DOI:
10.1039/C1PY00286D
(Paper)
Polym. Chem., 2011,
2, 2830-2834
Light-emitting poly(N-naphthyl-2,7-carbazole)
Received
23rd June 2011
, Accepted 21st August 2011
First published on 10th October 2011
Abstract
A blue-emitting poly(2,7-carbazole) having a naphthyl substituent at the N-position (PNCz) was newly synthesised by the Yamamoto method and used to fabricate an organic light emitting diode (OLED). PNCz was highly soluble in common organic solvent with the number average molecular weight (Mn) of 17600 and good thermal stability, with the decomposition temperature higher than 390 °C. PNCz exhibited intense blue photoluminescence with emission peak maxima at 415 nm in CHCl3 and 428 nm in the film state, and the fluorescence quantum efficiency in CHCl3 was extremely high (Φfl = 0.99). The HOMO level estimated by cyclic voltammetry was −5.50 eV and the band gap estimated from an absorption edge was 2.72 eV. The OLED device with a configuration of ITO/PEDOT:PSS/PNCz/CsF/Al exhibited bluish emission with several hundreds cd m−2 and CIE index (x = 0.20, y = 0.21) at low operating voltages in the range of 6–7 V and greenish emission with about 3000 cd m−2 and CIE index (0.25, 0.44) under high operating voltage around 11 V.
Introduction
Poly(2,7-carbazole) and poly(3,6-carbazole) derivatives have been extensively investigated for various organic electronic applications such as organic light emitting diodes (OLEDs), organic photovoltaic cells (OPCs) and organic field effect transistors (OFETs).1–6 In particular, poly(2,7-carbazole)s have attracted more attention for emitting materials in OLEDs than poly(3,6-carbazole)s, which is due to their effective conjugation and intense blue emission comparable to polyfluorenes.1,2,4 During past two decades, a variety of poly(2,7-carbazole)s have been synthesised and their electroluminescence characteristics were investigated. In general, there are two strategies to modify physical, optical and electronic properties of the poly(2,7-carbazole)s. One is copolymerisation, which is an easy method to modify optical and electronic properties. Therefore many poly(2,7-carbazole)-based copolymers have been designed to modify and improve electroluminescence characteristics such as brightness, efficiency and emission colour.7–11 Another is to functionalise the N-position of the carbazole ring by introducing alkyl or aryl groups. The N-alkyl groups mainly improve solubility of polymers in common organic solvents even though they have high molecular weights.12 On the other hand, the N-aryl groups generally decrease the solubility of polymers due to their rigid structures.13 However, introducing two flexible side chains on the phenyl ring afforded high-molecular-weight polymers with satisfactory solubility.2–4 Additionally, the substituents on the aryl rings have affected not only the solubility but also the electronic properties. Electron donating substituents such as alkoxy and amino groups enable the highest occupied molecular orbital (HOMO) level of the polycarbazoles to be raised and improve the hole-injection properties from the anode of ITO/PEDOT:PSS.2,14 On the other hand, some substituents lowered the HOMO levels, e.g., the result from a study of the polycarbazole with an oxadiazole pendant indicated that the electron deficient group lowered the energy levels of polycarbazoles.15 A similar tendency was observed in our results using silyl substituted phenyl ring that exhibited a low HOMO level of −5.70 eV.10 Among these studies for emitting materials using N-functionalised polycarbazoles, poly(N-phenyl-2,7-carbazole)s showed better performance in OLEDs than poly(N-alkyl-2,7-carbazole)s.2,5,13 Consequently, this motivates us to study the effect of introducing another aryl group at the N-position on polycarbazole.
Herein we focused on introducing a 1,5-naphthalene moiety in prospect of efficient blue luminescence affected by rigid steric effect, addition of n-type semiconducting properties induced by the naphthalene moiety,16 and ultimately efficient OLED performance with balanced carrier injection and transport. In this paper, poly(N-naphthyl-2,7-carbazole) was newly synthesised and its electronic and optical properties were compared with those of poly(N-phenyl-2,7-carbazole) to examine the effect of the aryl groups at the N-position.
