Targeted delivery of doxorubicin into cancer cells using a folic acid–dendrimer conjugate
Yin
Wang
ab,
Xueyan
Cao
b,
Rui
Guo
b,
Mingwu
Shen
b,
Mengen
Zhang
b,
Meifang
Zhu
a and
Xiangyang
Shi
*abc
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai, 201620, People's Republic of China
bCollege of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai, 201620, People's Republic of China. E-mail: xshi@dhu.edu.cn; Fax: +0086-21-67792306-804; Tel: +0086-21-67792656
cCQM- Centro de Química da Madeira, Universidade da Madeira, Campus da Penteada, 9000-390, Funchal, Portugal
First published on 9th June 2011
Abstract
We report here a general approach to using multifunctional poly(amidoamine) (PAMAM) dendrimer-based platform to encapsulate an anticancer drug doxorubicin (DOX) for targeted cancer therapy. In this approach, generation 5 (G5) PAMAM dendrimers modified with fluorescein isothiocyanate (FI) and folic acid (FA) via covalent conjugation, and with remaining terminal amines being acetylated (G5.NHAc-FI-FA) were used to complex DOX for targeted delivery of the drug to cancer cells overexpressing high-affinity folic acid receptors (FAR). We show that the formed G5.NHAc-FI-FA/DOX complexes with each dendrimer encapsulating approximately one DOX molecule are water soluble and stable. In vitro release studies show that DOX complexed with the multifunctional dendrimers can be released in a sustained manner. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability assay in conjunction with cell morphology observation demonstrates that the G5.NHAc-FI-FA/DOX complexes can specifically target and display specific therapeutic efficacy to cancer cells overexpressing high-affinity FAR. Findings from this study suggest that multifunctional dendrimers may be used as a general drug carrier to encapsulate various cancer drugs for targeting therapy of different types of cancer.
Introduction
The uses of polymeric nanoparticles (NPs) with functional properties have been of immense scientific and technological interest in a broad range of biomedical applications including but not limited to drug and gene delivery, cell and tissue engineering, diagnostic and therapeutic purposes.1,2 Among these applications, NP-based drug delivery systems with specific and rapid internalization into a target cell are particularly useful for cancer therapy.3,4 Formulating cancer drugs within polymer NPs not only preserves the therapeutic activity of the drug, but also facilitates a controlled release of the drug in a sustained manner.5,6Among many different polymer NP-based drug delivery systems, dendrimers have been paid much attention in the development of targeted drug delivery systems.7–10Dendrimers are a family of highly branched, monodispersed, and synthetic macromolecules with well-defined architecture and composition.11,12 The size of dendrimers is small (e.g., the diameter of a generation 5 (G5) poly(amidoamine) (PAMAM) dendrimer is 5.4 nm), which enables them to be easily cleared from the blood through the kidney,13 eliminating the need for biodegradability. For cancer drug carrier applications, dendrimers can be easily modified and conjugated with multiple functionalities such as targeting molecules, imaging agents, and drugs8,9,14 with desired water solubility and biocompatibility. In addition, the relatively hydrophobic interior of the dendrimers allows for physical encapsulation or complexation of hydrophobic cancer drugs, significantly improving the water solubility and bioavailability of the drugs.15–20
Doxorubicin (DOX) is a cancer drug widely used in cancer chemotherapy.21,22 Similar to other cancer drugs, free DOX displays prominent cytotoxicity to normal cells or tissues, limiting its clinical applications. To decrease the toxicity of DOX, development of various carrier systems to improve the water solubility and bioavailability is necessary to maintain the tumor inhibition effect.23,24Dendrimers have been used to either covalently link DOX molecules on their termini25–27 or physically encapsulate/complex DOX within the interior of the dendrimers19,28,29 to improve the therapeutic efficacy of the drug. To further improve the tumor inhibition effect, dendrimers have also been modified with targeting agents such as folic acid (FA),30,31peptides32 or galactose,33etc. prior to either covalently linking DOX on their termini or physically encapsulating/complexing DOX within their interiors. For FA-targeted delivery of DOX using dendrimers as a carrier, Gupta et al.31 physically loaded DOX into the interior of FA-conjugated G5 polypropylene imine dendrimers for targeted delivery of DOX to cancer cells. However, the targeting specificity and the targeted inhibition of cancer cells have not been fully illustrated. In another study, Baker and coworkers30 developed a FA-targeted PAMAM dendrimer conjugate that was covalently linked to DOX. The linked DOX can be released via a photochemical mechanism. The major drawback of the developed system is the requirement of UV light, which may not be suitable for practical medical applications. Although the research directed to targeted delivery of DOX using dendrimers as a carrier has progressed well, development of a desired, convenient, and cost-effective dendrimer-based targeted drug delivery system still remains a great challenge.
