Aromatic, microporous polymer networks with high surface area generated in Friedel–Crafts-type polycondensations†
Eduard
Preis
a,
Christian
Widling
a,
Ullrich
Scherf
*a,
Satish
Patil
b,
Gunther
Brunklaus
c,
Johannes
Schmidt
d and
Arne
Thomas
d
aMacromolecular Chemistry Group, Bergische Universität Wuppertal (BUW), Gauss-Str. 20, D-42097, Wuppertal, Germany. E-mail: preis@uni-wuppertal.de; christian.widling@uni-wuppertal.de; scherf@uni-wuppertal.de
bSolid State and Structural Chemistry Unit, Indian Institute of Science (IISc), Bangalore, India 560012. E-mail: satish@sscu.iisc.ernet.in
cMax-Planck-Institut für Polymerforschung Mainz (MPI-P), Ackermannweg 10, D-55128, Mainz, Germany. E-mail: brunklaus@mpip-mainz.mpg.de
dInstitut für Chemie, Technische Universität Berlin (TUB), Englische Str. 20, D-10587, Berlin, Germany. E-mail: johannes.schmidt@tu-berlin.de; arne.thomas@tu-berlin.de
First published on 15th August 2011
Abstract
A series of novel, microporous polymer networks (MPNs) have been generated in a simple, acid catalysed Friedel–Crafts-type self-condensation of A2B2- and A2B4-type fluorenone monomers. Two A2B4-type monomers with 2,7-bis(N,N-diphenylamino) A or 2,7-bis[4-(N,N-diphenylamino)phenyl] D substitution of the fluorenone cores lead to MPNs with high SBET surface areas of up to 1400 m2 g−1. Two MPNs made of binary monomer mixtures showed the highest Brunauer–Emmett–Teller (BET) surface areas SBET of our series (SBET of up to 1800 m2 g−1) after washing the powdery samples with supercritical carbon dioxide. Total pore volumes of up to 1.6 cm3 g−1 have been detected. It is observed that the substitution pattern of the monomers is strongly influencing the resulting physicochemical properties of the microporous polymer networks (MPNs).
Microporous polymer networks (MPNs) are covalently bound, highly crosslinked organic architectures with exceptionally high porosity and surface areas, which allow the incorporation of functional organic moieties into solid materials.1,2 The high porosities are in general generated from structure-directing monomers (called knots or tectons), mostly rigid molecules with multiple functional groups extending into two or three dimensions. These tectons can be polymerized directly or with aid of linear linkers to generate the two- or three-dimensional networks. Several protocols have been applied to connect tectons of various structures, including metal-catalyzed couplings, condensation reactions, oxidative polymerizations, trimerizations, click reactions and others.3–9 The high porosity and chemical stability as well as the possibility to introduce functional organic moieties into the frameworks make these materials interesting for applications like gas storage, catalysis and sensing.10
One important synthetic challenge is the straightforward generation of MPNs under mild reaction conditions involving moderate processing temperatures and the use of inexpensive catalysts and/or reagents, preferably in a metal-free regime.11 In this work, we describe the synthesis of a series of novel aromatic MPNs with high BET surface area in a simple self-condensation of ten multifunctional A4B2- and A2B2-type arylamino-substituted fluorenone monomers (Fig. 1), and binary mixtures of them, under acidic (Friedel–Crafts-type) conditions. Diarylamino-substituted fluorene units have been described as building blocks of blue light emitting polymers by improving the hole conducting properties.12,13 The synthesis of the 2-mono- and 2,7-bis(arylamino)-substituted fluorenone monomers is described in refs. 14 and 15 (monomers A–C) and ref. 15 (monomers D–F). The synthesis of the 3,6-substituted fluorenone derivatives G and I was accomplished analogous to the generation of the corresponding 2,7-derivatives A and B15 or D and E16 starting from 3,6-dibromofluorenone17,18 (for the procedures see the ESI†).