Experimental section
Materials
5-Amino-1-naphtol
1 was purchased from Kanto Chemical Co Inc. and used without any purification. 5-Iodo-1-naphtol 2, 2,7-dichlorocarbazole 4, and poly(N-(4-(2-ethylhexyloxy)phenyl)-2,7-carbazole) PPCz were synthesised according to the literature.13Bis(1,5-cyclooctadiene)nickel(0) (Ni(cod)2) was purchased from Kanto Chemical Co Inc., and used under nitrogen atmosphere. Other reagents and solvents were purchased from Kanto Chemical, Tokyo Chemical Industry, Aldrich and Nacalai Tesque Inc. N,N-Dimethylformamide (DMF), acetonitrile and tetrahydrofuran (THF) were purified by distillation using an appropriate drying reagent such as molecular sieves, sodium hydride and sodium, respectively. Other chemicals were used as received without further purification.
Characterisation
1H and 13C NMR data were collected on a JEOL EX-270 at 270 MHz using a CDCl3 solvent. Thermogravimetric analysis (TGA) was carried out at a heating rate of 10 °C min−1 under an argon atmosphere using a SII EXSTAR6000 TG/DTA. Decomposition temperature was determined at 5 wt % loss of the polymer. Photoluminescence (PL) and UV-vis absorption spectra were measured using a Hitachi F-4500 spectrophotometer and a Hitachi U-3500 spectrofluorometer, respectively. The fluorescence quantum yields in CHCl3 were estimated using 9,10-diphenylanthracene (Φfl = 0.90) in cyclohexane as a standard. Average molecular weights (Mn and Mw) of the polymers were determined by gel permeation chromatography (GPC) using polystyrene standards in THF. Electrochemical measurements of the polymers were performed by cyclic voltammetry equipped with a Pt disc electrode (diameter: 5 mm) as the working electrode, a Pt plate as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode. The measurements were carried out under a nitrogen atmosphere in an acetonitrile solution (0.1 M Et4NBF4) at a scan rate of 50 mV s−1. Ionisation potentials (vs. vacuum) of the polymers were estimated from the onset of their oxidation peaks in the cyclic voltammograms on the basis that ferrocene/ferrrocenium is 4.8 eV below the vacuum level, and the HOMO levels (EHOMO) basically followed the equation:17,18
Synthesis
1-(2-Ethylhexyloxy)-5-iodo-naphthalene (3).
Under nitrogen atmosphere, 2 (1.00 g, 3.70 mmol) and K2CO3 (0.614 g, 4.44 mmol) were added to DMF (5 ml). The reaction mixture was stirred at 80 °C for 10 min. Then 1-bromo-2-ethylhexane (0.766 ml, 4.44 mmol) was added dropwise to the mixture and stirred for 24 h. After the reaction, the mixture was extracted with CHCl3 and water. The organic phase was dried over anhydrous Na2SO4 and evaporated to obtain a crude product. After purification through a silica gel column chromatography (eluent: CHCl3/hexane= 1/1), 1.2 g of pure product was obtained as a clear oil. (Yield 85%). 1H NMR (270 MHz, CDCl3) δ = 0.90 (t, J = 6.91, 3H), 0.97 (t, J = 7.40, 3H), 1.32–1.64 (m, 8H), 1.81–1.88 (m, 1H), 4.03 (d, J = 5.45, 2H), 6.86 (d, J = 7.56, 2H), 7.13 (t, J = 7.43, 1H), 7.44 (t, J = 7.75, 1H), 7.62 (d, J = 8.75, 1H), 7.57 (d, J = 7.24, 1H), 8.28 (d, J = 8.40, 1H). 13C NMR (270 MHz, CDCl3) δ = 11.4, 14.2, 21.5, 23.1, 24.3, 29.2, 30.9, 39.6, 70.7, 98.9, 105.0, 122.9, 123.9, 125.8, 126.4, 127.4, 135.2, 137.9, 154.8. C18H24O2 (382.28): Calcd. C, 56.55; H, 6.06; I, 33.20; O, 4.19. Found. C, 56.74; H, 6.04; I, 29.72.
2,7-Dichloro-9-[5-(2-ethyl-hexyloxy)-naphthalen-1-yl]-9H-carbazole (5).
