Dispersion of single-walled carbon nanotubes with poly(pyridinium salt)s†
Tae Soo
Jo
a,
Haesook
Han
a,
Longzhou
Ma
b and
Pradip K.
Bhowmik
*a
aDepartment of Chemistry, University of Nevada Las Vegas, 4505 Maryland Parkway, Las Vegas, NV 89154, USA. E-mail: pradip.bhowmik@unlv.edu; Fax: +1 702 895 4072; Tel: +1 702 895 0885
bHarry Reid Center for Environmental Study, University of Nevada Las Vegas, 4505 Maryland Parkway, Las Vegas, NV 89154, USA
First published on 26th July 2011
Abstract
Dispersion of single-walled carbon nanotubes (SWNTs) with poly(pyridinium salt)s via non-covalent interactions in DMSO is demonstrated. The resulting composites display high quenching efficiency in the photoluminescent properties of this class of ionic polymers, and TEM study shows that SWNTs are wrapped by ionic polymers with thickness around 3 nm.
After the discovery of carbon nanotubes (CNTs) in 1991, CNTs have drawn considerable attention due to their unique physical properties1a,b including high electrical and thermal conductivity,1c field emission properties1d and high mechanical strength.1e The functionalization and dispersion of CNTs have received significant interest over the past decade and resulted in the development of a number of functionalization methods.1f,2 However, the dispersion and stabilization of CNTs in organic solvents or polymer matrices are still considered as one of the main challenges in CNTs research.
It is known that o-dichlorobenzene (DCB) is one of the most useful solvents for making stable dispersions of CNTs without any chemical processing or modification owing to its strong π–orbital interactions with the sidewalls of CNTs.3a,b Moreover, amide containing solvents such as N,N-dimethylformamide (DMF) and N-methylpyrrolidone (NMP), but not dimethyl sulfoxide (DMSO), have also shown a good dispersion of CNTs.3c Choosing the proper solvent is considered as a key step in the preparation of CNT dispersions. The enhanced dispersion of CNTs can occur via covalent functionalizations, requiring treatment with strong oxidizing reagents, or non-covalent modification of CNTs. In the former approach, the functionalization of CNTs inevitably disrupts the long range π-conjugation length of CNTs resulting in decreased electrical conductivity, diminished mechanical strength and other undesired properties.2b In the latter approach, non-covalent modification is an attractive approach because it can modify the material characteristics without altering the intrinsic properties of CNTs. Among many polymer matrices, conjugated polymers are promising candidates due to π–π interactions with CNTs. Notably such polymers include poly(m-phenylene vinylene),4a poly(3-alkylthiophene),4b and poly(arylene ethynylene).2aIonic polymers such as polysoap,5apolystyrene sulfonate,5b poly(N-cetyl-4-vinylpyridinium bromide-co-N-ethyl-4-vinylpyridinium bromide-co-4-vinylpyridine),5c and polycationic poly(diallyldimethylammonium chloride)5d also provided enhanced dispersion of CNTs with varying degrees of success.
We describe herein the first example of a high concentration dispersion of single-walled carbon nanotubes (SWNTs) in DMSO with poly(pyridinium salt)s and their lyotropic liquid-crystalline (LC) properties were examined.
Poly(pyridinium salt)s were synthesized by using a reported procedure6a and two aromatic diamines with different linkages between the phenyl rings were chosen to investigate their effect on SWNT dispersions by allowing for a difference in conjugation length (Scheme 1). Even though DMF or dimethylacetamide (DMAc) can dissolve the polymers and disperse SWNTs efficiently, poly(pyridinium salt)s can undergo photodecomposition in amide containing solvents.6b Therefore, DMSO was chosen for the dispersion of SWNTs with poly(pyridinium salt)s. Various amounts of SWNTs and polymers (I-1 or I-2) by weight to weight were solubilized in DMSO using a sonicator in a water bath at room temperature by using the coagulation method.7 For example, polymer I-1 (50 mg) was dissolved in DMSO and SWNTs (25 mg, 33 wt% of SWNTs content based on the total weight) were transferred into the polymer solution. The dark solution was mixed by sonication for 2 h with a water-bath temperature maintained at room temperature. The resulting solution was centrifuged (30 min under ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g) to settle down insoluble solids. The homogeneous dark solution was precipitated in ethyl acetate and the precipitated black solid was filtered and dried in vacuo at 80 °C for 48 h. However, note here that the compositions of composites are subjected to experimental errors because of 90% purity of SWNTs used in this study and mechanical loss. Therefore, it is difficult to determine the exact amount of the SWNTs present in the polymer matrix in each composition.
