DOI:
10.1039/C1PY00161B
(Paper)
Polym. Chem., 2011,
2, 1761-1768
Construction and application of pH-triggered cleavable hyperbranched polyacylhydrazone for drug delivery†
Received
11th April 2011
, Accepted 23rd May 2011
First published on 9th June 2011
Abstract
Polymeric drug carriers with high stability during long circulation and triggered degradation after drug release are particularly interesting in drug delivery. Here, a novel pH-triggered backbone-cleavable hyperbranched polyacylhydrazone (HPAH) was successfully prepared through a simple polycondensation of 2,3-butanedione and 1-(2-aminoethyl) piperazine tri-propionylhydrazine. The experimental results showed that the degree of branching (DB) of HPAH was 0.60, and the weight-average molecular weight (Mw) of end-capped HPAH was 4.0 × 103 with a polydipersity index (PDI) of 1.6. 2D DOSY NMR degradation experiments demonstrated that HPAH was stable in neutral conditions while cleavable in acidic environments. Owing to the existence of numerous acylhydrazine terminals, the anticancer drug doxorubicin (DOX) was conjugated to hydrophilic HPAH. The obtained HPAH-DOX conjugate could self-assemble into polymeric micelles with an average diameter of 20 nm, which were stable under physiological pH but cleavable after endocytosis. Cell viability of HPAH, monomers, and degradation products was maintained above 70% over the culture periods, even when the concentration was up to 3 mg mL−1 according to methyl tetrazolium (MTT) assay in NIH/3T3 cell line. Both flow cytometry and confocal laser scanning microscopy (CLSM) confirmed the high cellular uptake of HPAH-DOX. Anti-cancer effect was evaluated in HeLa cell line, and the DOX dose required for 50% cellular growth inhibition was found to be 3.5 μg mL−1 by MTT assay.
Introduction
As a potential method of drug delivery in disease therapy, polymeric drug delivery systems (PDDSs),1–4 such as polymeric micelles5–7 and polymer-drug conjugates,8–11 have gained significant attention over the past two decades. To realize an efficient delivery of drugs, the structural optimization of polymeric drug carriers must be performed. There are two basic requirements for the design of polymeric drug carriers.12 Firstly, with the help of carriers, the drugs should be efficiently delivered into target tissues and cells, and then released in a controlled manner. Until now, the majority of researches on PDDSs are focused on such a controlled delivery/release of drugs, which has already made a great achievement. For example, various PDDSs based on different stimuli-responsive polymers13–17 and targeting strategies (active or passive targeting)18,19 have been developed. Secondly, once the drugs are released, the carriers must be eliminated from the body to minimize the side effects. It has been well reported that the renal clearance is the main metabolic way for polymer degradation products. Considering that the maximum size for renal clearance is about 6 nm, it is necessary for polymers to degrade into low-molecular-weight oligomers or monomers after completion of a controlled drug release. According to these two requirements, the polymer carriers should remain stable during the transportation of drug in the body, but they must degrade quickly after the drug is released at the target sites. Therefore, design and preparation of polymer carriers with high stability and stimuli-responsive degradation has become very attractive.
As a newly emerging smart material, dynamic polymers (dynamers) connected by reversible covalent bonds20–23 show great potential in biomedical applications.24–26 Among the known reversible covalent bonds, acylhydrazone bonds are stable under physiological conditions but become labile with the trigger of acid. Ascribed to the perfect combination of high stability and pH-responsive degradation, dynamers with acylhydrazone connections provide a promising carrier for drug delivery. Here, we introduced the acylhydrazone bonds into the highly branched polymer to prepare a novel pH-triggered backbone-cleavable hyperbranched polyacylhydrazone (HPAH) by polycondensation of diketone and trihydrazine. Numerous acylhydrazine terminals of HPAH provided many active sites for binding hydrophobic drug doxorubicin (DOX) through acylhydrazone linkages. Because of the amphiphilicity, the obtained HPAH-DOX conjugate self-assembled into polymeric micelles in an aqueous solution. Importantly, the HPAH-DOX micelles remained stable under physiological pH value (pH = 7.4), while readily biodegraded into small molecules after cellular uptake by endocytosis (pH = 5–6).27,28 Cleavage of polymer backbone and intracellular pH-triggered release of DOX happened simultaneously. Therefore, HPAH can be used to construct promising PDDSs.
