Polymerization of 4-vinylpyridine and N,N-dimethylacrylamide using a system without organic initiator†
Sijing
Xia
,
Bin
Yang
,
Guangzhao
Li
,
Xiaoqing
Zhu
,
Anning
Wang
and
Jin
Zhu
*
Department of Polymer Science and Engineering, School of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing National Laboratory of Microstructures, Nanjing University, Nanjing, 210093, China. E-mail: jinz@nju.edu.cn; Tel: +86 25 83686291
First published on 27th July 2011
Abstract
Polymerization of monomer 4-vinylpyridine (4VP) and N,N-dimethylacrylamide (DMAA) could be carried out using a system without organic initiator. It was found that metal catalyst (CuCl) and water played a vital role in this process through a series of control experiments. Polymers with high degrees of polymerization (Mp = 128739 for P4VP and Mp = 355122 for PDMAA) have been obtained by the addition of Au nanoparticles (AuNPs). Radical inhibitor and electron spin resonance (ESR) spin-trapping techniques have been used to analyze the polymerization process and identify the involvement of radicals in the reaction.
1. Introduction
The synthesis of well-defined poly-(4-vinylpyridine) (P4VP) and poly-N,N-dimethylacrylamide (PDMAA) by copper-mediated atom transfer radical polymerization (ATRP) has been reported by the Matyjaszewski group.1,2 In their system, the polymerization of 4VP was performed at 40 °C in 2-propanol by employing 1-phenylethyl chloride (PECl) as the initiator and a complex formed between CuCl and tris[2-(dimethylamino)ethyl]amine (Me6TREN) as the catalyst, while the polymerization of DMAA was carried out in toluene by using methyl 2-chloropropionate (MCP) as the initiator and CuCl/Me6TREN as the catalyst. However, there are some limitations remaining for the system. Both reactions need the addition of some other organic reagents as initiators that should be purchased or synthesized. And the degree of polymerization is not very high. Herein we report the successful synthesis of P4VP and PDMAA by a system that does not require the addition of organic initiator under argon atmosphere. Importantly, a higher degree of polymerization has been obtained.First, the polymerization of 4VP was studied. Water/2-propanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, volume ratio) solution was chosen as the solvent for our system.32-Propanol can dissolve P4VP and reduce the coloring in the polymerization system, possibly through the hydrogen bonding to 4VP/P4VP, therefore decreasing the contamination of the catalyst.1Water is essential for the reaction. It was found that the reaction could not be carried out under otherwise identical conditions without water through control experiments. The catalytic application of copper(I) cations has been reported.4–7Water seems to promote the catalytic efficiency of the reaction.
1, volume ratio) solution was chosen as the solvent for our system.32-Propanol can dissolve P4VP and reduce the coloring in the polymerization system, possibly through the hydrogen bonding to 4VP/P4VP, therefore decreasing the contamination of the catalyst.1Water is essential for the reaction. It was found that the reaction could not be carried out under otherwise identical conditions without water through control experiments. The catalytic application of copper(I) cations has been reported.4–7Water seems to promote the catalytic efficiency of the reaction.
The roles of Me6TREN have been discussed previously.1,3 Since both 4VP and P4VP are strong coordinating ligands that can compete for the binding of the metal catalysts in these systems and in addition, pyridine-coordinated copper complexes are not effective catalysts, a stronger binding ligand such as Me6TREN is needed. When a complex formed by CuCl and Me6TREN was added, the polymerization was improved.