000g) to settle down insoluble solids. The homogeneous dark solution was precipitated in ethyl acetate and the precipitated black solid was filtered and dried in vacuo at 80 °C for 48 h. However, note here that the compositions of composites are subjected to experimental errors because of 90% purity of SWNTs used in this study and mechanical loss. Therefore, it is difficult to determine the exact amount of the SWNTs present in the polymer matrix in each composition.
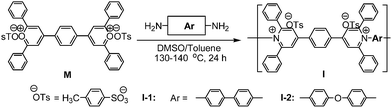 | ||
| Scheme 1 Synthesis of poly(pyridinium salt)s. | ||
Polymers I-1 and I-2 in DMSO had absorption maxima at 347 and 335 nm, respectively, in their UV-Vis spectra. Their corresponding emission spectra, recorded in DMSO solution at excitation wavelengths of 357 and 364 nm, showed a broad band at 518 and 510 nm, respectively (Fig. 1). There were no significant changes in the UV-Vis spectra of SWNT composites of I-1 and I-2. However, the photoluminescence spectra (PL) clearly supported strong π–π and cationic–π interactions between the polymers and SWNTs. The strong light emission of both I-1 and I-2 was quenched with the increased amounts of SWNTs. This behavior most likely arises from an efficient energy or electron transfer between the polymers and SWNTs, instead of disruption of π-conjugation.8 Pronounced quenching was observed in I-1/SWNT composites compared to that in I-2/SWNT composites. In the case of I-1/SWNT composites, the intensity was quenched by 88% while in the case of I-2/SWNT composites the intensity was quenched by 71%. These results imply that different types of interactions (vide supra) between polymers and SWNTs exist that are related to the conformational flexibility or rigidity of the polymer chains2a or better π–π interactions between polymer I-1 and SWNTs.
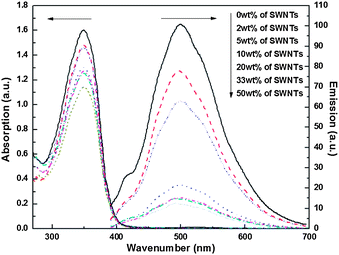 | ||
| Fig. 1 UV-Vis absorption spectra of I-1/SWNT composites in DMSO (left arrow) and emission spectra of I-1/SWNT composites at an excitation wavelength of 357 nm in DMSO (right arrow). | ||
The 1H NMR spectra of I-1/SWNT composites further revealed the strong interactions resulting in the broadening and reduced intensity of proton signals.4a,9 These results suggest the close contacts between the polymers and SWNTs. The composites of I-1/SWNTs showed broader peaks when compared with those of I-2/SWNTs. This broadness can provide indirect evidence for the stronger interactions in I-1/SWNT composites than in I-2/SWNTs, which is consistent with the results of PL (Fig. 2).
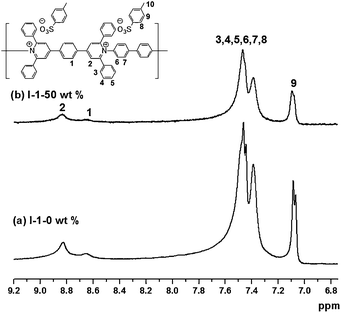 | ||
| Fig. 2 1H NMR spectra of (a) I-1-0 wt% (10 mg mL−1 in d6-DMSO) and (b) I-1-50 wt% (10 mg mL−1 in d6-DMSO). | ||
The interactions between the polymers and SWNTs were confirmed by the Raman spectroscopy (Fig. S3 and S4† in the ESI) study. The characteristic G and D bands of SWNTs appeared at 1565 and 1252 cm−1, respectively. The band intensity increased with a high concentration of SWNTs. Note here that, when SWNTs lose electrons, the G band shifts to a higher frequency.10 However, any shift in the G and D bands of composites was not observed that is suggestive of no loss of electrons occurred.
The dispersions of SWNTs in I-1 and I-2 were examined by the transmission electron microscopy (TEM) study. Some representative TEM images of SWNT dispersions are shown in Fig. 3a–d. SWNTs usually exist as bundles, which are heavily entangled (Fig. S5† in the ESI). Upon dispersion of SWNTs with I-1 and I-2 in DMSO, the entangled SWNT bundles exfoliated to form much finer bundles. The I-1/SWNT composites provided the more dispersed SWNTs than I-2/SWNT composites owing to their stronger π–π interactions, which are also supported by PL and 1H NMR spectra. A high resolution TEM image of polymer/SWNT composites (Fig. 3c and d) revealed the presence of a thin layer of an amorphous coating with a thickness around 3 nm on the surface of the nanotube walls in each case.