Experimental section
Materials
2,3-Butanedione (99%, BD) and methyl acrylate (MA) were purchased from Acros Organics. 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), HOECHST 33342 and 1-(2-aminoethyl) piperazine (AEP, 99%) were from Sigma-Aldrich. Doxorubicin hydrochloride (DOX·HCl) was purchased from Beijing Huafeng United Technology Corp. Methanol, ethanol, N,N′-dimethylformamide (DMF), disodium hydrogen phosphate, sodium dihydrogen phosphate, acetic acid, sodium acetate, and hydrazine hydrate (85%) were supplied by Sinopharm Chemical Reagent Co. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and phosphate buffered solution (PBS) were purchased from PAA Laboratories GmbH. MA, ethanol, DMF and hydrazine hydrate were purified according to standard procedure. Dialysis tube (MWCO, 1 kDa) was from Shanghai Lvniao Technology Corp. All the other chemicals were used as received without any further purification. Distilled water was used in all experiments. Clear polystyrene tissue culture treated 6-well and 96-well plates were obtained from Corning Costar.
Synthesis of HPAH and HPAH-DOX
HPAH was synthesized by a simple polycondensation of BD and 1-(2-aminoethyl) piperazine tri-propionylhydrazine (AEP-NHNH2), as shown in Scheme 1. After that, HPAH-DOX was obtained by conjugation of DOX onto the surface of HPAH through further condensation of ketone and acylhydrazine groups. The synthesis details of HPAH and HPAH-DOX are described in the ESI.†
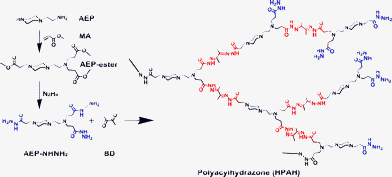 |
| | Scheme 1 Synthesis route of HPAH. | |
Nuclear magnetic resonance (NMR) spectroscopy
1H and 13C NMR spectra of intermediate products, HPAH and HPAH-DOX were carried out on Varian Mercury plus 400 NMR spectrometer (400 MHz, 298 K) with dimethyl sulfoxide-d6 (DMSO-d6) or deuterated chloroform (CDCl3) as solvents. Quantitative 13C NMR spectra were measured by the method of inverse gated 1H decoupling.
FTIR spectra were performed on a Bruker Equinox-55 FTIR spectrometer. All sample pellets were prepared by grinding the solid sample with solid potassium bromide (KBr) and applying great pressure to the dry mixture.
The molecular weights of the synthesized samples were evaluated by SEC-MALLS technique. The SEC-MALLS system consisted of a Waters 2690D Alliance liquid chromatography system, a Wyatt Optilab DSP differential refractometer detector, and a Wyatt MALLS detector. Two PL mix-D columns (Styragel HR3, HR4) were used in series with 80 °C external temperature (column temperature) and 50 °C internal temperature (RI temperature). DMF containing 0.5 M LiBr was used as eluent at a flow rate of 1 mL min−1. The data were processed with Astra software (Wyatt Technology).
Dynamic light scattering (DLS)
The average diameter and size distribution of the micelles were determined using a Zetasizer Nano-ZS90 (Malvern Instrument Ltd.) at 25 °C. The scattering angle was kept at 90° and the wavelength was set as 633 nm during the whole experiment. Intensity-average and number-average hydrodynamic diameters were adopted and all data were averaged over three measurements. All the samples were stabilized for several hours and then filtered with a 450 nm filter before the measurements.
Degradation experiments of HPAH were observed by in situ 2D DOSY NMR in NMR tube. HPAH (20 mg) was dissolved in deuterated phosphate buffered saline (PBS) solution (pH = 5.0 and pH = 7.4), respectively. Then, the results were recorded at predetermined time intervals. The 1H NMR DOSY spectra were collected on a Bruker AVANCE III-400M spectrometer using the standard ledbpgp2s sequence. Gradient strength in the z-direction was about 50 G cm−1. The gradients (G) were increased from 1 to 47.5 G cm−1 in 16 steps, and the diffusion time was set at 40 ms. Data were processed with topspin 2.0. All spectra were measured at 298 K with 8 accumulations.