Into o-dichlorobenzene (1.5 ml) were added 4 (0.380 g, 1.61 mmol), 2 (0.800 g, 2.09 mmol), K2CO3 (1.78 g, 12.9 mmol), Cu (0.409 g, 6.44 mmol), and 18-crown-6 (0.0270 g, 0.102 mmol), which was stirred at 180 °C for 25 h. After the reaction, the mixture was diluted with CHCl3 and filtered off to remove insoluble solid. The filtrate was concentrated under a reduced pressure, then purified with a silica gel column chromatography (eluent: hexane). The pure product (0.065 g) was obtained as a clear oil. (Yield 8%). 1H NMR (270 MHz, CDCl3) δ = 0.85 (t, J = 6.91, 3H), 0.93 (t, J = 7.26, 3H), 1.23–1.62 (m, 8H), 1.79–1.86 (m, 1H), 4.01 (d, J = 5.27, 2H), 6.62 (d, J = 8.56, 1H), 6.79 (d, J = 7.59, 1H), 6.86 (m, 2H). 13C NMR (270 MHz, CDCl3) δ = 25.9, 27.2, 29.0, 30.1, 43.0, 74.2, 108.5, 113.7, 118.7, 119.2, 120.3, 122.7, 125.5, 129.9, 130.9, 140.3, 152.5. C30H29Cl2NO (490.46): Calcd. C, 73.47; H, 5.96; Cl, 14.46; N, 2.86; O, 3.26. Found. C, 73.61; H, 6.23; N, 2.52.
Poly(N-(5-(2-ethylhexyloxy)-1-naphthyl)-2,7-carbazole) (PNCz).
Under nitrogen atmosphere, Ni(cod)2 (0.0943g, 0.343 mmol), 2,2′-bipyridine (0.0515 g, 0.343 mmol) and 1,5-cyclooctadiene (38.6 μl, 0.315 mmol) were dissolved in DMF (1.0 ml) and heated to 80 °C for 30 min. The monomer 5 (0.100 g, 0.143 mmol) dissolved in THF (1.0 ml) under nitrogen was added to the DMF solution. The reaction solution was kept heating at 80 °C for 3 days. After the reaction solution was cooled to room temperature, the resultant polymer was precipitated from methanol/aq. HCl, and reprecipitated from methanol/aq. NH3 and methanol, respectively, to afford 0.060 g of PNCz as a light yellow solid (Yield 100%). 1H NMR (270 MHz, CDCl3) δ = 0.99 (br, 3H), 1.05 (br, 3H), 1.44, 1.65 (br, 8H), 1.97 (br, 1H), 4.14 (d, J = 5.27, 2H), 6.81–6.88 (m, 2H), 7.04–7.21 (m, 3H), 7.43 (br, 2H), 7.61 (br, 2H), 8.09 (br, 2H), 8.51 (br, 1H). 13C NMR (270 MHz, CDCl3) δ = 11.4, 14.1, 23.1, 24.3, 29.3, 30.9, 39.7, 70.5, 105.1, 109.2, 115.5, 120.0, 120.2, 122.0, 123.2, 125.0, 127.3, 133.4, 140.1, 143.0, 155.3. (C30H29NO)n (419.56)n
Fabrication of OLED
The EL devices were fabricated on a patterned indium tin oxide (ITO) coated (150 nm) glass substrate with a sheet resistance of 10.2–11.8 Ω/sq. The substrate was cleaned with a 10% base solution (Clea 635N, Kanto Chemical Co Inc.) in an ultrasonic bath for 5 min, washed under running water for 5 min, dried using a spincoater, and irradiated with a UV light (NL-UV253, Filgen). On top of the substrate, an aqueous solution of poly(ethylenedioxthiophene) doped with poly(styrenesulfonate) (Baytron P VP AI 4083, H. C. Starck) was spin-coated in 90 nm thickness, then dried on a hot plate at 200 °C for 15 min.