In our previous work, we have encapsulated a potential anticancer drug 2-methoxyestradiol within FA-targeted G5 PAMAM dendrimers terminated with acetamide groups for targeted cancer therapy.20 Our results show that the targeting ligand FA covalently attached onto dendrimers enables intracellular delivery of the drug to FA receptor (FAR)-overexpressing cancer cells, resulting in targeted cancer cell death. It is anticipated that using a similar approach, DOX can be physically loaded into the interiors of the FA-targeted G5 PAMAM dendrimers, allowing for targeted delivery of DOX to cancer cells overexpressing FAR.
In this present study, we utilized fluorescein isothiocyanate (FI)- and FA-modified G5 PAMAM dendrimers with acetyl terminal groups (G5.NHAc-FI-FA) to encapsulate the anticancer drug DOX. The encapsulation efficiency, release kinetics, and targeted inhibition of cancer cells overexpressing high-affinity FAR were investigated in detail. Compared with the covalent conjugation of cancer drugs onto the dendrimer surfaces,8,9 this approach is simple and it is really not necessary to design suitable complicated linker system before the conjugation of the drug molecules. The results generated from this study provide a basis for a rational design of functional dendrimer/drug complexes for various therapeutic applications, especially for targeted cancer therapy.
Experimental section
Materials
Ethylenediamine core amine-terminated G5 PAMAM dendrimers (G5.NH2) with a polydispersity index less than 1.08 were purchased from Dendritech (Midland, MI). All other chemicals were obtained from Aldrich and used as received. The water used in all the experiments was purified using a Milli-Q Plus 185 water purification system (Millipore, Bedford, MA) with a resistivity higher than 18 MΩ cm. Regenerated cellulose dialysis membranes (molecular weight cutoff, MWCO = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were acquired from Fisher.
000) were acquired from Fisher.
Synthesis of G5.NHAc-FI-FA dendrimers
The G5.NH2 dendrimers were conjugated with FI and FA as described in our previous report.34,35 The remaining amines of the dendrimers were converted to acetyl groups by reacting with acetic anhydride according to previous reports.36,37 Following our previously published procedure, FI- and FA-modified G5 dendrimers with remaining amine transformed to acetamide groups (G5.NHAc-FI-FA) were obtained and characterized.20Characterization techniques
UV-Vis spectra were collected using a Lambda 25 UV-Vis spectrometer (PerkinElmer, USA). Samples were dissolved in water before measurements. 1H NMR spectra were recorded using Bruker AV-400 NMR spectrometer. Samples were dissolved in D2O before measurements. Zeta-potential measurements were carried out using a Zetasizer Nano ZS system (Malvern, UK) equipped with a standard 633 nm laser. Dendrimer samples with a concentration of 1 mg mL−1 were measured under different pH conditions (pH 5.0, 7.0, and 10.0).Encapsulation of DOX within G5.NHAc-FI-FA dendrimers
G5.NHAc-FI-FA dendrimers (10 mg) were dissolved in 1.5 mL water. Doxorubicin hydrochloride (DOX·HCl) with 10 molar equivalents of dendrimers was dissolved in 300 μL methanol and was neutralized with 5 μL triethylamine to generate DOX, which is insoluble in water. Then the DOX solution was mixed with the 1.5 mL dendrimer aqueous solution. The mixture solution was vigorously stirred overnight to allow the evaporation of the methanol solvent. The dendrimer/DOX mixture solution was centrifuged (7000 rpm for 10 min) to remove the precipitates related to non-complexed free DOX. The precipitate was collected and dissolved into 1 mL methanol for UV-Vis analysis. The supernatant was lyophilized for 3 d to obtain the dendrimer/DOX complex.In vitro release kinetic study
Five-millilitre solution of G5.NHAc-FI-FA/DOX complex in water or free DOX in ethanol was placed in a dialysis bag with MWCO of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, hermetically tied, and suspended in 45 mL of aqueous medium, which was PBS buffer (pH 7.4) or acetate buffer (pH 5.0). The entire system was kept in a vapor-bathing constant temperature vibrator at 37 °C. Three millilitres of the buffer medium was taken out at each predetermined time interval and measured by UV-Vis spectrometer. The volume of the outer phase buffer medium was maintained constant by replenishing 3 mL of the corresponding buffer solution.