Morphology and size of micelles were observed on a JEOL 2010 microscope at an accelerating voltage of 200 kV. A drop of aqueous HPAH-DOX solution (1 mg mL−1) was spread onto an amorphous holey-carbon film supported by a copper grid (XinXingBaiRui, Beijing, China), then lyophilized by a freeze-dryer (Christ Alpha 1–4 LD plus, Germany) for observation. To determine the particle size, over 200 particles were counted in multiple pictures from different areas of the TEM grid.
Cell cultures
NIH/3T3 cells (a mouse embryonic fibroblast cell line) and HeLa cells (a human uterine cervix carcinoma cell line) were cultured in DMEM supplied with 10% FBS, and antibiotics (50 units mL−1penicillin and 50 units mL−1streptomycin) at 37 °C in a humidified atmosphere containing 5% CO2.
Degradation experiments
Degradation of HPAH was carried out in PBS solution (pH = 7.4) at 37 °C, a mimic of physiological conditions. The concentration of HPAH was set at 15 mg mL−1. At the predetermined intervals (5 d, 13 d, 19 d, 26 d), 1.5 mL PBS solution of degradation product was taken out, then kept frozen, and finally tested by MTT assay.
In vitro cytotoxicity assay
The cytotoxicity of HPAH, the degradation products of HPAH, and monomer BD and AEP-NHNH2 against cultured NIH/3T3 cells was evaluated in vitro by MTT assay. NIH/3T3 cells were seeded into 96-well plates at an initial seeding density of 8.0 × 103cells/well in 200 μL medium. After 24 h incubation, the culture medium was removed and replaced with 200 μL medium containing serial dilutions of samples. The cells were grown for another 24 h. Then, 20 μL of 5 mg mL−1 MTT solution in PBS was added to each well. After incubating the cells for 4 h, the medium containing unreacted dye was removed carefully. The obtained purple formazan crystals were dissolved in 200 μL per well DMSO and the absorbance was measured in a BioTek Elx800 at a wavelength of 490 nm.
Drug release experiments
The release studies were performed at 37 °C with both mimetic physiological and lysosome conditions: pH = 7.4 PBS solution and pH = 5.0 acetate buffer medium. Firstly, HPAH-DOX (40 mg) was dissolved in medium (5 mL) and placed in a dialysis bag with a molecular weight cut-off of 1 kDa. The dialysis bag was then immersed in 195 mL of the release medium and stirred at a constant temperature. Samples (2 mL) were periodically sucked up and filled the same volume of fresh medium. The amount of released DOX was analyzed with UV/Vis spectroscopy at 485 nm.
Cellular uptake studies
Flow cytometry
.
Flow cytometry was used to provide statistics on the uptake of HPAH-DOX into HeLa cells. HeLa cells (5.0 × 105cells per well) were seeded in six-well culture plates and grown overnight. Then, the DOX-conjugated polymer dissolved in DMEM culture medium with a polymer concentration of 0.327 mg mL−1 and the free DOX with concentration of 0.02 mg mL−1 were added to different wells, and the cells were incubated at 37 °C for 5 and 60 min. After the incubation, samples were prepared for flow cytometry analysis by removing the cell growth media, rinsing with cold PBS, and treating with trypsin. Data for 1.0 × 104 gated events were collected and analysis was performed by means of a BD FACS Calibur flow cytometer and CELLQuest software.
Confocal laser scanning microscopy (CLSM).
For the CLSM studies, HeLa cells (2.0 × 105) were seeded on cell culture coverslips in a 12-well tissue culture plate. After 24 h culture, the DOX-conjugated polymer dissolved in DMEM culture medium with a polymer concentration of 0.327 mg mL−1 and the free DOX with concentration of 0.02 mg mL−1 were added to different wells, and the cells were incubated at 37 °C for predetermined time intervals. Then, the cells were washed with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature, and the slides were rinsed with cold PBS for three times. Finally, the cells were stained with HOECHST 33342 for 5 min and the slides were mounted and observed with a LSM510 META.
In vitro anticancer experiments
The HeLa cells with a density of 8.0 × 103cells/well in 200 μL medium were cultured for 24 h after seeding into a 96-well plate. The culture medium was removed and replaced with 200 μL fresh medium containing serial dilutions of the polymer and DOX-conjugated polymer, respectively. The cells were grown for another 48 h. Then, the wells were quickly washed three times with PBS. Thereafter, 20 μL of 5 mg mL−1 MTT assays stock solution in PBS was added to each well, and incubated again for another 4 h. After discarding the culture medium, the obtained purple formazan crystals were dissolved in 200 μL per well DMSO and the absorbance was measured in a BioTek Elx800 at a wavelength of 490 nm.