![Chemical structures of the monomers A–J: 2,7-bis(N,N-diphenylamino)fluorenone (A, A4B2-type), 2,7-bis(N-phenyl-N-methylamino)fluorenone (B, A2B2-type), 2-(N,N-diphenylamino)fluorenone (C, A2B2-type), 2,7-bis[4-(N,N-diphenylamino)phenyl]fluorenone (D, A4B2-type), 2,7-bis[4-(N-phenyl-N-methylamino)phenyl]fluorenone (E, A2B2-type), 2-[4-(N,N-diphenylamino)phenyl]fluorenone (F, A2B2-type), 3,6-bis(N,N-diphenylamino)fluorenone (G, A4B2-type), 3,6-bis(N-phenyl-N-methylamino)fluorenone (H, A2B2-type), 3,6-bis[4-(N,N-diphenylamino)phenyl]fluorenone (I, A4B2-type), and 3,6-bis[4-(N-phenyl-N-methylamino)phenyl]fluorenone (J, A2B2-type).](/image/article/2011/PY/c1py00251a/c1py00251a-f1.gif) | ||
| Fig. 1 Chemical structures of the monomers A–J: 2,7-bis(N,N-diphenylamino)fluorenone (A, A4B2-type), 2,7-bis(N-phenyl-N-methylamino)fluorenone (B, A2B2-type), 2-(N,N-diphenylamino)fluorenone (C, A2B2-type), 2,7-bis[4-(N,N-diphenylamino)phenyl]fluorenone (D, A4B2-type), 2,7-bis[4-(N-phenyl-N-methylamino)phenyl]fluorenone (E, A2B2-type), 2-[4-(N,N-diphenylamino)phenyl]fluorenone (F, A2B2-type), 3,6-bis(N,N-diphenylamino)fluorenone (G, A4B2-type), 3,6-bis(N-phenyl-N-methylamino)fluorenone (H, A2B2-type), 3,6-bis[4-(N,N-diphenylamino)phenyl]fluorenone (I, A4B2-type), and 3,6-bis[4-(N-phenyl-N-methylamino)phenyl]fluorenone (J, A2B2-type). | ||
Related, acid-catalysed A2 + B2polycondensations after Friedel–Crafts reaction between aromatic ketones (e.g. N-methylisatin or acenaphthoquinone) and bisfunctional aromatic building blocks into linear, high molecular weight condensation polymers have been extensively described by Zolotukhin and co-workers.19–22 The corresponding self-condensation of related AB2 monomers into hyperbranched condensation polymers was reported by Smet and co-workers.23–25
The generation of our novel MPNs (Schemes 1 and 2 show the idealized structures for the MPNs starting from monomers A–C) follows similar reaction conditions as described by Müllen and co-workers for the acidic condensation of 2,7-dibromofluorenone with 2 equivalents of N,N,N-triphenylamine towards 2,7-dibromo-9,9-bis[4-(N,N-diphenylamino)phenyl]fluorene (solvent: 1,2-dichlorobenzene, catalyst: methane sulfonic acid MSA, temperature: 140 °C).12Schemes 1 and 2 show the proposed three-dimensional (3D) structure of the MPNs, which contain regularly arranged tetragonal carbons (in 9-position of the 9,9-diarylfluorene building blocks) that are connected by N,N-diphenylamine-3,4′-diyl bridges for monomers A–C and N,N-diphenylamine-4,4′-diyl bridges for monomers G and H, or more extended N-(4-biphen-3′-yl)-N-(phen-4-yl)amine and N-(4-biphen-4′-yl)-N-(phen-4-yl)amine bridges for monomers D–F and I/J, respectively. The structure of the monomers allows for the formation of cross-linked networks in a single reaction step. Hereby, the in situ generation of 9,9-diphenylfluorene units with their rigid and three-dimensional structure represents suitable tectons for the generation of open network structures with a high intrinsic porosity. The triphenylamine units of monomers A, C, D, F, G and I when incorporated into the MPNs will act as additional structure directing tectons.