000, hermetically tied, and suspended in 45 mL of aqueous medium, which was PBS buffer (pH 7.4) or acetate buffer (pH 5.0). The entire system was kept in a vapor-bathing constant temperature vibrator at 37 °C. Three millilitres of the buffer medium was taken out at each predetermined time interval and measured by UV-Vis spectrometer. The volume of the outer phase buffer medium was maintained constant by replenishing 3 mL of the corresponding buffer solution.
Cell biological evaluation
KB cells (a human epithelial carcinoma cell line) were continuously grown in two 50 mL culture flask, one in FA-free media and the other in regular RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U mL−1penicillin, 100 U mL−1streptomycin, and 2.5 μM FA. The cells grown in FA-free media express high-level FAR (denoted as KB-HFAR), while the cells grown in FA-containing media express low-level FAR (denoted as KB-LFAR).To check if G5.NHAc-FI-FA/DOX complex is therapeutically active, one day before experiments, cells (1 × 104cells per well) were plated into a 96-well plate in a complete medium. The next day, DOX·HCl (500 nM) or G5.NHAc-FI-FA/DOX complex with the same DOX concentration in PBS buffer (10 μL) was added to cells and then incubated for 48 h at 37 °C before 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Note that for the DOX encapsulation and release studies, water-insoluble DOX was used, while for cell biological evaluation, water-soluble DOX·HCl was used as a control to check the therapeutic activity of the free drug.
In order to investigate the targeted inhibition of KB-HFAR cells, the G5.NHAc-FI-FA/DOX complex with the same DOX concentration (500 nM) was added to both KB-HFAR and KB-LFAR cells, the medium in wells containing dendrimer/DOX complexes was totally taken out and replenished with the same volume of fresh medium after 1 h incubation. The cells were then incubated for 48 h at 37 °C before MTT assay. Parallel cell samples after 1 h incubation of G5.NHAc-FI-FA/DOX complex and subsequent change with fresh medium was observed by confocal microscopy (Carl Zeiss LSM 700 laser scanning confocal microscope, Jena, Germany) to check the binding specificity of the G5.NHAc-FI-FA/DOX complex to KB cells. The FI fluorescence was excited with a 488 nm argon blue laser, and the red fluorescence of DOX was excited using a 543 nm Helium-Neon laser. The optical section thickness was set at 5 μm. Samples were imaged using a 63× water-immersion objective lens.
An MTT assay was used to quantify the viability of cells. After 48 h incubation with DOX or dendrimer/DOX complex, the metabolically active cells were then detected by adding MTT to each well. Then, the plates were read at 570 nm. Mean and standard deviation for the triplicate wells were reported. One way ANOVA statistical analysis was performed to evaluate the significance of the therapeutic efficacy of the DOX drug. After treatment with DOX or dendrimer/DOX complexes, cell morphology was observed by phase contrast microscopy (Leica DMIRB fluorescent inverted microscope). The magnification was set at 200× for all samples.
Results and discussion
Encapsulation of DOX within G5.NHAc-FI-FA dendrimers
G5 PAMAM dendrimer was selected for the synthesis of multifunctional drug carrier because of its relatively small size, comparable to hemoglobin (5.4 nm), and sufficient terminal amine groups for multiple conjugation reactions.8,9 The FI moieties covalently attached to the dendrimer surface are used to fluorescently detect the binding and intracellular uptake of the dendrimer carriers. The FA attached onto the dendrimer surfaces can be used for targeting cancer cells overexpressing high-affinity FAR.10,14 The final acetylation is a key step to not only improve the biocompatibility of the dendrimer species38 but also minimize non-specific binding of the dendrimers to cell membranes.39 The chemical structure of the final G5.NHAc-FI-FA dendrimers was characterized using both 1H NMR spectroscopy and UV-Vis spectrometry. Based on NMR integration, the number of FI and FA moieties attached to each dendrimer molecule was estimated to be 4 and 5, respectively. These results are consistent with those reported in our previous study.20The neutralization of DOX·HCl to form DOX with very low water solubility enables efficient encapsulation of the drug within the relatively hydrophobic interior of the dendrimers. The formed dendrimer/DOX complexes as a new formulation are expected to have improved water-solubility and thus enhanced bioavailability. The formed G5.NHAc-FI-FA/DOX complexes were characterized with UV-Vis spectroscopy (Fig. 1). The UV-Vis spectra of DOX dissolved in ethanol and G5.NHAc-FI-FA dendrimers without DOX were also recorded for comparison. It is clear that DOX shows a strong absorption peak at 481 nm. After encapsulation with DOX, the G5.NHAc-FI-FA/DOX complexes have an absorption enhancement at the 481 nm peak when compared with G5.NHAc-FI-FA dendrimers without DOX under similar dendrimer concentration. This indicates that DOX has been successfully encapsulated within the G5.NHAc-FI-FA dendrimers. The payload of DOX encapsulated within G5.NHAc-FI-FA dendrimers was analyzed with UV-Vis spectroscopy. Approximately one DOX molecule was found to be complexed within each G5.NHAc-FI-FA dendrimer. The lower encapsulation efficiency of DOX compared to that of 2-methoxyestadiol reported in our previous work20 may be due to the larger molecular scaffold of DOX (Mw = 543.5) than that of 2-methoxyestadiol (Mw = 302.4), limiting the entrance and retention of DOX within the dendrimer interiors.
