Poly(N-protected ethylene imine-alt-ethylene sulfide) block to functionalize polymeric materials†
Yoichi
Hori
,
Na
Pei
,
Ryota
Kumagai
and
Yuji
Sasanuma
*
Department of Applied Chemistry and Biotechnology, Faculty of Engineering, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba 263-8522, Japan. E-mail: sasanuma@faculty.chiba-u.jp; Fax: +81-43-290-3394; Tel: +81-43-290-3394
First published on 11th August 2011
Abstract
Poly(N-tosyl-ethylene imine-alt-ethylene sulfide) has been synthesized by thiol-ene photopolymerization between N,N-bis(2-mercaptoethyl)-4-methylbenzenesulfonamide and 4-methyl-N,N-divinylbenzenesulfonamide. Its weight-average molecular weight was determined by static light scattering to be 2.8 × 104. The alternating copolymer has –SH and –CH=CH2 end groups and exhibits high solubility in common organic solvents such as chloroform, tetrahydrofuran, acetone, and dimethyl sulfoxide. The N-tosylated copolymer was deprotected to yield poly(ethylene imine-alt-ethylene sulfide), which is fully soluble in dimethyl sulfoxide, acetic acid, and ethanol. Both N-tosylated and deprotected polymers are hydrophobic, and the former and latter polymers are thermally decomposed at 272 and 244 °C, respectively.
Nitrogenous polymers such as
–[–NHCH2CH2XCH2CH2–]x– (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and S) 1 O, NH, and S) 1 |
In nonpolar media, the polymers form intramolecular N–H⋯X attractions; the interaction energies (designated as Eη) were evaluated as −1.75 kcal mol−1 for N–H⋯O,3 −1.54 kcal mol−1 for N–H⋯N,4 and −0.97 kcal mol−1 for N–H⋯S.5 Their configurational properties are greatly affected by the N–H⋯X strengths; characteristic ratios at 25 °C were calculated from the above Eη values to be 1.33 (X = O), 3.09 (NH), and 5.15 (S).5 If the N–H⋯X attractions were completely ineffective (i.e., Eη = 0), the three polymers would give characteristic ratios (6.4–7.3) close to that (6.8) of polyethylene in the Θ state.6
The N–H⋯X strengths may be qualitatively interpreted according to the hard and soft acids and bases principle.7,8Ethers are classified into hard bases, thioethers into soft ones, and amines into hard or intermediate ones. Protons (hydrogen), hard acids, prefer hard bases rather than soft ones; hence, the N–H⋯X attraction increases in the order S < N < O. This tendency has long been paid attention to in molecular design of macrocycles containing O, N, and S atoms for selective complexation with metal cations.8–11
If polymer 1 is dissolved in polar or protic solvents, the intramolecular N–H⋯X attraction is switched to intermolecular ones with the solvents. In aqueous phases, the NH site is often protonated and exerts electrostatic forces, which may attract anionic phosphate groups of DNA to form DNA–polymer complexes; therefore, such cationic polymers have been used as nonviral vectors for gene delivery.1 Solubilities of polymer 1 also depend on properties intrinsic to the –CH2–CH2–X – moiety of the repeating unit.4,12 Poly(ethylene oxide) (X = O) is soluble in a number of solvents, and poly(ethylene imine) (X = NH) in hot water, methanol, ethanol, and chloroform, whereas poly(ethylene sulfide) (X = S) is soluble in only a few solvents such as nitrobenzene and dimethyl sulfoxide at temperatures above 140 °C.13 From the above discussion, we can expect that copolymers composed of different –[NHCH2CH2XCH2CH2]x– and – [CH2CH2X]y– blocks to show a variety of aggregate structures, phase behaviors, physical properties, and functions, depending on solvent, temperature, and pH.