After cooling to room temperature, polymers in toluene/o-xylene (7/3, 0.75 wt %) were spin-coated on the substrate. After the spin-coating, the devices were dried under a pressure of 0.3 Torr at room temperature for 90 min. The emitting layers were obtained in 64 nm thickness. After that, CsF was vacuum-deposited in 2 nm thickness and finally Al was deposited in 150 nm thickness under 1.5–2.3 × 10−6 Torr.
Results and discussion
Synthesis
The route for synthesis of the polymer is shown in Scheme 1. The naphthyl part 3 was easily prepared from 1 through 2 by traditional synthetic procedures using Sandmeyer reaction and Williamson etherification as described in the experimental section. Finally, we obtained the monomer 5 using traditional N-arylation of Ullmann type reaction. The homopolymer PNCz prepared from 5 by the standard Yamamoto method of Ni(0)-mediated dehalogenative polycondensation showed number average molecular weights (Mn) of 17600 and weight average molecular weight (Mw) of 42100 (polydispersity index (PDI) = 2.4, degree of polymerisations (D.P.) = 41) determined by gel permeation chromatography (GPC). The polymer was very soluble in common organic solvent such as THF, CHCl3, toluene, DMFetc. The thermogravimetric analysis (Fig. 1) suggests that PNCz has good thermal stability showing a high decomposition temperature (Td = 396 °C).
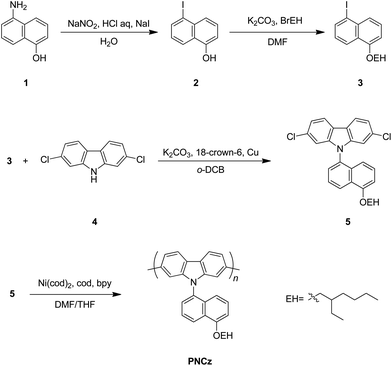 |
| | Scheme 1 Synthesis of poly(N-naphthyl-2,7-carbazole). | |
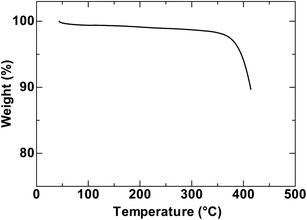 |
| | Fig. 1
TGA traces of PNCz. | |
Photophysical properties
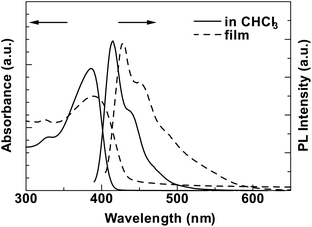
Fig. 2 shows UV-vis and PL spectra of PNCz in CHCl3 and the film state. The photophysical data are compared to those of PPCz reported previously13 and summarised in Table 1. The absorption spectra of PNCz exhibit π–π* transitions peaked at 386 nm in solution and 390 nm in the film state, respectively.
Table 1 Comparison of photophysical data of PNCz and PPCz
| |
UV-vis
λmax (nm) in CHCl3 |
film |
PL
λmax (nm) in CHCL3 |
film |
Φ
fl
a
|
E
g (eV)b |
CIE (x, y)c |
|
Fluorescence quantum yield in CHCl3. (9,10-Diphenylantheracene in cyclohexane as a standard).
Estimated from the edge of the absorption spectra in the film state.
Estimated from PL spectral data in the solid state.
|
|
PNCz
|
386 |
390 |
415 |
428 |
0.99 |
2.72 |
(0.16, 0.13) |
|
PPCz
|
379 |
381 |
420 |
432 |
1.00 |
2.90 |
(0.16, 0.11) |
They showed slight red-shifts of about 7–9 nm compared to those of PPCz. This red-shift is attributed to more regular conformation of the polymer main chain controlled by steric interaction of the rigid and planar naphthalene side group that should be stronger than the case of PPCz. The optical band gap (Eg) estimated from the onset of the absorption spectrum in the film state was 2.72 eV, which was narrower than that of PPCz. The PL spectra show typical vibronic structures of polycarbazoles reported previously.7,12,13 From the result of the absorption spectra, the emission peaks of PNCz are considered to be at longer wavelengths compared to those of PPCz, since the optical band gap of PNCz was smaller than that of PPCz. However, the emission peaks were observed at 415 nm in CHCl3 and 428 nm in the film state, which were about 4–5 nm blue-shifted from those of PPCz. Stokes shifts of PNCz in the solution and film state were 29 nm and 38 nm, respectively, which were much smaller than those of PPCz (41 nm and 51 nm). These small Stokes shifts also support PNCz having a rigid and regular conformation induced by the naphthalene substituent, which enabled utilisation of absorbed energy to emit PL efficiently. PNCz showed intense blue PL emission with CIE coordinates (x, y = 0.16, 0.13) in the film state and a high relative fluorescence quantum yield (Φfl) of 0.99 in CHCl3, which suggest that PNCz will be one of the good polymeric blue emitters comparable to a series of polyfluorenes derivatives.