Results and discussion
As one newly emerging smart material, dynamic polymers (dynamers) combine high stability and stimuli-responsive properties. Here, we introduced the labile acylhydrazone bonds into the backbone of highly branched polymer to prepare a novel backbone pH-sensitive hyperbranched polyacylhydrazone (HPAH) through A2 + B3polycondensation of diketone and trihydrazine. The synthesis route is given in Scheme 1.
Briefly, the biodegradable HPAH with pH-sensitivity was synthesized from BD (A2 monomer) and AEP-NHNH2 (B3 monomer) by one-pot polymerization with a feed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Through polycondensation of acylhydrazine and ketone groups, HPAH with plentiful acylhydrazine terminals was obtained. By adjusting the feeding ratio and reaction time, the gelation could be avoided. Both FTIR and 1H NMR analyses (Fig. S1 and Fig. S2†) confirm the formation of HPAH. The quantitative 13C NMR data (Fig. S3 and Fig. S4†) show that the degree of branching (DB) of HPAH is 0.60. SEC-MALLS measurement suggests that the weight-average molecular weight (Mw) of HPAH end-capped with benzaldehyde is around 4.0 × 103, with a polydispersity index (PDI) of 1.6 and dn/dc value of 0.07. The details of polymer characterization are given in the ESI.†
1. Through polycondensation of acylhydrazine and ketone groups, HPAH with plentiful acylhydrazine terminals was obtained. By adjusting the feeding ratio and reaction time, the gelation could be avoided. Both FTIR and 1H NMR analyses (Fig. S1 and Fig. S2†) confirm the formation of HPAH. The quantitative 13C NMR data (Fig. S3 and Fig. S4†) show that the degree of branching (DB) of HPAH is 0.60. SEC-MALLS measurement suggests that the weight-average molecular weight (Mw) of HPAH end-capped with benzaldehyde is around 4.0 × 103, with a polydispersity index (PDI) of 1.6 and dn/dc value of 0.07. The details of polymer characterization are given in the ESI.†
Degradation of HPAH by 2D DOSY NMR studies
The acylhydrazone linkages in HPAH endow this polymer with pH-sensitive properties. Acylhydrazone bonds are stable under neutral and basic conditions. However, with the trigger of acid (pH < 5.5), the hydrolysis of acylhydrazone bonds leads to the degradation of HPAH. With the help of the 2D DOSY NMR technique,29 the degradation behavior of HPAH was studied. Fig. 1 gives the diffusion coefficients (D) of HPAH in different buffer solutions at various time intervals. As shown in Fig. 1A, all methylenes at 2.0–2.1 ppm exhibit similar diffusion coefficients around 6.31 × 10−11 m2s−1. After HPAH is kept in pH = 7.4 buffer solution for 144 h, the D value only changes to 7.08 × 10−11 m2s−1, suggesting only a slight degradation of HPAH. In contrast, in pH = 5.0 buffer solution, the diffusion coefficients of HPAH increase from 5.01 × 10−11 to 1.48 × 10−10 m2s−1, as displayed in Fig. 1B. The enhancement of the D value reflects the degradation of HPAH because of the cleavage of acylhydrazone connections.
Cytotoxicity of HPAH, degradation products of HPAH, monomer AEP-NHNH2 and BD
Owing to the existence of nitrogen atoms in AEP units and acylhydrazone linkages, HPAH can be regarded as a polycation. Generally speaking, polycations with high nitrogen content are highly toxic. However, according to MTT assay in NIH/3T3 cells, in vitro cytotoxicity of monomer BD, AEP-NHNH2, and HPAH is very low. Fig. 2 shows that cell viability after 24 h incubation with HPAH, AEP-NHNH2 and BD up to 3 mg mL−1 remains still above 80%, 90% and 70% compared to untreated cells. This suggests the low cytotoxicity of HPAH and the reactant monomers. After the endocytosis of HPAH, pH-sensitive acylhydrazone linkages in polymer backbone are readily cleaved under acidic conditions. The cytotoxicity of degradation products of HPAH was also evaluated by MTT assay. Both Fig. 2 and Fig. S6† give the in vitro cytotoxicity of HPAH samples at different degradation stages. It can be found that after 27 days degradation, the cell viability is still higher than 80%, indicating the low cytotoxicity of degradation products. The MTT results show that all HPAH, monomer AEP-NHNH2 and BD, and degradation products of HPAH display low cytotoxicity to NIH/3T3 cells.