To the best of our knowledge, neither poly(ethylene imine-alt-ethylene sulfide) nor its N-substituted polymers seem to have been synthesized. Our theoretical predictions5 about structures, properties, and functions of the nonexistent polymers may be considered to be molecular design of new polymers. In this study, in order to materialize the molecular design, we have synthesized and characterized poly(N-tosyl-ethylene imine-alt-ethylene sulfide) block, –[N(Ts)CH2CH2SCH2CH2]x– (referred herein to as 7, Ts = tosyl group, see Fig. 1) as a precursor of the –[NHCH2CH2SCH2CH2]x– block (designated as 1S: polymer 1 with X = S). For the sake of (1) introducing sulfur into the main chain, (2) exact alternation of ethylene-imine and ethylene-sulfide subunits, and (3) no side reactions at the nitrogen site, we have adopted thiol-ene photopolymerization between N,N-bis(2-mercaptoethyl)-4-methylbenzenesulfonamide and 4-methyl-N,N-divinylbenzenesulfonamide (reaction VI in Fig. 1), because the polymerization of a stoichiometric mixture of dithiol and diene monomers may lead to a linear alternating copolymer.14–17 In the polymerization, ultraviolet light of 375 nm and an initiator of 2,2-dimethoxy-2-phenylacetophenone were used. The total synthesis route is shown in Fig. 1. Details of the individual reactions are explained in the ESI.†
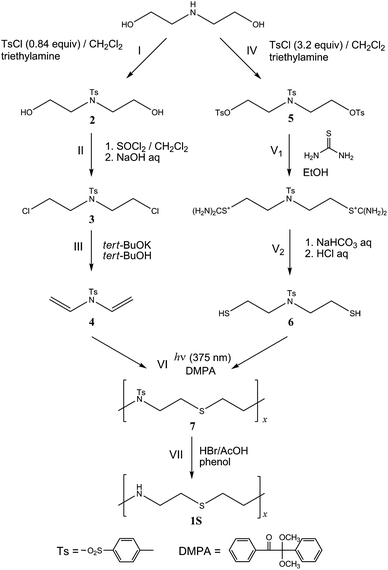 | ||
| Fig. 1 Total synthetic route of poly(N-tosyl-ethylene imine-alt-ethylene sulfide) (7) and deprotection. Diethanolamine is the starting material for both monomers, 4 and 6. The synthetic procedures are described in the ESI,† and 1H NMR spectra of the intermediate compounds, 2, 3, 4, 5, and 6, are also shown there.† | ||
Fig. 2 shows a 1H NMR spectrum observed from 7. Even at 500 MHz, the CH2 signals that ought to be triplets appear as broad singlets (cf.NMR spectra in Fig. S2–S5†). This is probably because the molecular motions have short relaxation times, and this fact attests the formation of a polymer. The large peaks in the spectrum can be assigned to the methylene and tosyl protons of the repeating unit as indicated. The step-growth polymerization between bifunctional thiol and ene is known to yield (1) one ene and one thiol, (2) two ene, or (3) two thiol ends.
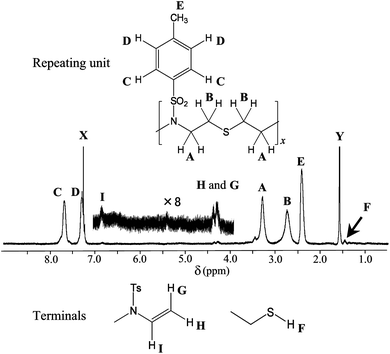 | ||
| Fig. 2 1H NMR spectrum of 7 with assignment of observed peaks: A–E, from the repeating unit; F–I, from the terminal groups; X and Y, from CHCl3 and water immixed in the solvent, CDCl3, respectively. | ||
A weak signal (F) at 1.4 ppm undoubtedly stems from the thiol proton (cf. that (1.41 ppm) of 6, Fig. S5†). Molecular orbital calculations for a model compound of the ene terminal, CH3CH2N(Ts)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, were carried out to evaluate 1H NMR chemical shifts of the –CH
CH2, were carried out to evaluate 1H NMR chemical shifts of the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 part. The δ values were obtained as 4.09 ppm (transmethylene proton G in Fig. 2), 4.21 ppm (cismethylene H), and 7.59 ppm (methine I). The δ values of divinyl protons of 4 were also calculated to be 4.98 (4.93) ppm (transmethylene), 4.85 (4.79) ppm (cismethylene), and 7.34 (6.41) ppm (methine), where the corresponding experimental data are given in the parentheses (see Fig. S3†); the MO calculations satisfactorily reproduced the observations for the C
CH2 part. The δ values were obtained as 4.09 ppm (transmethylene proton G in Fig. 2), 4.21 ppm (cismethylene H), and 7.59 ppm (methine I). The δ values of divinyl protons of 4 were also calculated to be 4.98 (4.93) ppm (transmethylene), 4.85 (4.79) ppm (cismethylene), and 7.34 (6.41) ppm (methine), where the corresponding experimental data are given in the parentheses (see Fig. S3†); the MO calculations satisfactorily reproduced the observations for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2protons but overestimated the δ value of the –CH