 | ||
| Fig. 1 UV-Vis spectra of free DOX dissolved in ethanol, G5.NHAc-FI-FA dendrimers dissolved in water, and G5.NHAc-FI-FA/DOX complexes dissolved in water. | ||
The stability of the G5.NHAc-FI-FA/DOX complexes is of paramount importance for their biological applications. We showed that the lyophilized powder of G5.NHAc-FI-FA/DOX complexes could be dissolved in aqueous solution, and was stable under different pH conditions (pH = 5.0, 7.0, and 10.0), similar to the G5.NHAc-FI-FA dendrimers without DOX (Fig. 2). This suggests that the G5.NHAc-FI-FA/DOX complexes have similar colloidal stability to that of the G5.NHAc-FI-FA dendrimers without drug complexed under the selected pH conditions. The surface potentials of G5.NHAc-FI-FA dendrimers and G5.NHAc-FI-FA/DOX complexes under different pH conditions are listed in Table 1. This information is crucial for understanding the cellular interactions of the complexes. The larger values at pH 5.0 for both dendrimers and complexes compared to those at pH 7.0 should be ascribed to the protonation of a portion of the dendrimer tertiary amines.40 The change of the surface potential of both the dendrimers and complexes with pH followed the same trend as that of G5.NHAc dendrimers as described in our previous work.19 In addition, the G5.NHAc-FI-FA/DOX complexes in aqueous solution (e.g., water, PBS buffer with a pH value of 7.4, and cell culture medium) stored at 4 °C were stable for at least 12 months, which is essential for their further biological applications.
 | ||
| Fig. 2 Photographs of the aqueous solutions of G5.NHAc-FI-FA dendrimers (a) and G5.NHAc-FI-FA/DOX complexes (b) under different pH conditions (pH = 5.0, 7.0, and 10.0 from left to right for both panels). | ||
| Materials | Zeta-potential (mV) | ||
|---|---|---|---|
| pH = 5.0 | pH = 7.0 | pH = 10.0 | |
| G5.NHAc-FI-FA dendrimer | 15.9 ± 6.0 | 9.1 ± 4.5 | −14.4 ± 3.3 |
| G5.NHAc-FI-FA/DOX complex | 14.0 ± 6.7 | 11.1 ± 5.5 | −6.2 ± 6.4 |
In vitro release kinetic studies
The in vitro release study of the G5.NHAc-FI-FA/DOX complexes was performed in PBS buffer (pH = 7.4) or acetate buffer (pH = 5.0) medium at 37 °C. The cumulative release of DOX from the complexes showed that the drug was released in a sustained manner (Fig. 3). In contrast, the free DOX drug dissolved in ethanol was quickly released to the outer phase of the dialysis tube in PBS buffer. About 65% was released within just 2 h. In both buffers with different pH conditions, the release of DOX from the G5.NHAc-FI-FA/DOX complexes followed a biphasic pattern which was characterized by an initial faster release followed by a sustained release profile. In PBS buffer (pH = 7.4), about 11.5% of the DOX drug was released within 1 h from the complexes, and about 25.7% of the DOX was released within 24 h. For comparison, the drug release rate was slightly increased in acetate buffer with a pH value of 5.0. About 16.2% of DOX was released within 1 h and approximately 28.2% of the drug was released within 24 h. The prolonged release profile of DOX from the complexes under both buffer conditions implies that the relatively hydrophobic interior of dendrimer molecules is extremely useful for effective encapsulation and retention of the hydrophobic DOX drug. The release rate of DOX from the complexes under pH 5.0 was higher than that under pH 7.4. Under pH 5.0, the drug DOX was protonated and has positive charges. The positively charged DOX molecules could be repelled by the protonated dendrimer branches with positive charges under pH 5.0 (Table 1), slightly speeding up the release of DOX drugs from the hydrophobic dendrimer interiors. | ||
