2,6-Substituted pyridine derivative-containing conjugated polymers: synthesis, photoluminescence and ion-sensing properties†
Bin
Liu
,
Huiguang
Dai
,
Yinyin
Bao
,
Fanfan
Du
,
Jiao
Tian
and
Ruke
Bai
*
CAS Key Laboratory of Soft Matter Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, P. R. China 230026. E-mail: bairk@ustc.edu.cn; Fax: +0086-551-3631760; Tel: +0086-551-3600722
First published on 18th May 2011
Abstract
This paper describes a strategy to design and synthesize novel 2,6-substituted pyridine derivative-containing conjugated polymers for metal ion sensing. Two polymers P-1 and P-2 were prepared and the ion sensing properties of the monomers and the polymers were examined by UV-Vis absorption and fluorescence spectra respectively. It was found that polymer sensor P-1 exhibited a high selectivity for palladium ions among various transitional metals. The main absorbance peak was enhanced and a new peak appeared with the addition of palladium ions, meanwhile a linear calibration curve was observed. Moreover, the fluorescence intensity of emission maximum also decreased dramatically with a detection limit of 1 × 10−6 M in aqueous solution. These results indicate the polymer P-1 can be used as a novel sensor for Pd2+ detection with high sensitivity and selectivity. The excellent performance of the polymer for Pd2+ detection can be attributed to the structure of the modular-based conjugated polymer with the meta-substituted pyridine, which provides a proper spatial matching for selective binding of metal ions and this is very important for designing polymer sensors for metal ions.
Introduction
Although chemical sensors have been widely utilized as popular tools for chemical, biological, and medical detection,1 the design and synthesis of new sensing materials with high sensitivity and selectivity is still a critical challenge. Recently, fluorescent conjugated polymers (CPs) as sensing probes have received considerable attention because of their sensing ability in response to interaction with analytes such as metal ions, toxic chemicals, and biomolecules.2 Swager and coworkers have demonstrated that a delocalizable π-electronic conjugated “molecular wire” polymer can greatly amplify the fluorescence response signal because of facile energy migration along the polymer backbone upon light excitation. Therefore, increasing the number of possible exciton migration pathways has been considered as an efficient way to enhance the amplified signals of the sensor.2c Besides the amplified sensitivity, comparing with small molecule chemosensors, the CPs also display more advantages such as excellent film-forming properties and systematical modification with different functional groups.To develop new sensors for detection of metal ions is one of the current research interests in chemosensors because of the importance in revealing a number of biological processes, disease states, and environmental pollutions.2,3 Palladium is widely used in various materials such as dental crowns, catalysts, jewellery, and fuel cells.4 Especially in chemistry, Pd-catalyzed reactions represent powerful transformations for the synthesis of complex molecules, such as refecoxib and eniluracil.5 The high level of palladium ions together with the resultant compounds was emitted from these reactions. Owing to the ability of Pd2+ ions to form complexes, some biomacromolecules such as proteins, DNA, and RNA seem to be most sensitive targets, which cause major cellular functions to be inhibited, as seen in vivo and in vitro.6 Moreover, palladium is capable of eliciting a series of cytotoxic effects which may cause severe primary skin and eye irritations. In spite of the fact that the metallic form is not cytotoxic, Pd ions, especially PdCl2, are among the most frequent reacting sensitizers within metals. As a result, the proposed maximum dietary intake of palladium is <1.5–15 μg day−1 per person, and its threshold in drugs is 5–10 ppm.7 Conventional methods for palladium detection include atomic absorption spectrometry, plasma emission spectroscopy, solid phase microextraction-high performance liquid chromatography, and X-ray fluorescence. For nonliving samples, inductively coupled plasma mass spectrometry (ICP-MS) continues to be a widely used method because of its superior sensitivity and robustness.8 Although fast measurement and high sensitivity, ICP-MS analyses suffer from the high cost of the instrument, isotope effects, and spectral and nonspectral interferences due to matrix effects.9 Colormetric and fluorimetric methods for Pd2+ detection would be more desirable because the measurement becomes relatively easier and cheaper.10
Up to now, most of the sensors for palladium ions detection are based on two major mechanisms: chemical reaction between Pd2+ and detector, or the complexation of Pd2+ with ligands. Koide group developed a fluorescence method to detect palladium on the basis of the Tsuji–Trost reaction which can be used to measure palladium in various forms.10h Peng’s group investigated a series of fluorescent sensors used to detect Pd2+ through fluorescence quenching.10o However, most of the sensors reported in the literature, such as N-9-anthrylmethyl-N-methyl-N′-benzoyl thiourea,10a bis(naphthalenemethyleneoxy)tetrathia-16-crown-4,10b bathop henanthroline,10c and meso-tetrakis(4-(carboxymethyleneoxy) phenyl)porphyrin,10d exhibit poor selectivities for Pd2+ among other transition metallic cations. There are only three ligands, including 1,2-dithioethene derivatives,10g thiophenemethyl-amino derivatives,10i and diallylamino derivatives,10m proved to be highly selective sensors for Pd2+ ions. As a result, the investigation on design and preparation of new sensors for highly sensitive and selective detection of Pd2+ has attracted more and more attention.