Importantly, we have successfully applied the system to another reaction: the polymerization of DMAA. Again, a polymer with higher degree of polymerization was achieved compared with previous reports.8,9
2. Experimental section
2.1 Materials and measurements
The monomer, 4VP (Alfa Aesar, 96%) was purified through an alumina column in order to remove the polymerization inhibitor. Another monomer, DMAA (Aldrich, 99%) was distilled under vacuum. PECl (Aldrich) and dimethyl pyridine N-oxide (DMPO, Aldrich) was used as received. CuCl (Nanjing Chemical Reagent Co. Ltd.) was purified according to the published procedure. Me6TREN was prepared from TREN by a procedure similar to that reported by Ciampolini and Nardi.10Water was purified through Unique S15. 13 nm AuNPs was prepared in laboratory by the citrate reduction of HAuCl4. All of the solvents were used without further purification. 1H NMR spectrum was obtained by using a Bruker DRX-500 NMR spectrometer with chemical shifts reported as ppm (TMS as the internal standard). Molecular weights of polymer P4VP with DMF as solvent and PDMAA with water as solvent were determined by GPC with Waters 1515 Isocratic HPLC Pump and poly(ethylene glycol) as the relative standard. Electron spin resonance (ESR) spectrum was obtained from Bruker EMX-10/12 electron paramagnetic resonance spectrometer.2.2 Polymerization of 4VP
All the polymerization reactions were carried out under an argon atmosphere in a round bottom flask with a stirring bar. Monomer 4VP (1 mL) and solvent 2-propanol (1 mL) were added to the flask first. After the solution was bubbled with argon for 30 min, 13 nm AuNPs (15 pmol in 1 mL H2O) were added. Catalyst CuCl (2.6 mg) complexed with Me6TREN (7.5 μL) was then added. The flask was immersed in an oil bath held at 40 °C by a thermostat. After 24 h, the sample was moved out of the flask and added to ethanol, passing the solution through an alumina column to remove the metal containing residues. Precipitation in cold hexane yielded the polymer. The polymer was dried at 80 °C under vacuum for 48 h. Atom transfer radical polymerization (ATRP) of 4VP was carried out with the same procedure as reported in ref. 1.2.3 Polymerization of DMAA
Monomer DMAA (1 mL) and solvent 2-propanol (1 mL) with water (1 mL) or AuNPs water solution (1 mL, 15 pmol) were added to a round bottom flask with a stirring bar under an argon atmosphere. Catalyst CuCl (2.6 mg) complexed with Me6TREN (7.5 μL) was then added. The reaction was carried out at ambient temperature. After 24 h, the flask was open and the sample was solved in water. AuNPs were removed through centrifugation. The solution was dialyzed in water for three days to remove the copper ions and dried in a freeze dryer for 24 h.2.4 Preparation of 13 nm AuNPs
AuNPs with an average diameter of 13 nm were prepared by the citrate reduction of HAuCl4. An aqueous solution of HAuCl4 (300 mL, 1 mM) was added to a round bottom flask and heated to reflux with stirring in an oil bath. An aqueous solution of trisodium citrate (30 mL, 38.8 mM) was immediately added to the flask. After the color changed from pale yellow to deep red, the solution was refluxed for an additional 15 min, then allowed to cool to room temperature. We washed the AuNPs with deionized water to remove the citrate residue.2.5 ESR spin-trapping experiment11–15
To obtain the ESR spectrum of DMPO radical adduct in the process of polymerization, the experiment was carried out under an argon atmosphere. After the addition of monomer 4VP or DMAA (1 mL), the solvents 2-propanol (1 mL) and water (1 mL) along with catalyst CuCl (2.6 mg) complexed with Me6TREN (7.5 μL) were added to a round bottom flask with a stirring bar and allowed to react for 15 min under an argon atmosphere, followed by the addition of 10 μL DMPO in the system.16–18 An aliquot of solution was quickly taken out from the flask by 1 mL injector to do ESR spectrum analysis in order to determine the amount of free radicals in the system.For the ESR spectrum of Cu(II) in the reaction solution, the solvents 2-propanol (1 mL) and water (1 mL) along with catalyst CuCl (2.6 mg) complexed with Me6TREN (7.5 μL) were mixed to do ESR spectrum analysis before the reaction. Then the mixture was added to the monomer 4VP or DMAA (1 mL) and allowed to react for 24 h under an argon atmosphere. Then an aliquot of solution was taken out of the flask by 1 mL injector to do ESR spectrum analysis of Cu(II).