| Fig. 3 Cumulative release of DOX from G5.NHAc-FI-FA/DOX complexes in PBS buffer (pH = 7.4) and acetate buffer (pH = 5.0) at 37 °C. The control experiment was performed by dialyzing the free DOX dissolved in ethanol against PBS buffer (pH = 7.4). The data are expressed as mean ± S.D. (n = 3). | ||
Therapeutic efficacy of DOX encapsulated within G5.NHAc-FI-FA dendrimers
The therapeutic efficacy of DOX complexed with G5.NHAc-FI-FA dendrimers was tested using KB cells, a human epithelial carcinoma cell line. After incubation of the dendrimer/DOX complexes with cells for 48 h, an MTT assay was performed to evaluate the viability of KB cells (Fig. 4). It appeared that both free DOX·HCl (500 nM) and G5.NHAc-FI-FA/DOX complexes with DOX concentration of 500 nM caused a significant loss of cell viability in KB cells when compared with the untreated control cells and the cells treated with PBS buffer (p value < 0.0001 for each). To exclude the possible inherent toxicity of the dendrimers, the multifunctional G5.NHAc-FI-FA dendrimers without DOX were also tested. The concentration of the multifunctional dendrimers tested was similar to that of the dendrimer used to complex with DOX (500 nM) for the MTT assay. It is clear that G5.NHAc-FI-FA dendrimers are non-toxic (p > 0.05). These results indicate that the therapeutic activity of G5.NHAc-FI-FA/DOX complexes is solely related to the complexed drug DOX. | ||
| Fig. 4 MTT assay of KB cell viability after treatment with 10 μL PBS buffer, free DOX.HCl dissolved in 10 μL PBS (500 nM), G5.NHAc-FI-FA/DOX complexes with DOX concentration of 500 nM, and G5.NHAc-FI-FA dendrimers without DOX but with the same concentration to those used to encapsulate 500 nM DOX. The data are expressed as mean ± S.D. (n = 3). | ||
The cytotoxicity effect of the G5.NHAc-FI-FA/DOX complexes was further confirmed by microscopic visualization of the cell morphology change after treatment with the complexes. Fig. 5 shows the morphology of untreated KB cells, KB cells treated with PBS buffer, KB cells treated with free DOX·HCl, and KB cells treated with G5.NHAc-FI-FA/DOX complexes, respectively. DOX·HCl in PBS (Fig. 5c), G5.NHAc-FI-FA/DOX complexes (Fig. 5d) with similar DOX concentration (500 nM) induced similar cell morphology changes. A significant portion of the cells became rounded and non-adherent, indicative of the fact that cells have undergone apoptosis. In contrast, no rounded and detached cells can be visualized in control cells without DOX treatment (Fig. 5a) and cells treated with 10 μL PBS (Fig. 5b). In addition, the KB cells were also treated with G5.NHAc-FI-FA dendrimers (without the complexation of DOX) with a dendrimer concentration similar to that of dendrimer used for complexation of DOX (500 nM). It was found that the multifunctional G5.NHAc-FI-FA dendrimers did not exhibit any prominent toxic effect (Fig. 5e). This further suggests that the bioactivity of G5.NHAc-FI-FA/DOX complexes is solely related to the complexed drug DOX. These results are consistent with the MTT assay data.