 |
| | Fig. 2 Cytotoxicity of HPAH, degradation products of HPAH (27 d), monomer AEP-NHNH2 and BD in NIH/3T3 cell line by MTT assay. | |
Preparation and micellization of HPAH-DOX
The pH-sensitive biodegradability and low cytotoxicity make HPAH a promising biomaterial for biomedical applications. Benefiting from the good hydrophilicity and many functional acylhydrazine terminals, HPAH can be used as an efficient carrier for hydrophobic drugs. Among the well-known anticancer drugs, DOX has a ketone group, which facilitates the conjugation with acylhydrazine terminals of HPAH to form pH-sensitive acylhydrazone bonds. As shown in Fig. S2D,† the proton signals of benzene ring at 8.03, 7.93 and 7.65 ppm of DOX and 11.18–10.08 of acylhydrazone in HPAH illustrate the successful conjugation of DOX and HPAH. No trace of free DOX level appeared in the thin layer chromatography analysis of HPAH-DOX (Fig. S7†), suggesting the formation of HPAH-DOX conjugate.
HPAH-DOX has a hydrophilic HPAH core and pendant hydrophobic DOX molecules. In an aqueous solution, the amphiphilicity of HPAH-DOX makes the conjugate spontaneously self-assemble into micelles. The TEM image in Fig. 3A shows the formation of spherical micelles with a uniform size. The mean diameter of these micelles is about 19.5 nm with a Gaussian size distribution from 12 to 36 nm. As shown in Fig. 3B, the DLS size distribution curve of the micelles (1 mg mL−1 in aqueous solution) displays a monomodal size distribution with a hydrodynamic diameter of 20 nm with PDI of 0.35.
 |
| | Fig. 3 (A) Representative TEM image of HPAH-DOX micelles and (B) DLS size distributions of HPAH-DOX micelles. | |
When amphiphilic HPAH-DOX conjugate self-assembles into micelles in aqueous solution, the formed micelles can permeate cytomembrane to endosome/lysosome through cell endocytosis (Scheme 2). The low pH of the endosome/lysosome (pH = 5–6) imparts a structural change of HPAH-DOX. Under the acidic environment, the acylhydrazone bonds between DOX and HPAH can be cleaved and the same acylhydrazone bonds in the backbone of HPAH will be degraded. Accordingly, both DOX and degradation products diffuse into the cytoplasm.
 |
| | Scheme 2
Self-assembly of HPAH-DOX conjugate into cleavable shell/core micelles and subsequent drug delivery for efficient pH-triggered intracellular release of DOX based on dynamic acylhydrazone bonds. | |
 |
| | Fig. 4 Cumulative release curve of DOX from HPAH-DOX under different pH values at 37 °C: (A) pH = 7.4 PBS buffer solution and (B) pH = 5.0 acetate buffer medium. | |
Cellular internalization studies
Flow cytometry was performed to evaluate and compare cellular uptake of HPAH-DOX and free DOX by HeLa cell line. HeLa cells were cultured for predetermined time intervals with DOX concentration of 20 μg mL−1. In this measurement, DOX itself was used as a fluorescence probe.30,31 Histograms of cell-associated DOX fluorescence of HeLa cells are shown in Fig. 5. Cells with free DOX treatment were served as a negative control. In Fig. 5A, after 5 min of incubation, mean fluorescence intensities of DOX-pretreated cells are about 2-fold of non-pretreated cells, while relative geometrical mean fluorescence intensities of cells pretreated by HPAH-DOX are about 60-fold. Fig. 5 shows that the longer the pretreated time of free DOX is, the higher the mean fluorescence intensity will be. It suggests that the fluorescence intensity of free DOX increases with time, due to a passive diffusion mechanism of free DOX transported into cells. On the contrary, the fluorescence intensity of HPAH-DOX has no such a time dependency. Compared with the control, the mean fluorescence intensities of free DOX and HPAH-DOX in the M2 region increase from 1.92% to 9.07% and 98.93% in 5 min (Fig. S8 and Fig. S9†), respectively. Enhancement of fluorescence signals indicates the fast cellular uptake of HPAH-DOX in HeLa cells, which can be easily explained by the cationic characteristics of HPAH carrier.