Electrochemical and electroluminescence properties
The HOMO level (EHOMO) of PNCz was estimated by cyclic voltammerty (CV) from the onset potential of an oxidation peak using the equation: EHOMO (eV) = −Eox (V vs.SCE) − 4.4.17,18 The onset of oxidation potential of PNCz was 1.10 V (vs.SCE), which corresponded to the EHOMO of −5.50 eV. Since it was difficult to observe the reduction peak of PNCz in the film state, the LUMO level (ELUMO) was estimated from the EHOMO and optical band gap (Eg) using the equation, ELUMO = EHOMO + Eg, being −2.78 eV. These energy levels were somewhat lower than those of PPCz (EHOMO = −5.44 eV and ELUMO = −2.61 eV).
To evaluate electroluminescence characteristics of PNCz, an OLED device was fabricated with the structure ITO/PEDOT:PSS/PNCz/CsF/Al. Fig. 3 shows the I–V–L curves and Fig. 4 shows EL spectra at various voltages. The drive voltage showing an electroluminescence above 1 cd m−2 was 5 V. The maximum brightness (Lmax) of the PNCz device was 3200 cd m−2 at an operating voltage of 12 V with the maximum current efficiency (ηmax) of 0.2 cd/A. The EL spectra at various voltages indicated that an emission colour was variable from blue to green, so the emission peak and CIE index (x, y) considerably changed from 432 nm (0.20, 0.21) at 7 V to 509 nm (0.27, 0.47) at 12 V. The performance of the OLED of PNCz was ordinary but lower than those of PPCz, i.e., Lmax = 31500 cd m−2, ηmax = 1.7 cd A−1, and CIE change (4–10 V) between (0.18, 0.15) and (0.26, 0.46).
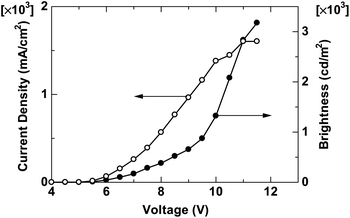 |
| | Fig. 3
I–V–L curves of the device with PNCz. | |
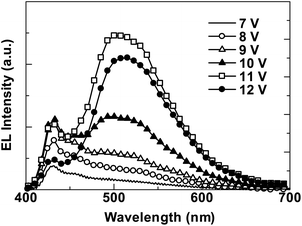 |
| | Fig. 4
EL spectra of PNCz at different voltages (7–12 V). | |
These results suggest that the stackable naphthalene portion that is expected to function as an ambipolar semiconductor in the same way as phenylnapthalene16 does not work effectively for a balanced carrier injection and transport to form exciton in the PNCz device, and might lower the purity of blue EL emission colour due to energy transfer for various excimeric interactions of luminescent naphthalene macromolecules.19,20 Additionally, unbalanced carrier injection and transport in polycarbazole, typical of p-type polymers, where excitons generate near the surface of cathode owing to higher mobility of hole than electron, would bring about irreversible reactions due to interaction between the emissive species and cathode. This could lead not only considerable changes of EL spectra but also apparent difference between PL and EL spectra, which has been a main drawback of OLED performances using polycarbazole derivatives.4
Conclusion
In summary, we successfully synthesised PNCz, a new N-naphthyl substituted poly(2,7-carbazole) by Yamamoto method using Ni(cod)2. PNCz exhibited excellent photophysical properties similar to those of PPCz, i.e., intense blue PL, high Φfl, in solution, and appropriate CIE index (0.16, 0.11) in the film state. The narrower Eg and smaller Stokes shifts of PNCz observed in the solution and film state than those of PPCz might be characterised by the rigid and arranged structure of the N-naphthyl moiety. However, the EL performance of the PNCz device was unremarkable even though it was not fully optimised. The attempt to add electron injection and transport ability of PPCz by replacement of the N-phenyl moiety by the N-naphthyl one might be successful, because electronic substituent effects of the N-naphthalene one surely heightened electron affinity by lowering the ELUMO level and enough current was observed in the I–V curve of the OLED device (Fig. 3). Matters that should be improved are the low current efficiency and decreased purity of blue EL emissions of the PNCz device. Nevertheless, regulation of stacking structures of the ambipolar naphthalene side group should enable to enhance EL efficiency and control emission colour from blue to white in combination with the blue light emitting p-type polycarbazole main structure.