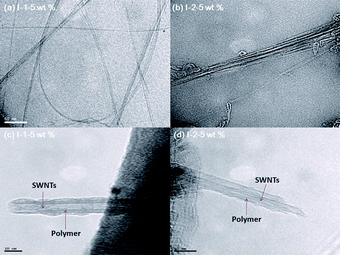 | ||
| Fig. 3 TEM images of (a) I-1-5 wt% and (b) I-2-5 wt%; and HRTEM images of (c) I-1-5 wt% and (d) I-2-50 wt% (The scale bars in a, b and c, d represent 50 and 10 nm, respectively). | ||
Polymers I-1 and I-2 exhibited lyotropic properties in DMSO and methanol.6a Their lyotropic properties were also examined when they are mixed with SWNTs using polarizing optical microscope (POM) study. For the entire range of concentrations, any entanglement of SWNTs was not observed. Their LC structures composed of small and large bâtonnets, polygonal arrays, and rounded droplets were identified as anisotropic and isotropic phases. These unique textures are indicative of their lamellar phase.11 However, by increasing the amounts of SWNTs, I-1-20 wt% exhibited a biphasic image (an LC and an isotropic phase). This result is possibly due to an interruption of dispersed SWNTs in the formation of the lyotropic phase. However, at the identical concentration of SWNTs (5 wt%), I-2/SWNT composites required a little higher concentration of polymer for the development of the fully grown lyotropic phase that means the rod-like structure is conducive to form the LC properties (Fig. 4).
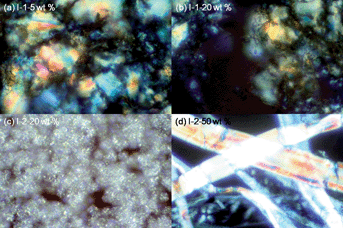 | ||
| Fig. 4 Photomicrographs of (a) 30 wt% of composite I-1-5 wt% SWNTs in DMSO, (b) 30 wt% of composite I-1-20 wt% SWNTs in DMSO, (c) 40 wt% of composite I-2-20 wt% SWNTs in DMSO, and (d) 40 wt% of composite I-2-50 wt% SWNTs in DMSO under crossed polarizers (magnification 400×). | ||
To investigate the morphology of the composites, we examined the microstructures with the X-ray scattering technique. In Fig. S7†, the representative X-ray power diffraction (XRD) plots with the scattering angle range between 5 and 60° of composites I-1-0 wt% and I-1-50 wt% in 30 wt% DMSO were recorded at room temperature and are displayed. Broad diffraction peaks with relatively low intensities at 2θ = 20° (d-spacing in the range of 4.43–4.96 Å) were observed, which are the characteristic feature of separation of polymer chains both in the lyotropic phase and biphasic state.12
Conclusions
In summary, we demonstrated a new method for the dispersion of SWNTs using poly(pyridinium salt)s via non-covalent interactions in DMSO. Unlike other SWNT dispersion methods in organic solvents, to our knowledge, these results are the first examples of SWNT dispersions in this solvent without any chemical modification of the SWNTs. The non-covalent interactions of ionic polymers with SWNTs allow the convenient preparation of a new family of SWNT dispersions, which can provide a general approach to disperse carbon nanotubes for the development of high-performance materials.Acknowledgements
P.K.B. acknowledges the University of Nevada Las Vegas (UNLV) for Applied Research Initiative (ARI) grant, and the donors of the Petroleum Research Fund, administered by the American Chemical Society, and an award (CCSA# CC5589) from Research Corporation for the support of this research. T.S.J. acknowledges the Graduate College (UNLV) for providing him a SPGRA for the academic year 2010–2011.Notes and references
- (a) P. M. Ajayan, Chem. Rev., 1999, 99, 1787–1800 CrossRef CAS; (b) R. H. Baughman, A. A. Zakhidov and W. A. deHeer, Science, 2002, 297, 787–792 CrossRef CAS; (c) S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS; (d) J. Hone, M. Whitney, C. Piskoti and A. Zettl, Phys. Rev. B: Condens. Matter, 1999, 59, R2514–R2518 CrossRef CAS; (e) R. B. Sharma, V. N. Tondare, D. S. Joag, A. Govindaraj and C. N. R. Rao, Chem. Phys. Lett., 2001, 344, 283–286 CrossRef CAS; (f) M. M. J. Treacy, T. W. Ebbesen and J. M. Gibson, Nature, 1996, 381, 678–680 CrossRef CAS.
- (a) J. Chen, H. Y. Liu, W. A. Weimer, M. D. Halls, D. H. Waldec and G. C. Walker, J. Am. Chem. Soc., 2002, 124, 9034–9035 CrossRef CAS; (b) D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS; (c) K. J. Ziegler, Z. N. Gu, H. Q. Peng, E. L. Flor, R. H. Hauge and R. E. Smalley, J. Am. Chem. Soc., 2005, 127, 1541–1547 CrossRef CAS; (d) J. Lagerwall, G. Scalia, M. Haluska, U. Dettlaf-Weglikowska, S. Roth and F. Fiesselmann, Adv. Mater., 2007, 19, 359–364 CrossRef CAS; (e) M. C. LeMieux, M. Roberts, S. Barman, Y. W. Jin, J. M. Kim and Z. Bao, Science, 2008, 321, 101–104 CrossRef CAS.