 |
| | Fig. 5
Flow cytometry histogram profiles of HeLa cells incubated without and with free DOX and HPAH-DOX (A) 5 min; (B) 1 h; and (C) 3 h at 37 °C (DOX concentration: 20 μg mL−1). | |
The cellular uptake of free DOX and HPAH-DOX by HeLa cells was further evaluated by CLSM. HeLa cells were incubated with free DOX and HPAH-DOX at 37 °C for 5 min and 1 h respectively, and then fixed with paraformaldehyde. After that, the treated samples were observed directly with CLSM in the fluorescent mode. As seen in Fig.6A1 and Fig.6B1, cells exposed to free DOX show no signal of DOX fluorescence in both cytoplasm and nucleus after 5 min, while cells exposed to HPAH-DOX have high fluorescence intensity in the cytoplasm but no signal in the nucleus. The high DOX fluorescence excitation in the cytoplasm of CLSM image B1 should be related to the positive charges of HPAH which helps the cell penetration. Because DOX molecules are loosely packed in shell/core micelles, the fluorescence quenching can be avoided.32 After 1 h incubation, free DOX enters the nucleus while the HPAH-DOX still remains in the cytoplasm (Fig.6A2 and Fig.6B2), which is similar to the previous reports.32 Because of rapid transportation of the DOX molecules from the cytosol to the nucleus, free DOX accumulates in the nucleus. After 5 min and 1 h incubation, HPAH-DOX appears in the perinuclear region instead of nucleus. These experimental results indicate that the cellular uptake of HPAH-DOX is based on an endocytosis mechanism, not the simple passive diffusion of small molecules between the extracellular and intracellular milieu. Furthermore, the DOX departs from HPAH-DOX micelles through a pH-triggered sustained release rather than a burst release.
 |
| | Fig. 6
CLSM images of HeLa cells incubated with (A1) free DOX, 5 min; (B1) HPAH-DOX, 5 min; and (A2) free DOX, 1 h; (B2) HPAH-DOX, 1 h at 37 °C (DOX concentration: 20 μg mL−1) (Red: DOX, Blue: HOECHST 33342). | |
Anticancer effect assay
The ability of HPAH-DOX to inhibit the proliferation of HeLa cells was evaluated by MTT assay and free DOX was used as a control. The HeLa cells were treated with HPAH-DOX and free drug at different DOX dose from 1 to 20 μg mL−1 for 48 h. Fig. 7 shows that the dose of the conjugated DOX required for 50% cellular growth inhibition (IC50) is 3.5 μg mL−1. This demonstrates that HPAH-DOX can efficiently enter the cell and produce the desired pharmacological activities. As a comparison, the IC50 of the free DOX is 1 μg mL−1, indicating a better anticancer effect of free DOX than conjugated DOX. It has been well documented that the free drug often shows higher activity than that of polymer-drug conjugate in cells.33 Just as for all anthracyclines, DOX is known to interact with DNA by intercalation and inhibition of macromolecular biosynthesis.34,35 Here, fast passive diffusion of free DOX into the cell nuclei could explain this observation.
 |
| | Fig. 7
Cell viability of HeLa cells against HPAH-DOX and free DOX after culturing for 48 h. | |
It is well known that endosomes/lysosomes contain a series of acidic hydrolytic enzymes. Once HPAH-DOX conjugate enters the cell, it would be encapsulated by endosomes/lysosomes in an acidic environment (pH = 5–6), which provides an opportunity for DOX controlled release from the conjugate via the cleavage of pH-sensitive acylhydrazone linkages to reach therapeutic effect.