3. Results and discussion
3.1 GPC chromatograms of P4VP and PDMAA prepared by our method
From the 1H NMR spectra (Fig S1†) of P4VP obtained by our method and ATRP, it was inferred that the two polymers contained analogous chain structures from their similar shape. But the GPC chromatogram shows that our reaction system provides a polymer with a much higher degree of polymerization than that prepared from ATRP (Fig. 1). GPC chromatogram of PDMAA shows a high degree of polymerization too (Fig. 2). | ||
| Fig. 1 GPC chromatograms of P4VP through polymerization by a) our method and b) ATRP. Reaction conditions and results are as follows: a) 40 °C, 4VP = 1 mL, CuCl = 2.6 mg, Me6TREN = 7.5 μL, H2O/2-propanol = 1 mL/1 mL, AuNPs = 15 pmol, Mn = 59344, Mw = 136062, Mp = 128739, PDI = 2.29; b) 40 °C, 4VP = 1 mL, PECl = 3.7 mg, CuCl = 2.6 mg, Me6TREN = 7.5 μL, 2-propanol = 2 mL, Mn = 25231, Mw = 43626, Mp = 36740, PDI = 1.73. Both reactions are carried out under argon atmosphere. | ||
 | ||
| Fig. 2 GPC chromatogram of PDMAA through a) entry 1′ and b) entry 3′. Reaction conditions and results are as follows: a) 20 °C, DMAA = 1 mL, CuCl = 2.6 mg, Me6TREN = 7.5 μL, H2O/2-propanol = 1 mL/1 mL, Mn = 112566, Mw = 510282, Mp = 194232, PDI = 4.53; b) 20 °C, DMAA = 1 mL, CuCl = 2.6 mg, Me6TREN = 7.5 μL, H2O/2-propanol = 1 mL/1 mL, AuNPs = 15 pmol, Mn = 164623, Mw = 801616, Mp = 355122, PDI = 4.87. Both reactions are carried out under argon atmosphere. | ||
3.2 Regarding the mechanism of the polymerization without organic initiator
From entries 3, 5, and 2′ (Table 1), it was found that water and metal catalyst were the key elements for the polymerization of 4VP and DMAA. Without them, neither reaction could be carried out so that it was hypothesized they played a vital role in the reaction. To gain further insight into our novel polymerization, a radical inhibitor such as 1,1-diphenylethylene (diyldibenzene),19,20 was employed in the two reactions (Table 2). Indeed, the reactions were completely shut down, which could indicate that our polymerization may involve radical intermediates though it seems different from ATRP.| Entry | Monomer (mL) | CuCl (mg) | Me6TREN (μL) | H2O (mL) | 2-Propanol (mL) | AuNPs (pmol) | Yield (g) | |
|---|---|---|---|---|---|---|---|---|
| 4VP | 1 | 1 | — | — | 1 | 1 | 15 | — |
| 2 | 1 | 2.6 | 7.5 | 1 | 1 | — | low | |
| 3 | 1 | 2.6 | 7.5 | 1 | 1 | 15 | 0.153 | |
| 4 | 1 | 2.6 | — | 1 | 1 | 15 | low | |
| 5 | 1 | 2.6 | 7.5 | — | 2 | 15 | — | |
| DMAA | 1′ | 1 | 2.6 | 7.5 | 1 | 1 | — | 0.747 |
| 2′ | 1 | 2.6 | 7.5 | — | 2 | — | — | |
| 3′ | 1 | 2.6 | 7.5 | 1 | 1 | 15 | 0.468 |
| Monomer (mL) | CuCl (mg) | Me6TREN (μL) | H2O (mL) | 2-Propanol (mL) | Diyldibenzene (μL) | Product | |
|---|---|---|---|---|---|---|---|
| 4VP | 1 | 2.6 | 7.5 | 1 | 1 | — | YES |
| 1 | 2.6 | 7.5 | 1 | 1 | 5 | NO | |
| DMAA | 1 | 2.6 | 7.5 | 1 | 1 | — | YES |
| 1 | 2.6 | 7.5 | 1 | 1 | 5 | NO |
In order to get further understanding of our reactions, an ESR spin-trapping technique was used to determine whether and what free radical are formed in the system. The ESR spectrum of the DMPO–C radical adduct was detected in the polymerization of 4VP. And the ESR spectrum of DMPO–C radical and DMPO–O–C radical adduct was detected in the polymerization of DMAA (Fig. 3). It is because there are different radicals which may form different polymers in the polymerization of DMAA that the product PDMAA has a larger polydispersity index. Besides, the altered ESR spectrum of Cu(II) before and after the polymerization of 4VP and DMAA were also collected (Fig. 4), which indicated the increasing amount of Cu(II) in the reaction.