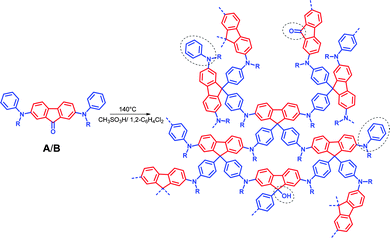 | ||
| Scheme 1 Idealized structure of the MPNs obtained by acid-catalysed self-condensation after Friedel–Crafts reaction starting from monomers A (R = phenyl) and B (R = methyl), unreacted aromatic and keto units, as well as (only) 9-monosubstituted fluorene cores are encircled; please note that for monomer A also two phenyls at one triarylamine nitrogen can react. | ||
 | ||
| Scheme 2 Idealized structure of the MPN obtained by acid-catalysed self-condensation after Friedel–Crafts reaction starting from monomer C; unreacted aromatic and keto units, as well as 9-monosubstituted fluorene cores are encircled. | ||
The aromatic MPNs were obtained in nearly quantitative yields as reddish brown colored, powdery solids. The insoluble products have been carefully extracted with water, ethanol, acetone, toluene, and chloroform to remove reagents, solvents and possible low molecular weight by-products.
The MPNs have been characterized by solid state NMR spectroscopy, thermogravimetry and nitrogen sorption measurements. To give an example for the solid-state MAS NMR analysis, the solid state 13C {1H} CP/MAS NMR spectrum of the condensation product of monomer A (see ESI†) displays a broad feature in the aromatic region between 105 and 160 ppm with three maxima at 129, 138 and 148 ppm. A weak signal at 63 ppm is assigned to the aliphatic 9-carbons of the fluorene building blocks. Leftover keto groups are not detectable within the detection limit of the method (ca. 1%, no signals in the 160–220 ppm region). The condensation product of monomer B displays a similar signal pattern in the aromatic region (peaks at 122, 129, 138, 150 ppm), a signal at 65 ppm for the aliphatic 9-fluorene carbons, and an additional aliphatic signal for the >N–CH3methyls. Again, no signals are observed in the 160–220 ppm region (see ESI†). Also the 13C {1H} CP/MAS NMR spectrum of the condensation product of the “chain-extended” tetraphenyl monomer D (see ESI†) does not show leftover keto groups (160–210 ppm region). The aromatic region is dominated by three broad maxima peaking at 128, 140 and 148 ppm. The aliphatic 9-fluorene carbon is detected at 65 ppm. Thermogravimetry showed a thermal stability of all MPNs up to temperatures of >250 °C (see ESI†).
To test the porosity of the polymeric products nitrogen sorption measurements were carried out and the Brunauer–Emmett–Teller (BET) surface areas SBET and total pore volumes extracted (Table 1). The condensation product of monomer A showed a high SBET value of up to 1420 m2 g−1 (pore volume: 1.3 cm3 g−1). The condensation product of monomer B made by replacing one N-phenyl of the N,N-diphenylamino groups by N-methyl displayed a dramatically reduced SBET value of only 57 m2 g−1 most probably caused by the increased flexibility of the N,N-diaryl-N-methylamino building blocks (MPN from monomer B) in relation to the corresponding N,N,N-triarylamino units (MPN from monomer A) thus leading to a “softer” polymer network and, subsequently, to a collapse of the porous solid state structure. Interestingly, the network generated from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) mixture of monomers A and B leads to a network with a SBET surface area of 716 m2 g−1 (pore volume: 0.47 cm3 g−1), an average value located between the numbers for the networks made from A or B. As the monomers A and B can be co-polymerized in any feed ratio, surface areas and porosities will be continuously tuneable over a wide range. The MPN made from the unsymmetrical 2-(N,N-diphenylamino)fluorenone monomer (C, A2B2-type) showed an only moderate SBET value of 228 m2 g−1 probably due to symmetry reduction caused by the reduced degree of substitution allowing for a denser packing of the structural building blocks. Distinct differences are also observed for materials made from monomers with different substitution patterns (2,7- vs. 3,6-) of the diarylamino substituents at the fluorenone core. Thus, switching from monomer A to G surprisingly yields a non-porous material. On the other hand the more extended monomer D with 2,7-substitution also gives a highly porous material with just a slightly decreased surface area (compare A and D).