Conclusions
A novel backbone-degradable branched dynamer, hyperbranched polyacylhydrazone (HPAH), has been successfully prepared by the polycondensation of 2,3-butanedione and 1-(2-aminoethyl) piperazine tri-propionylhydrazine. HPAH shows good water-solubility and low cytotoxicity, making it as an excellent carrier for drug delivery. Benefiting from numerous acylhydrazine terminals of HPAH, anticancer drug DOX can be readily conjugated onto HPAHviaacylhydrazone linkages. The obtained amphiphilic HPAH-DOX conjugate self-assembles into uniform micelles with an average diameter of 20 nm in water. Since the acylhydrazone bonds are stable in physiological conditions (pH = 7.4), the HPAH-DOX micelles with nanosize can avoid the removal of the reticuloendothelial system (RES), kidneys, and intestines. Importantly, after the endocytosis of HPAH-DOX micelles, the acidic lysosomes (pH = 5–6) trigger the cleavage of acylhydrazone bonds, resulting in the release of DOX drugs. In the meantime, the HPAH carrier is degraded into oligomers or monomers, which can be readily removed by the kidney and other organs. In short, HPAH carrier combines the high stability during long circulation with the pH-responsive degradation after drug release. Thus, HPAH can serve as a kind of safe and efficient drug delivery carrier in the future.
Acknowledgements
This work is sponsored by the National Natural Science Foundation of China (20974062, 30700175) and National Basic Research Program 2009CB930400, the Interdisciplinary Foundation of Medical and Engineering (or Science) of Shanghai Jiao Tong University (YG2009MS48), Shuguang Program (08SG14), Shanghai Leading Academic Discipline Project (Project Number: B202), and China National Funds for Distinguished Young Scientists (21025417).
References
- J. S. Román, A. Gallardo and B. Levenfeld, Adv. Mater., 1995, 7, 203–208 Search PubMed.
- K. E. Uhrich, S. M. Cannizzaro, R. S. Langer and K. M. Shakesheff, Chem. Rev., 1999, 99, 3181–3198 CrossRef CAS.
- R. Duncan, Nat. Rev. Drug Discovery, 2003, 2, 347–360 CrossRef CAS.
- K. Katrin, H. Richard, F. Dagmar and S. S. Ulrich, Angew. Chem., Int. Ed., 2010, 49, 6288–6308 CrossRef CAS.
- J. Akimoto, M. Nakayama, K. Sakai and T. Okano, Biomacromolecules, 2009, 10, 1331–1336 CrossRef CAS.
- A. N. Lukyanov and V. P. Torchilin, Adv. Drug Delivery Rev., 2004, 56, 1273–1289 CrossRef CAS.
- Y. Chan, T. Wong, F. Byrne, M. Kavallaris and V. Bulmus, Biomacromolecules, 2008, 9, 1826–1836 CrossRef CAS.
- I. J. Majoros, A. Myc, T. Thomas, C. B. Mehta and J. R. Baker, Biomacromolecules, 2006, 7, 572–579 CrossRef CAS.
- M. J. Vicent and R. Duncan, Trends Biotechnol., 2006, 24, 39–47 CrossRef CAS.
- M. Prabaharan, J. J. Grailer, S. Pilla, D. A. Steeber and S. Gong, Biomaterials, 2009, 30, 57–66 Search PubMed.
- Y. L. Li, L. Zhu, Z. Z. Liu, R. Cheng, F. H. Meng, J. H. Cui, S. J. Ji and Z. Y. Zhong, Angew. Chem., Int. Ed., 2009, 48, 9914–9918 CrossRef CAS.
- M. E. Fox, F. C. Szoka and J. M. J. Fréchet, Acc. Chem. Res., 2009, 42, 1141–1151 CrossRef CAS.
- A. J. Convertine, C. Diab, M. Prieve, A. Paschal, A. S. Hoffman, P. H. Johnson and P. S. Stayton, Biomacromolecules, 2010, 11, 2904–2911 CrossRef CAS.
- J. Kost and R. Langer, Adv. Drug Delivery Rev., 2001, 46, 125–148 CrossRef CAS.
- K. Ulbrich and V. Subr, Adv. Drug Delivery Rev., 2004, 56, 1023–1050 CrossRef CAS.
- D. Schmaljohann, Adv. Drug Delivery Rev., 2006, 58, 1655–1670 CrossRef CAS.
- R. Haag, Angew. Chem., Int. Ed., 2004, 43, 278–282 CrossRef CAS.
- R. I. Pinhassi, Y. G. Assaraf, S. Farber, M. Stark, D. Ickowicz, S. Drori, A. J. Domb and Y. D. Livney, Biomacromolecules, 2009, 11, 294–303 Search PubMed.
- A. Chilkoti, M. R. Drehera, D. E. Meyera and D. Raucherb, Adv. Drug Delivery Rev., 2002, 54, 613–630 CrossRef CAS.