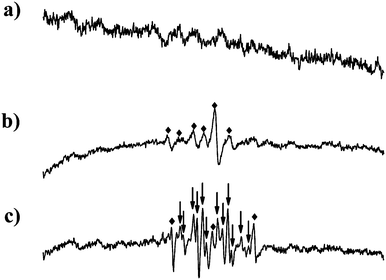 | ||
| Fig. 3 The ESR spectra of a) DMPO background; b) DMPO radical adduct in the process of polymerization of 4VP; c) DMPO radical adduct in the process of polymerization of DMAA. Diamonds represent the peaks of the DMPO–C radical adduct and arrows represent the peaks of the DMPO–O–C radical adduct. | ||
 | ||
| Fig. 4 The ESR spectrum of Cu(II) in the reaction solution: a) background; b) before reaction; c) after polymerization of 4VP for 24 h; d) after polymerization of DMAA for 24 h. The height of the peaks in c) and d) increases, which reflects the increase of the concentration of Cu(II). | ||
There is a transformation from Cu(I) to Cu(II). Thus, we propose that the radical comes from the reaction between metal catalyst, water and monomers.21–24 As shown in Scheme 1, when the vinyl groups of monomers react with CuCl and water, they may form unstable intermediates. The radicals are generated through a reversible redox process catalyzed by a transition metal complex CuCl/Me6TREN which undergoes a one-electron oxidation with concomitant abstraction of a halogen atom Cl from the intermediate. Then the polymer chains grow by addition of the radicals to the monomers.
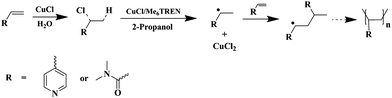 | ||
| Scheme 1 The proposed mechanism of polymerization of 4VP and DMAA. | ||
3.3 Discussion of the effect of 13 nm AuNPs
From entry 1 and entry 2 (Table 1), the reaction without Me6TREN can hardly proceed, so little product was obtained. When CuCl complexed by Me6TREN was added, the polymerization was improved but monomer conversion was low. After the polymer samples were passed through the alumina column, the solution was green and little product was obtained, presumably due to the competitive coordination of 4VP or P4VP to copper compared with Me6TREN.AuNPs with negatively charged layers could attract the positively charged Cu(I) species (Fig. S2†) and supply a larger surface area to form active centers, which could also decrease the influence of counterions on the polymerization or inhibit the coordination of 4VP or P4VP to copper to some degree. Besides, AuNPs are relatively stable metal and they were not changed in the reaction from our observation. Based on these considerations, 13 nm AuNPs were added to the polymerization system of 4VP in order to improve the conversion efficiency of monomer. In fact, after the addition of AuNPs, the polymerization was greatly improved and a higher conversion of 4VP was achieved (entries 2 and 3, Table 1).
For monomer DMAA, whose coordinating ability is weak, AuNPs are not necessary. Even without them, we get a high conversion of PDMAA. But AuNPs may have good effect to increase the polymerization degree (Fig. 2b).
4. Conclusion
We have discovered a new polymerization method for monomers 4VP and DMAA using a metal-catalyst system without organic initiator. Evidence suggests that the process involves radicals in the reaction. This finding offers an excellent option for obtaining products with a high degree of polymerization, which may have applications in industry. Work is ongoing to determine the full extent of these reactions as well as a detailed mechanism of the process.Acknowledgements
J.Z. acknowledges support from the National Natural Science Foundation of China (20604011, 20974044, 90923006) and the National Basic Research Program of China (2007CB925103).References
- J. H. Xia, X. Zhang and K. Matyjaszewski, Macromolecules, 1999, 32, 3531–3533 CrossRef CAS.