- (a) J. L. Bahr, E. T. Mickelson, M. J. Bronikowski, R. E. Smalley and J. M. Tour, Chem. Commun., 2001, 193–194 RSC; (b) D. S. Kim, D. Nepal and K. E. Geckeler, Small, 2005, 1, 1117–1124 CrossRef CAS; (c) C. A. Furtado, U. J. Kim, H. R. Gutierrez, L. Pan, E. C. Dickey and P. C. Eklund, J. Am. Chem. Soc., 2004, 126, 6095–6105 CrossRef.
- (a) A. Star, J. F. Stoddart, D. Steuerman, M. Diehl, A. Boukai, E. W. Wong, X. Yang, S.-W. Chung, H. Choi and J. R. Heath, Angew. Chem., Int. Ed., 2001, 40, 1721–1725 CrossRef CAS; (b) A. Ikeda, K. Nobusawa, T. Hamano and J. Kikuchi, Org. Lett., 2006, 8, 5489–5492 CrossRef CAS.
- (a) D. Wang, W.-X. Ji, Z.-C. Li and L. Chen, J. Am. Chem. Soc., 2006, 128, 6556–6557 CrossRef CAS; (b) M. J. O'Connell, P. Boul, L. M. Ericson, C. Huffman, Y. Wang, E. Haroz, C. Kuper, J. Tour, K. D. Ausman and R. E. Smalley, Chem. Phys. Lett., 2001, 342, 265–271 CrossRef CAS; (c) V. A. Sinani, M. K. Gheith, A. A. Yaroslavov, A. A. Rakhnyanskaya, K. Sun, A. A. Mamedov, J. P. Wicksted and N. A. Kotov, J. Am. Chem. Soc., 2005, 127, 3463–3472 CrossRef CAS; (d) J. H. Rouse and P. T. Lillehei, Nano Lett., 2003, 3, 59–62 CrossRef CAS.
- (a) P. K. Bhowmik, R. A. Burchett, H. Han and J. J. Cebe, Macromolecules, 2001, 34, 7579–7581 CrossRef CAS; (b) F. Lin, S. Z. D. Cheng and F. W. Harris, Polymer, 2002, 43, 3421–3430 CrossRef CAS.
- (a) F. Du, J. E. Fischer and K. I. Winey, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 3333–3338 CrossRef CAS; (b) Y. Sabba and E. L. Thomas, Macromolecules, 2004, 37, 4815–4820 CrossRef CAS.
- (a) J. Zou, L. Liu, H. Chen, S. I. Khondaker, R. D. McCullough, Q. Huo and L. Zhai, Adv. Mater., 2008, 20, 2055–2060 CrossRef CAS; (b) B. K. Kulia, S. Malik, S. K. Batabyal and A. K. Nandi, Macromolecules, 2007, 40, 278–287 CrossRef CAS; (c) R. Koizhaiganova, H. J. Kim, T. Vasudevan, S. Kudaibergenov and M. S. Lee, J. Appl. Polym. Sci., 2010, 115, 2448–2454 CrossRef CAS.
- (a) A. B. Dalton, C. Stephan, J. N. Coleman, B. McCarthy, P. M. Ajayan, S. Sefrant, P. Bernier, W. J. Blau and H. J. Byrne, J. Phys. Chem. B, 2000, 104, 10012–10016 CrossRef CAS; (b) D. E. Hill, Y. Lin, A. M. Rao, L. F. Allard and Y.-P. Sun, Macromolecules, 2002, 35, 9466–9471 CrossRef CAS; (c) Y. Lin, B. Zhou, K. A. S. Fernando, P. Liu, L. F. Allard and Y.-P. Sun, Macromolecules, 2003, 36, 7199–7204 CrossRef CAS; (d) Y.-P. Sun, K. Fu, Y. Lin and W. Huang, Acc. Chem. Res., 2002, 35, 1096–1104 CrossRef CAS.
- A. M. Rao, P. C. Eklund, S. Bandow, A. Thess and R. E. Smalley, Nature, 1997, 388, 257–259 CrossRef CAS.
- F. B. Rosevear, J. Am. Oil Chem. Soc., 1954, 31, 628–639 CrossRef CAS.
- A. K. Nedeltchev, H. Han and P. K. Bhowmik, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4408–4412 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, 1H NMR, UV-Vis, photoluminescence, Raman spectra, TEM and POM. See DOI: 10.1039/c1py00212k |
| This journal is © The Royal Society of Chemistry 2011 |