CH2protons but overestimated the δ value of the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C one by 0.93 ppm. According to our experiences, the MO calculations have occasionally failed to predict NMR chemical shifts of nuclei adjacent to nitrogen. This is partly because the calculations are performed for only a selected conformation; in fact, the nitrogen inversion leads to rapid conformational and configurational changes.4 From the above-mentioned difference (0.93 ppm) between theory and experiment, the chemical shift of the methine proton of N-ethyl-4-methyl-N-vinylbenzenesulfonamide may be corrected: the δ value would be calibrated as 7.59–0.93 = 6.66 ppm. In Fig. 2, a small peak can be found at 6.84 ppm, thus being reasonably assigned to proton I, and two signals observed at 4.27 and 4.35 ppm are assigned to the terminal –C
C one by 0.93 ppm. According to our experiences, the MO calculations have occasionally failed to predict NMR chemical shifts of nuclei adjacent to nitrogen. This is partly because the calculations are performed for only a selected conformation; in fact, the nitrogen inversion leads to rapid conformational and configurational changes.4 From the above-mentioned difference (0.93 ppm) between theory and experiment, the chemical shift of the methine proton of N-ethyl-4-methyl-N-vinylbenzenesulfonamide may be corrected: the δ value would be calibrated as 7.59–0.93 = 6.66 ppm. In Fig. 2, a small peak can be found at 6.84 ppm, thus being reasonably assigned to proton I, and two signals observed at 4.27 and 4.35 ppm are assigned to the terminal –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2protons, G and H. Accordingly, it is reasonable to conclude that 7 keeps both –SH and –CH
CH2protons, G and H. Accordingly, it is reasonable to conclude that 7 keeps both –SH and –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 ends. This polymer may be chemically modified and combined with other chemical species or polymer blocks by thiol-ene “click” chemistry.15,18 A better way to control the end groups would be to use a small incorporation of monofunctional ene or thiol and conduct the polymerization with an imbalance in the stoichiometry of the two monomers.
CH2 ends. This polymer may be chemically modified and combined with other chemical species or polymer blocks by thiol-ene “click” chemistry.15,18 A better way to control the end groups would be to use a small incorporation of monofunctional ene or thiol and conduct the polymerization with an imbalance in the stoichiometry of the two monomers.
Static light scattering experiments for 7 determined its weight-average molecular weight as 2.77 × 104, i.e., the degree of polymerization is 108. On DTA curves of 7 and 1S, no well-defined melting peaks can be found (Fig. S6†). The TG/DTA curves show that the thermal decomposition of 7 begins at 272 °C and finishes at 315 °C and that 1S is decomposed between 244 and 312 °C.
Solubilities of 7 and 1S were examined for a variety of solvents; nonpolar, polar, and protic media were employed in the tests. Copolymer 7 is insoluble in n-hexane, acetic acid, ethanol, and water, partially soluble in toluene, and fully soluble in chloroform, tetrahydrofuran, acetone, and dimethyl sulfoxide. The deprotected polymer 1S is insoluble in n-hexane and diethyl ether, partially soluble in toluene, chloroform, and acetone, and completely soluble in dimethyl sulfoxide, acetic acid, and ethanol, but insoluble in cold and hot water. Polymer 1S, being free of tosyl group, seems to be less organic than 7 but still remains hydrophobic.