 | ||
| Fig. 5 Photomicrographs of the control KB cells without treatment (a) and the KB cells treated with 10 μL PBS (b), free DOX·HCl (500 nM) (c), DOX (500 nM) complexed with G5.NHAc-FI-FA dendrimers (d), and G5.NHAc-FI-FA dendrimers without DOX but with the same concentration as those used to encapsulate 500 nM DOX (e), respectively. | ||
Targeted inhibition of cancer cells using G5.NHAc-FI-FA/DOX complexes
FA was selected as a targeting ligand to be conjugated onto the dendrimer surfaces in order to afford specific delivery of the DOX drugvia the dendrimer carrier to cancer cells overexpressing high-affinity FAR. FAR has been known to be overexpressed in many types of cancer cells, including ovary, kidney, uterus, testis, brain, colon, lung, and myelocytic blood.41–43 The high-affinity of FAR for FA affords specific binding and internalization of FA-modified dendrimers to cancer cells in the presence of normal cells through receptor-mediated endocytosis. KB cells were selected for the specific binding and treatment with the G5.NHAc-FI-FA/DOX complexes.The conjugation of the FI moiety onto G5 dendrimers enables fluorescence microscopic imaging of the cellular uptake of the G5.NHAc-FI-FA/DOX complexes. Fig. 6 shows that after 1 h incubation of cells with the G5.NHAc-FI-FA/DOX complexes, only KB-HFAR cells display significant fluorescence signals, which is associated with the specific internalization of G5.NHAc-FI-FA/DOX complexes into the cytoplasm of the cells (Fig. 6a–d). The green (Fig. 6a) and red (Fig. 6b) fluorescence signals indicate that both the FI-labeled dendrimer and the complexed DOX were internalized within the KB-HFAR cells. This can be further demonstrated by orange signals in the merged images shown in Fig. 6d. In contrast, under similar microscopic conditions, the KB-LFAR cells treated with the same complexes do not show apparent orange fluorescence signals in the merged confocal microscopic images (Fig. 6f), which is quite close to the PBS-treated KB-HFAR cells (Fig. 6e). This result suggests that the complexation with DOX drug within the G5.NHAc-FI-FA dendrimers does not compromise the targeting specificity of the FA-modified dendrimers, in agreement with our previous results.20
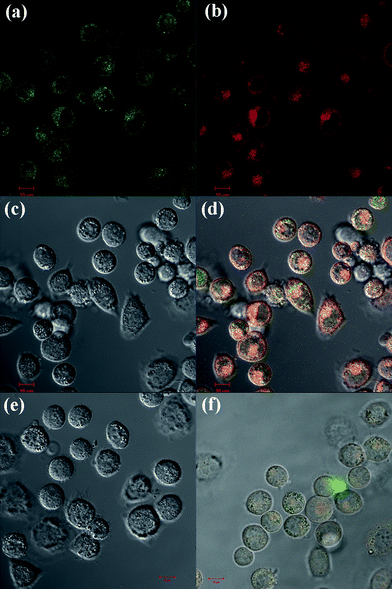 | ||
| Fig. 6 Confocal microscopic images of KB-HFAR cells treated with G5.NHAc-FI-FA/DOX complexes for 1 h (a, green fluorescence channel detecting the dye FI; b, red fluorescence channel detecting DOX; c, DIC image of the cells; and d, merged images with the above three modes), the control KB-HFAR cells treated with PBS buffer (e), and KB-LFAR cells treated with G5.NHAc-FI-FA/DOX complexes for 1 h (f). Both (e) and (f) show the merged images with the green fluorescence channel, red fluorescence channel, and DIC images. All images were collected under similar instrumental conditions. | ||
To prove our hypothesis that the G5.NHAc-FI-FA/DOX complexes are able to specifically inhibit KB-HFAR cells, after incubation of cells with the complexes for 1 hour, the medium in wells was replaced with the same volume of fresh medium. Then, the cells were incubated for 48 h at 37 °C and MTT assay was performed to detect the viability of cells. Fig. 7 shows the viability of untreated KB-HFAR cells (control) and both KB-HFAR cells and KB-LFAR cells treated with the G5.NHAc-FI-FA/DOX complexes. It is clear that the treatment of KB-HFAR cells with G5.NHAc-FI-FA/DOX complexes results in a significant decrease (about 55%) in the cell viability (p value <0.001 versus control). In contrast, around 88% of KB-LFAR cells were still alive after the same treatment (p value < 0.05 versus control), suggesting a less therapeutic effect of DOX complexed with G5.NHAc-FI-FA dendrimers on KB-LFAR cells. These results clearly indicate that the G5.NHAc-FI-FA/DOX complexes afford a targeted inhibition of the growth of cancer cellsviareceptor-mediated binding and intracellular uptake.
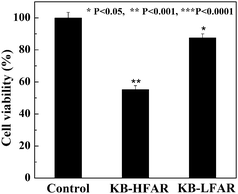 | ||
| Fig. 7 MTT assay of the viability of KB-HFAR and KB-LFAR cells after treatment with G5.NHAc-FI-FA/DOX complexes. The data are expressed as mean ± S.D. (n = 3). | ||
Conclusions
In summary, we developed a facile approach to encapsulating an antitumor agent DOX within multifunctional FI- and FA-modified G5 dendrimers (G5.NHAc-FI-FA). The formed G5.NHAc-FI-FA/DOX complexes are stable and display a sustained release profile of the drug molecules. Importantly, the formed G5.NHAc-FI-FA/DOX complexes are able to specifically target to KB cells with high-level FAR and display specific toxicity to the targeted KB cells. To further prove the efficacy of the developed multifunctional dendrimer/drug complexes, in vivo studies will be performed in our future work. With the facile approach to encapsulating hydrophobic cancer drugs with improved water solubility and drug bioavailability, we envisage that the multifunctional dendrimers may serve as a general carrier for sustained release and targeted delivery of various anticancer drugs for a range of cancer therapeutics applications.Acknowledgements
This research is financially supported by the National Natural Science Foundation of China (20974019 and 50925312), the National Basic Research Program of China (973 Program, 2007CB936000), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, and the Fundamental Research Funds for the Central Universities (For R. G. and M. S.). R. G. thanks the support from Shanghai Natural Science Foundation (10ZR1400800). X. S. gratefully acknowledges the Fundação para a Ciência e a Tecnologia (FCT) and Santander bank for the Invited Chair in Nanotechnology.References
- K. E. Uhrich, S. M. Cannizzaro, R. S. Langer and K. M. Shakesheff, Chem. Rev., 1999, 99, 3181–3198 CrossRef CAS.