Acknowledgements
This work was partially supported by Research Fellowships for Young Scientists, JSPS. We thank Chemical Analysis Division, Research Facility Center for Science and Technology, University of Tsukuba for elemental analysis, PL, NMR, and TGA measurements.
References
- J.-F. Morin, P.-L. Boudreault and M. Leclerc, Macromol. Rapid Commun., 2002, 23, 1032 CrossRef CAS.
- N. Kobayashi, R. Koguchi and M. Kijima, Macromolecules, 2006, 39, 9102 CrossRef CAS.
- S. Wakim, N. Blouin, E. Gingras, Y. Tao and M. Leclerc, Macromol. Rapid Commun., 2007, 28, 1798 CrossRef CAS.
- R. Koguchi, N. Kobayashi, T. Shinnai, K. Oikawa, K. Tsuchiya and M. Kijima, Macromol. Chem. Phys., 2008, 209, 439 CrossRef CAS.
- N. Blouin and M. Leclerc, Acc. Chem. Res., 2008, 41, 1110 CrossRef CAS.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. Neagu-Plesu, M. Belletête, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732 CrossRef CAS.
- J.-F. Morin and M. Leclerc, Macromolecules, 2001, 34, 4680 CrossRef CAS.
- J.-F. Morin and M. Leclerc, Macromolecules, 2002, 35, 8413 CrossRef CAS.
- J. Bouchard, M. Belletête, G. Durocher and M. Leclerc, Macromolecules, 2003, 36, 4624 CrossRef CAS.
- R. Koguchi, N. Kobayashi and M. Kijima, Macromolecules, 2009, 42, 5946 CrossRef CAS.
- T. Mori and M. Kijima, Eur. Polym. J., 2009, 45, 1149 CrossRef CAS.
- Y. Fu and Z. Bo, Macromol. Rapid Commun., 2005, 26, 1704 CrossRef CAS.
- M. Kijima, R. Koguchi and S. Abe, Chem. Lett., 2005, 34, 900 CrossRef CAS.
- A. Iraqi, T. G. Simmance, H. Yi, M. Stevenson and D. G. Lidzey, Chem. Mater., 2006, 18, 5789 CrossRef CAS.
- K. Zhang, Y. Tao, C. Yang, H. You, Y. Zou, J. Qin and D. Ma, Chem. Mater., 2008, 20, 7324 CrossRef CAS.
- M. Funahashi and J. Hanna, Appl. Phys. Lett., 1997, 71, 602 CrossRef CAS.
- J. Pommerehne, H. Vestweber, W. Guss, R. F. Mahrt, H. Bässler, M. Porsch and J. Daub, Adv. Mater., 1995, 7, 551 CrossRef CAS.
- A. K. Agrawal and S. A. Jenekhe, Chem. Mater., 1996, 8, 579 CrossRef CAS.
- T. Mori and M. Kijima, Opt. Mater., 2007, 30, 545 CrossRef CAS.
- T. Mori and M. Kijima, Mol. Cryst. Liq. Cryst., 2008, 489, 246 CAS.
Footnote |
| † Current affiliation: Industrial Technology Center of Wakayama Prefecture, 60 Ogura, Wakayama, 649-6261, Japan. E-mail: tmori@wakayama-kg.go.jp |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here.