In this communication, we have briefly described synthesis and characterization of the alternating copolymers. Future studies are expected to deal with the polymerization conditions, e.g., initiator and UV irradiation,19,20 in more detail to control and optimize molecular characteristics of the product.
In summary, the alternating copolymer 7 was synthesized by thiol-ene photopolymerization between 4 and 6 with ultraviolet light of 375 nm and an initiator of 2,2-dimethoxy-2-phenylacetophenone and characterized as follows. The terminal ends were identified by 1H NMR as –SH and –CH = CH2 groups, and the weight-average molecular weight was evaluated to be 2.8 × 104. Copolymer 7 shows high solubility in organic solvents and thermal stability. The alternating copolymer was deprotected to be 1S, which is completely soluble in dimethyl sulfoxide, acetic acid, and ethanol but insoluble in cold and hot water. The copolymer blocks, if being chemically modified or connected to other polymer blocks as needed, are expected to be used as parts of functional polymeric materials.
We thank Dr Joachim H. G. Steinke of Imperial College, UK for helpful advice on the polymerization and reviewers of this communication for helpful comments. This study was partly supported by a Grant-in-Aid for Scientific Research (C) (22550190) from the Japan Society for the Promotion of Science.
References
- M. A. Mintzer and E. E. Simanek, Chem. Rev., 2009, 109, 259 CrossRef CAS.
- I. B. Pehlivan, R. Marsal, P. Georén, C. G. Granqvist and G. A. Niklasson, J. Appl. Phys., 2010, 108, 74102 CrossRef.
- Y. Sasanuma, R. Kumagai and K. Nakata, Macromolecules, 2006, 39, 6752 CrossRef CAS.
- Y. Sasanuma, S. Hattori, S. Imazu, S. Ikeda, T. Kaizuka, T. Iijima, M. Sawanobori, M. A. Azam, R. V. Law and J. H. G. Steinke, Macromolecules, 2004, 37, 9169 CrossRef CAS.
- Y. Sasanuma and R. Kumagai, Macromolecules, 2007, 40, 7393 CrossRef CAS.
- A. Abe, R. L. Jernigan and P. J. Flory, J. Am. Chem. Soc., 1966, 88, 631 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533 CrossRef CAS.
- R. D. Hancock and A. E. Martell, Chem. Rev., 1989, 89, 1875 CrossRef CAS.
- J. M. Lehn, Acc. Chem. Res., 1978, 11, 49 CrossRef CAS.
- J. M. Lehn, Pure Appl. Chem., 1980, 52, 2441 CrossRef CAS.
- R. M. Izatt, J. S. Bradshaw, S. A. Nielsen, J. D. Lamb and J. J. Christensen, Chem. Rev., 1985, 85, 271 CrossRef CAS.
- Y. Sasanuma, H. Ohta, I. Touma, H. Matoba, Y. Hayashi and A. Kaito, Macromolecules, 2002, 35, 3748 CrossRef CAS.
- Polymer Data Handbook, ed. J. E. Mark, Oxford University Press, Inc., New York, 1999 Search PubMed.
- F. O. Davis and E. M. Fetters, in Polyethers. Part III. Polyalkylene Sulfides and Other Polythioethers, ed. N. G. Gaylord, Wiley-Interscience, New York, 1962, ch. 12 Search PubMed.
- C. E. Hoyle, T. Y. Lee and T. Roper, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5301 CrossRef CAS.
- S. K. Reddy, N. B. Cramer and C. N. Bowman, Macromolecules, 2006, 39, 3673 CrossRef CAS.
- T. F. Scott, C. J. Kloxin, R. B. Draughon and C. N. Bowman, Macromolecules, 2008, 41, 2987 CrossRef CAS.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540 CrossRef CAS.
- D. L. Kurdikar and N. A. Peppas, Macromolecules, 1994, 27, 733 CrossRef CAS.
- N. T. Brummelhuis, C. Diehl and H. Schlaad, Macromolecules, 2008, 41, 9946 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Description of methods, 1H NMR spectra of intermediate compounds, and thermogravimetry and differential thermal analysis curves. See DOI: 10.1039/c1py00264c |
| This journal is © The Royal Society of Chemistry 2011 |