1 (w/w) mixture of monomers A and B leads to a network with a SBET surface area of 716 m2 g−1 (pore volume: 0.47 cm3 g−1), an average value located between the numbers for the networks made from A or B. As the monomers A and B can be co-polymerized in any feed ratio, surface areas and porosities will be continuously tuneable over a wide range. The MPN made from the unsymmetrical 2-(N,N-diphenylamino)fluorenone monomer (C, A2B2-type) showed an only moderate SBET value of 228 m2 g−1 probably due to symmetry reduction caused by the reduced degree of substitution allowing for a denser packing of the structural building blocks. Distinct differences are also observed for materials made from monomers with different substitution patterns (2,7- vs. 3,6-) of the diarylamino substituents at the fluorenone core. Thus, switching from monomer A to G surprisingly yields a non-porous material. On the other hand the more extended monomer D with 2,7-substitution also gives a highly porous material with just a slightly decreased surface area (compare A and D).
| Entry | Monomer(s) | Yield | S BET surface areaa/m2 g−1 | S BET after supercritical CO2 washing/m2 g−1 | Total pore volumeb/cm3 g−1 [after SC CO2 washing] |
|---|---|---|---|---|---|
| a All BET data have been obtained on a Quantochrome Instruments machine (Autosorb 1). b The total pore volume is determined at a relative pressure (p/p0) of 0.95. c Measurement performed with a Belsorp Max machine; total pore volume at p/p0 0.305. d Not determined. | |||||
| 1 | A | Quant. | 1420 | 1243 | 1.31 [0.90] |
| 1260c | — | 0.60c | |||
| 2 | B | Quant. | 57 | — | 0.06 |
| 3 |
A/B (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 716 | 924 | 0.47 [0.53] |
| 4 | C | Quant. | 228 | — | 0.16 |
| 5 |
A/C (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 718 | 1775 | 0.43 [1.26] |
| 6 |
B/C (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 9 | — | 0.009 |
| 7 | D | Quant. | 1161 | 1394 | 0.80 [0.85] |
| 8 | E | Quant. | 12 | — | 0.01 |
| 9 | F | Quant. | 16 | — | 0.02 |
| 10 |
D/E (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 9 | — | 0.01 |
| 11 |
D/F (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 372 | — | 0.28 |
| 12 |
E/F (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 14 | — | 0.02 |
| 13 | G | Quant. | 9 | — | 0.01 |
| 14 | H | Quant. | 18 | — | 0.02 |
| 15 |
G/H (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 3 | — | 0.01 |
| 16 | I | Quant. | 17 | — | 0.02 |
| 17 | J | Quant. | 163 | — | 0.30 |
| 18 |
I/J (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 947 | 1447 | 0.67 [0.97] |
| 19 |
A/D (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 1081 | 1103 | —d |
| 20 |
A/D (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) 1 w/w) |
Quant. | 968 | 1748 | 0.77 [1.6] |
| 21 |
A/D (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) 3 w/w) |
Quant. | 1221 | 1408 | —d |
Comparing the chemical structures of the monomers some obvious trends towards microporous networks with high surface area can be derived: first, N,N-diphenylamino groups directly connected to the fluorene cores or N,N,N-triphenylamino substituents are essential for the generation of high surface areas, as they act as additional tectons due to their rigid, 2-dimensional structure. Second, the substitution pattern (2,7- vs. 3,6-) at the fluorenone core has a distinct influence on the generation of a porous network. Third, the use of monomer mixtures allows for tuning the porosity and surface area of the resulting networks. The most unexpected finding, however, was the generation of a high surface area material from a mixture of two monomers, which are forming non-porous networks when homopolymerized. Indeed, the I/J mixture of the two 3,6-bis[4-(N,N-diphenylamino)phenyl] derivatives shows a high SBET surface area of 947 m2 g−1 (pore volume: 0.67 cm3 g−1). There is no straightforward explanation for this finding and currently experiments are carried