- N. V. Tsarevsky, W. A. Braunecker, S. J. Brooks and K. Matyjaszewski, Macromolecules, 2006, 39, 6817–6824 CrossRef CAS.
- W. M. Wan and C. Y. Pan, Macromolecules, 2007, 40, 8897–8905 CrossRef CAS.
- Y. M. Zhang, W. Sun, A. M. Santos and F. E. Kuhn, Catal. Lett., 2005, 101, 35–41 CrossRef CAS.
- T. Yang, A. Ferrali, F. Sladojevich, L. Campbell and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 9140 CrossRef CAS.
- M. Yu, Y. Wang, C. J. Li and X. Q. Yao, Tetrahedron Lett., 2009, 50, 6791–6794 CrossRef CAS.
- Y. M. Zhang, W. Sun, C. Freund, A. M. Santos, E. Herdtweck, J. Mink and F. E. Kuhn, Inorg. Chim. Acta, 2006, 359, 4723–4729 CrossRef CAS.
- M. Teodorescu and K. Matyjaszewski, Macromol. Rapid Commun., 2000, 21, 190–194 CrossRef CAS.
- M. Ciampoli and N. Nardi, Inorg. Chem., 1966, 5, 41 CrossRef.
- M. Teodorescu and K. Matyjaszewski, Macromolecules, 1999, 32, 4826–4831 CrossRef CAS.
- M. Kamachi and A. Kajiwara, Macromol. Symp., 2002, 179, 53–74 CrossRef CAS.
- Y. Inaki, M. Ishiyama, K. Hibino and K. Takemoto, Makromol. Chem., 1975, 176, 3135–3151 CrossRef CAS.
- A. Kajiwara and K. Matyjaszewski, Macromol. Rapid Commun., 1998, 19, 319–321 CrossRef CAS.
- J. Barth and M. Buback, Macromol. Rapid Commun., 2009, 30, 1805–1811 CrossRef CAS.
- M. B. Kadiiska, K. S. De Costa, R. P. Mason and J. M. Mathews, Chem. Res. Toxicol., 2000, 13, 1082–1086 CrossRef CAS.
- Q. Guo, G. H. Gao, S. Y. Qian and R. P. Mason, Chem. Res. Toxicol., 2004, 17, 1481–1490 CrossRef CAS.
- D. C. Goodwin, S. D. Aust and T. A. Grover, Chem. Res. Toxicol., 1996, 9, 1333–1339 CrossRef CAS.
- N. Tsutsumi, K. Inami and M. Mochizuki, Bioorg. Med. Chem., 2010, 18, 8284–8288 CrossRef CAS.
- W. Liu, H. Cao, H. Zhang, H. Zhang, K. H. Chung, C. A. He, H. B. Wang, F. Y. Kwong and A. W. Lei, J. Am. Chem. Soc., 2010, 132, 16737–16740 CrossRef CAS.
- M. Ochiai, T. Shu, T. Nagaoka and Y. Kitagawa, J. Org. Chem., 1997, 62, 2130–2138 CrossRef CAS.
- M. Ouchi, T. Terashima and M. Sawamoto, Chem. Rev., 2009, 109, 4963–5050 CrossRef CAS.
- G. Coullerez, A. Carlmark, E. Malmstrom and M. Jonsson, J. Phys. Chem. A, 2004, 108, 7129–7131 CrossRef CAS.
- M. A. Allodi, M. E. Dunn, J. Livada, K. N. Kirschner and G. C. Shields, J. Phys. Chem. A, 2006, 110, 13283–13289 CrossRef CAS.
- C. H. Peng, M. Fryd and B. B. Wayland, Macromolecules, 2007, 40, 6814–6819 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures. See DOI: 10.1039/c1py00223f |
| This journal is © The Royal Society of Chemistry 2011 |