- J.-M. Lehn and A. V. Eliseev, Science, 2001, 291, 2331–2332 CrossRef CAS.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 899–952 CAS.
- J.-M. Lehn, Prog. Polym. Sci., 2005, 30, 814–831 CrossRef CAS.
- C. D. Meyer, C. S. Joiner and J. F. Stoddart, Chem. Soc. Rev., 2007, 36, 1705–1723 RSC.
- A. Aissaoui, B. Martin, E. Kan, N. Oudrhiri, M. Hauchecorne, J. P. Vigneron, J.-M. Lehn and P. Lehn, J. Med. Chem., 2004, 47, 5210–5223 CrossRef CAS.
- M. Hrubý, Č. Koňák and K. Ulbrich, J. Controlled Release, 2005, 103, 137–148 CrossRef CAS.
- V. T. Bhat, A. M. Caniard, T. Luksch, R. Brenk, D. J. Campopiano and M. F. Greaney, Nat. Chem., 2010, 2, 490–497 CrossRef CAS.
- S. A. Brockman and R. F. Murphy, Pharm. Biotech., 1993, 4, 51–70 Search PubMed.
- L. J. Zhu, Y. F. Shi, C. L. Tu, R. B. Wang, Y. Pang, F. Qiu, X. Y. Zhu, D. Y. Yan, L. He, C. Y. Jin and B. S. Zhu, Langmuir, 2010, 26, 8875–8881 CrossRef CAS.
- C. S. Johnson Jr, Prog. Nucl. Magn. Reson. Spectrosc., 1999, 34, 203–256 CrossRef.
- Y. Bae, S. Fukushima, A. Harada and K. Kataoka, Angew. Chem., Int. Ed., 2003, 42, 4640–4643 CrossRef CAS.
- M. Wartenberg, J. Hescheler, H. Acker, H. Diedershagen and H. Sauer, Cytometry, 1998, 31, 137–145 Search PubMed.
- G. Xiong, Y. Chen and E. A. Arriaga, Anal. Chem., 2005, 77, 3488–3493 CrossRef CAS.
- S. Ibsen, E. Zahavy, W. Wrasdilo, M. Berns, M. Chan and S. Esener, Pharm. Res., 2010, 27, 1848–1860 Search PubMed.
- A. Akinc, D. G. Anderson, D. M. Lynn and R. Langer, Bioconjugate Chem., 2003, 14, 979–988 CrossRef CAS.
- D. A. Gewirtz, Biochem. Pharmacol., 1999, 57, 727–741 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Sample preparation and FTIR and 1H NMR characterization. Chemical structure, quantitative 13C NMR spectra are given to calculate branched degree of HPAH. Cell cytotoxicity of HPAH after degradation. Thin layer chromatography (TLC) of HPAH-DOX and HPAH. Flow cytometry histogram profiles of HeLa cells incubated with free DOX and HPAH-DOX. See DOI: 10.1039/c1py00161b |
| ‡ These authors are joint first authors. |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Through polycondensation of acylhydrazine and ketone groups, HPAH with plentiful acylhydrazine terminals was obtained. By adjusting the feeding ratio and reaction time, the gelation could be avoided. Both FTIR and 1H NMR analyses (Fig. S1 and Fig. S2†) confirm the formation of HPAH. The quantitative 13C NMR data (Fig. S3 and Fig. S4†) show that the degree of branching (DB) of HPAH is 0.60. SEC-MALLS measurement suggests that the weight-average molecular weight (Mw) of HPAH end-capped with benzaldehyde is around 4.0 × 103, with a polydispersity index (PDI) of 1.6 and dn/dc value of 0.07. The details of polymer characterization are given in the ESI.†
1. Through polycondensation of acylhydrazine and ketone groups, HPAH with plentiful acylhydrazine terminals was obtained. By adjusting the feeding ratio and reaction time, the gelation could be avoided. Both FTIR and 1H NMR analyses (Fig. S1 and Fig. S2†) confirm the formation of HPAH. The quantitative 13C NMR data (Fig. S3 and Fig. S4†) show that the degree of branching (DB) of HPAH is 0.60. SEC-MALLS measurement suggests that the weight-average molecular weight (Mw) of HPAH end-capped with benzaldehyde is around 4.0 × 103, with a polydispersity index (PDI) of 1.6 and dn/dc value of 0.07. The details of polymer characterization are given in the ESI.†