out to investigate if porous materials can be also generated starting from other monomer feed ratios. It has been frequently shown that solvents and subsequent drying procedures can have a remarkable influence on the porosity of the resulting microporous organic networks.26 For metal–organic frameworks (MOFs) supercritical CO2 treatment (washing) is described as a mild method to increase the surface area due to a complete activation of all pores, involving the removal of residues (solvent molecules, catalysts) inside the framework.27Polymer networks, which are more flexible than MOFs, often swell when infiltrated with solvents, but the resulting extended pore structure cannot be preserved after drying in vacuum. The exchange of organic solvents by supercritical CO2 may thus increase the surface area of the polymer network due to a preservation of the swollen state. Therefore, solvent exchange and washing with supercritical CO2 were performed on selected polymer networks (for details see ESI†). After drying the samples surface areas and pore volumes were again measured by nitrogen sorption. A significant increase of the BET surface area could be observed for some samples and remarkably high SBET values of up to 1800 m2 g−1 have been calculated (especially for entries 5, 18, and 20 of Table 1; starting from binary mixtures of two monomers: A/C, I/J, or A/D). On the other hand for some polymer networks the surface area stays mainly unchanged.
It is interesting to note that the porosity of the network derived from monomer A showing the highest surface area without further treatment is only weakly influenced by the supercritical CO2 treatment. However, more “irregular” MPNs made from binary monomer mixtures (e.g.I/J) are very sensitive to the supercritical CO2 treatment. Those MPNs may show a more pronounced expansion under supercritical CO2 treatment if compared to the more regular and compact network made from monomer A. More flexible networks (e.g. made from D as extended monomer) show a moderate increase of the surface area after supercritical CO2 treatment. For the A/D-couple (entries 19–21) no clear trend was observed upon variation of the monomer feed ratio. Still, it can already be concluded that flexibility and rigidity of the networks should be carefully balanced to reach porous materials of high surface area.
In summary, two A2B4-type monomers with 2,7-bis(N,N-diphenylamino) A or 2,7-bis[4-(N,N-diphenylamino)phenyl] D substitution of the fluorenone cores quantitatively form MPNs with high SBET surface areas of up to 1400 m2 g−1 in a simple Friedel–Crafts-type self-condensation. 2,7-Disubstitution with N-phenyl-N-methylamino groups as well as 3,6-disubstitution give rise to drastically reduced SBET values except for the mixture of the two extended monomers I and J. Supercritical CO2 (sCO2) treatment can distinctly increase the porosity of the samples especially for MPNs made of binary monomer mixtures containing A and C or D.27 The supercritical CO2 treatment seems to stabilize an expanded pore structure corresponding to the swollen state of the polymer network. Further ongoing modelling studies may help to explain the strong influence of the substitution pattern at the fluorene core and the influence of the sCO2 treatment.
References
- A. Thomas, Angew. Chem., Int. Ed., 2010, 49, 8328–8344 CrossRef CAS.
- J.-X. Jiang and A. Cooper, Topics in Current Chemistry, Springer Berlin/Heidelberg, 2009, vol. 293, pp. 1–33 Search PubMed.
- J. Weber, M. Antonietti and A. Thomas, Macromolecules, 2008, 41, 2880–2885 CrossRef CAS.
- J.-X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574–8578 CrossRef.
- L. Chen, Y. Honsho, S. Seki and D. L. Jiang, J. Am. Chem. Soc., 2010, 132, 6742–6748 CrossRef CAS.
- J. Schmidt, M. Werner and A. Thomas, Macromolecules, 2009, 42, 4426–4429 CrossRef CAS.
- J. Schmidt, J. Weber, J. D. Epping, M. Antonietti and A. Thomas, Adv. Mater., 2009, 21, 702–705 CrossRef CAS.