- J. Panyam and V. Labhasetwar, Adv. Drug Delivery Rev., 2003, 55, 329–347 CrossRef CAS.
- H. Maeda, G. Y. Bharate and J. Daruwalla, Eur. J. Pharm. Biopharm., 2009, 71, 409–419 CrossRef CAS.
- L. Brannon-Peppas and J. O. Blanchette, Adv. Drug Delivery Rev., 2004, 56, 1649–1659 CrossRef CAS.
- C. Vauthier, C. Dubernet, C. Chauvierre, I. Brigger and P. Couvreur, J. Controlled Release, 2003, 93, 151–160 Search PubMed.
- H. F. Liang, C. T. Chen, S. C. Chen, A. R. Kulkarni, Y. L. Chiu, M. C. Chen and H. W. Sung, Biomaterials, 2006, 27, 2051–2059 CrossRef CAS.
- S. H. Medina and M. E. H. El-Sayed, Chem. Rev., 2009, 109, 3141–3157 CrossRef CAS.
- I. J. Majoros, A. Myc, T. Thomas, C. B. Mehta and J. R. Baker, Jr, Biomacromolecules, 2006, 7, 572–579 CrossRef CAS.
- I. J. Majoros, T. P. Thomas, C. B. Mehta and J. R. Baker, Jr, J. Med. Chem., 2005, 48, 5892–5899 CrossRef CAS.
- T. P. Thomas, I. J. Majoros, A. Kotlyar, J. F. Kukowska-Latallo, A. Bielinska, A. Myc and J. R. Baker, Jr, J. Med. Chem., 2005, 48, 3729–3735 CrossRef CAS.
- R. Esfand and D. A. Tomalia, Drug Discovery Today, 2001, 6, 427–436 CrossRef CAS.
- D. A. Tomalia, A. M. Naylor and W. A. Goddard III, Angew. Chem., Int. Ed. Engl., 1990, 29, 138–175 CrossRef.
- D. Peer, J. M. Karp, S. Hong, O. C. FarokHzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS.
- J. F. Kukowska-Latallo, K. A. Candido, Z. Y. Cao, S. S. Nigavekar, I. J. Majoros, T. P. Thomas, L. P. Balogh, M. K. Khan and J. R. Baker, Jr, Cancer Res., 2005, 65, 5317–5324 CrossRef CAS.
- M. T. Morgan, M. A. Carnahan, C. E. Immoos, A. A. Ribeiro, S. Finkelstein, S. J. Lee and M. W. Grinstaff, J. Am. Chem. Soc., 2003, 125, 15485–15489 CrossRef CAS.
- M. T. Morgan, Y. Nakanishi, D. J. Kroll, A. P. Griset, M. A. Carnahan, M. Wathier, N. H. Oberlies, G. Manikumar, M. C. Wani and M. W. Grinstaff, Cancer Res., 2006, 66, 11913–11921 CrossRef CAS.
- R. S. Dhanikula, A. Argaw, J. F. Bouchard and P. Hildgen, Mol. Pharmaceutics, 2008, 5, 105–116 CrossRef CAS.
- C. Kojima, K. Kono, K. Maruyama and T. Takagishi, Bioconjugate Chem., 2000, 11, 910–917 CrossRef CAS.
- X. Shi, I. Lee, X. S. Chen, M. W. Shen, S. L. Xiao, M. F. Zhu, J. R. Baker, Jr and S. H. Wang, Soft Matter, 2010, 6, 2539–2545 RSC.
- Y. Wang, R. Guo, X. Cao, M. Shen and X. Shi, Biomaterials, 2011, 32, 3322–3329 Search PubMed.
