 74, 381–393 CrossRef CAS.
- B. Andreasson, L. Nordenskiöld and W. H. Braunlin, Biopolymers, 1996, 38, 505–513 CrossRef CAS.
- H. Robinson and A. H. J. Wang, Nucleic Acids Res., 1996, 24, 676–682 CrossRef CAS.
- H. Deng, V. A. Bloomfield, J. M. Benevides and G. J. Thomas, Jr, Nucleic Acids Res., 2000, 28, 3379–3385 CrossRef CAS.
- L. Lomozik, R. Jastrzab and A. Gasowska, Polyhedron, 2000, 19, 1145–1154 CrossRef CAS.
- A. Gąsowska and L. Łomozik, J. Coord. Chem., 2001, 52, 375–382 CrossRef.
- A. Gąsowska, R. Jastrząb, R. Bregier-Jarzębowska and L. Łomozik, Polyhedron, 2001, 20, 2305–2313 CrossRef.
- J. P. Glusker, Adv. Protein Chem., 1991, 42, 1–76 CrossRef CAS.
- V. K. Misra and D. E. Draper, Biopolymers, 1998, 48, 113–135 CrossRef CAS.
- R. H. Holm, P. Kennepohl and E. I. Solomon, Chem. Rev., 1996, 96, 2239–2314 CrossRef CAS.
- J. Pelta, Jr, D. Durand, J. Doucet and F. Livolant, Biophys. J., 1996, 71, 48–63 CrossRef.
- N. Sundaresan, C. H. Suresh, C. K. S. Pillai, T. Thomas and T. J. Thomas, Biomacromolecules, 2008, 9(7), 1860–1869 CrossRef CAS.
- T. E. Strzelecka and R. L. Rill, Biopolymers, 1990, 30, 57–71 CrossRef CAS.
- P. L. Jorgensen, K. O. Hakansson and S. J. Karlish, Annu. Rev. Physiol., 2003, 65, 817–849 CrossRef CAS.
- I. Koltover, K. Wagner and C. R. Safinya, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 14046–14051 CrossRef CAS.
- Y. M. Dong, Q. Yuan and Z. L. Xiao, Chem. J. Chinese. Univ., 1999, 20, 140 CAS.
- N. Sundaresan, T. Thomas, T. J. Thomas and C. K. S. Pillai, Macromol. Biosci., 2006, 6, 27–32 CrossRef CAS.
- B. R. Puri, L. R. Sharma and K. C. Kalia, Principles of Inorganic Chemistry, Shobhan Lal Nagin Chand & Co., 1998 Search PubMed.
- N. Sundaresan and C. H. Suresh, J. Chem. Theory Comput., 2007, 3(3), 1172–1182 CrossRef CAS.
- J. Widom, Annu. Rev. Biophys. Biomol. Struct., 1998, 27, 285–327 CrossRef CAS.
- N. Sundaresan, C. K. S. Pillai and C. H. Suresh, J. Phys. Chem. A, 2006, 110(28), 8826–8831 CrossRef CAS.
- V. I. Salyanov, P. I. Lavrent'ev, B. A. Chernuka and Y. M. Evdokimov, Mol. Biol., 1995, 28, 796–800 Search PubMed.
- F. Livolant, Eur. J. Cell Biol., 1984, 33, 300–311 CAS.
- Y. Bouligand, M. O. Soyer and S. Puiseux-Dao, Chromosoma, 1968, 24, 251–287 CrossRef CAS.
- R. L. Rill, F. Livolant, H. C. Aldrich and M. W. Davidson, Chromosoma, 1989, 98, 280–286 CrossRef CAS.
- J. P. Gourett, Biol. Cell, 1978, 32, 299–306 Search PubMed.
- J. Lepault, W. Dubochet, W. Baschong and E. Kellenberger, EMBO J., 1987, 6, 1507–1512 CAS.
| This journal is © The Royal Society of Chemistry 2011 |