- J. Weber and A. Thomas, J. Am. Chem. Soc., 2008, 130, 6334–6335 CrossRef CAS.
- J. R. Holst, E. Stöckel, D. J. Adams and A. I. Cooper, Macromolecules, 2010, 43, 8531–8538 CrossRef CAS.
- A. Thomas, P. Kuhn, J. Weber, M. M. Titirici and M. Antonietti, Macromol. Rapid Commun., 2009, 30, 221–236 CrossRef CAS.
- R. S. Sprick, A. Thomas and U. Scherf, Polym. Chem., 2010, 1, 283–285 RSC.
- C. Ego, A. C. Grimsdale, F. Uckert, G. Yu, G. Srdanov and K. Müllen, Adv. Mater., 2002, 14, 809–811 CrossRef CAS.
- T. Miteva, A. Meisel, W. Knoll, H.-G. Nothofer, U. Scherf, D. C. Müller, K. Meerholz, A. Yasuda and D. Neher, Adv. Mater., 2001, 13, 565–570 CrossRef.
- J. Ipaktschi and A. Sharifi, Monatsh. Chem., 1998, 129, 915–920 CAS.
- M. Kimura, S. Inoue, K. Shimada, S. Tokito, K. Noda, Y. Taga and Y. Sawaki, Chem. Lett., 2000, 192–193 CrossRef CAS.
- Y. Liu, X. Tao, F. Wang, X. Dang, D. Zou, Y. Ren and M. Jiang, J. Phys. Chem. C, 2008, 112, 3975–3981 CrossRef CAS.
- K. Brunner, A. van Dijken, H. Börner, J. J. A. M. Bastiaansen, N. M. M. Kiggen and B. M. W. Langeveld, J. Am. Chem. Soc., 2004, 126, 6035–6042 CrossRef CAS.
- Z. Wu, Y. Xiong, J. Zou, L. Wang, J. Liu, Q. Chen, W. Yang, J. Peng and Y. Cao, Adv. Mater., 2008, 20, 2359–2364 CrossRef CAS.
- M. G. Zolotukhin, L. Fomina, R. Salcedo, L. E. Sansores, H. M. Colquhoun and L. M. Khalilov, Macromolecules, 2004, 37, 5140–5141 CrossRef CAS.
- M. G. Zolotukhin, S. Fomine, R. Salcedo and L. M. Khalilov, Chem. Commun., 2004, 1030–1031 RSC.
- M. G. Zolotukhin, S. Fomine, L. M. Lazo, R. Salcedo, L. E. Sansores and G. Cedillo, Macromolecules, 2005, 38, 6005–6014 CrossRef CAS.
- M. C. G. Hernández, M. G. Zolotukhin, J. L. Maldonado, N. Rehmann, K. Meerholz, S. King, A. P. Monkman, N. Fröhlich, C. Kudla and U. Scherf, Macromolecules, 2009, 42, 9225–9230 CrossRef CAS.
- M. Smet, E. H. Schacht and W. Dehaen, Angew. Chem., Int. Ed., 2002, 41, 4547–4550 CrossRef CAS.
- Y. Fu, A. Vandendriessche, W. Dehaen and M. Smet, Macromolecules, 2006, 39, 5183–5186 CrossRef CAS.
- Y. Fu, C. V. Oosterwijck, A. Vandendriessche, A. Kowalczuk-Bleja, X. Zhang, A. Dworak, W. Dehaen and M. Smet, Macromolecules, 2008, 41, 2388–2393 CrossRef CAS.
- R. Dawson, A. Laybourn, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Macromolecules, 2010, 43, 8524–8530 CrossRef CAS.
- A. P. Nelson, O. K. Farha, K. L. Mulfort and J. T. Hupp, J. Am. Chem. Soc., 2009, 131, 458–460 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, solid state NMR measurements, supercritical carbon dioxide washing, monomer and polymer synthesis, nitrogen physisorption isotherms, and solid state NMR spectra. See DOI: 10.1039/c1py00251a |
| This journal is © The Royal Society of Chemistry 2011 |