- T. Minko, E. V. Batrakova, S. Li, Y. L. Li, R. I. Pakunlu, V. Y. Alakhov and A. V. Kabanov, J. Controlled Release, 2005, 105, 269–278 Search PubMed.
- Y. J. Wang, V. Bansal, A. N. Zelikin and F. Caruso, Nano Lett., 2008, 8, 1741–1745 CrossRef CAS.
- P. S. Lai, P. J. Lou, C. L. Peng, C. L. Pai, W. N. Yen, M. Y. Huang, T. H. Young and M. J. Shieh, J. Controlled Release, 2007, 122, 39–46 CrossRef CAS.
- K. Kono, C. Kojima, N. Hayashi, E. Nishisaka, K. Kiura, S. Wataral and A. Harada, Biomaterials, 2008, 29, 1664–1675 CrossRef CAS.
- O. L. Padilla De Jesus, H. R. Ihre, L. Gagne, J. M. J. Frechet and F. C. Szoka, Jr, Bioconjugate Chem., 2002, 13, 453–461 CrossRef CAS.
- C. C. Lee, E. R. Gillies, M. E. Fox, S. J. Guillaudeu, J. M. J. Frechet, E. E. Dy and F. C. Szoka, Jr, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 16649–16654 CrossRef CAS.
- S. Zhu, M. Hong, G. Tang, L. Qian, J. Lin, Y. Jiang and Y. Pei, Biomaterials, 2010, 31, 1360–1371 CrossRef CAS.
- S. Chandra, S. Dietrich, H. Lang and D. Bahadur, J. Mater. Chem., 2011, 21, 5729–5737 RSC.
- A. Agarwal, U. Gupta, A. Asthana and N. K. Jain, Biomaterials, 2009, 30, 3588–3596 Search PubMed.
- S. K. Choi, T. Thomas, M. H. Li, A. Kotlyar, A. Desai and J. R. Baker, Jr, Chem. Commun., 2010, 46, 2632–2634 RSC.
- U. Gupta, S. K. D. Dwivedi, H. K. Bid, R. Konwar and N. K. Jain, Int. J. Pharm., 2010, 393, 185–196 Search PubMed.
- L. Han, R. Huang, S. Liu, S. Huang and C. Jiang, Mol. Pharmaceutics, 2010, 7, 2156–2165 Search PubMed.
- J. Huang, F. Gao, X. Tang, J. Yu, D. Wang, S. Liu and Y. Li, Polym. Int., 2010, 59, 1390–1396 Search PubMed.
- X. Shi, S. H. Wang, S. D. Swanson, S. Ge, Z. Y. Cao, M. E. Van Antwerp, K. J. Landmark and J. R. Baker, Jr, Adv. Mater., 2008, 20, 1671–1678 CrossRef CAS.
- S. H. Wang, X. Y. Shi, M. Van Antwerp, Z. Y. Cao, S. D. Swanson, X. D. Bi and J. R. Baker, Jr, Adv. Funct. Mater., 2007, 17, 3043–3050 CrossRef CAS.
- I. J. Majoros, B. Keszler, S. Woehler, T. Bull and J. R. Baker, Jr, Macromolecules, 2003, 36, 5526–5529 CrossRef CAS.
- X. Shi, I. Banyai, K. Rodriguez, M. T. Islam, W. Lesniak, P. Balogh, L. P. Balogh and J. R. Baker, Jr, Electrophoresis, 2006, 27, 1758–1767 CrossRef CAS.
- X. Y. Shi, S. H. Wang, H. P. Sun and J. R. Baker, Jr, Soft Matter, 2007, 3, 71–74 RSC.
- X. Shi, S. H. Wang, S. Meshinchi, M. E. Van Antwerp, X. D. Bi, I. H. Lee and J. R. Baker, Jr, Small, 2007, 3, 1245–1252 CrossRef CAS.
- D. Cakara, J. Kleimann and M. Borkovec, Macromolecules, 2003, 36, 4201–4207 CrossRef CAS.
- I. G. Campbell, T. A. Jones, W. D. Foulkes and J. Trowsdale, Cancer Res., 1991, 51, 5329–5338 CAS.
- J. F. Ross, P. K. Chaudhuri and M. Ratnam, Cancer, 1994, 73, 2432–2443 CAS.
- S. D. Weitman, R. H. Lark, L. R. Coney, D. W. Fort, V. Frasca, V. R. Zurawski and B. A. Kamen, Cancer Res., 1992, 52, 3396–3401 CAS.
| This journal is © The Royal Society of Chemistry 2011 |