In this paper, encouraged by our previous study in which the fluorescence polymer P-1 exhibited significant sensitivity and selectivity for palladium ions (see Fig. 1),10p we synthesized and examined the light-emitting and ion-sensing properties of two fluorescent conjugated polymers P-1 and P-2 containing 2,6-substituted pyridine derivative. The effects of two building blocks, 2,6-dithienyl-4-phenylpyridine (TPP) and 2,6-diphenyl-4-phenylpyridine (PPP), as binding sites for the conjugated polymers, were investigated for palladium ion-sensing properties. We found that the conjugated polymer (P-2) lost its response to palladium ions, when the thiophene groups were replaced by benzene groups. These results indicated that thiophene moieties played a very important role for binding palladium ions. The fluorescence quenching is ascribed to the intrachain linkage between Pd2+ and TPP units.
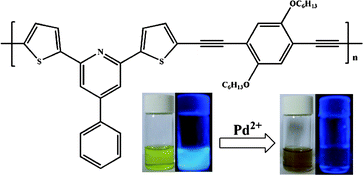 | ||
| Fig. 1 Visible emission of P-1 observed in the presence of Pd2+ without excitation and with UV-lamp excitation. | ||
Experimental
Materials and measurement
1H NMR and 13C NMR spectra were obtained using a Bruker AVANCE II and elemental analyses were measured by VARIO ELIII. UV-Vis spectra were recorded with a general UV-Vis TU-1901 spectrometer and fluorescence spectra were measured by RF-5301PC spectrometer. Molecular weight was determined by GPC on a Waters systems equipped with a Waters 1515 pump, a Water 2414 differential refractive index detector, and three styragel columns. THF was used as solvent and relative to polystyrene standards. All solvents and reagents were commercially available A.R. grade.Thermal analyses
Thermogravimetric analysis (TGA) studies were carried out on a Hi-Res TGA 2950 thermogravimetric analyser (TA Instruments) with a 10 °C min−1 ramp to 700 °C under nitrogen atmosphere.Ion sensing
A titration experiment was started with 5.0 mL polymer in THF solution with a known concentration (4.0 × 10−6mol L−1). Analyte solutions were prepared from AgNO3, Co(NO3)2·6H2O, CdCl2·1.5H2O, NiCl2·6H2O, CuAc2·H2O, ZnCl2, HgCl2, FeCl3·6H2O, and CaCl2·2H2O (4.0 × 10−3 mol L−1) by dissolving in DMF respectively. The solution of Pd2+ and Pt4+ in the form of Na2PdCl4 and Na2PtCl6 (4.0 × 10−3 mol L−1) was also prepared in DMF used for titration. Polymer-metal complexes were produced by adding aliquots of a solution of the selected metal salt to a THF solution of polymer. All kinds of measurements were monitored after sonicating the mixture for 1 h.Synthesis
The syntheses of 1–6 and P-1 were prepared according to our previous work.10pSynthesis of 1-phenyl-3-(4-bromophenyl)-1-propen-3-one(8)
To a mixture of 2-acetyl-4-bromobenzene 7 (0.04 mol) and benzaldehyde (0.04 mol) in ethanol (50 ml), a solution of potassium hydroxide (5%, 50 ml) was added slowly. The mixture was stirred for 24 h. The precipitated solid was filtered, washed with water, dried, and recrystallized from ethanol. The product was used without further purification. Yield = 92%.1H NMR (400 MHz, CDCl3) δ = 8.10 (d, 2H), 7.95–7.83 (m, 2H), 7.93 (d, 1H), 7.79 (d, 2H), 7.75 (d, 1H), 7.53–7.40 (m, 3H)Synthesis of 2,6-bis(4-bromophenyl)-4-phenylpyridine(9)
An equimolar amount of compound 8 (10 mmol) and 2-acetyl-4-bromobenzene 7 (10 mmol) was heated under reflux for 8 h in the presence of ammonium acetate (5.0 g) and glacial acetic acid (10.0 mL). After the reaction mixture was maintained at room temperature overnight, ice cold water (30.0 mL) was added to it. The precipitate was filtered, washed with methanol, dried, and recrystallized from appropriate solvent to afford compound 9. Yield = 42%. 1H NMR (400 MHz, DMSO-d6) δ = 9.21 (s, 2H), 8.62 (d, 2H), 8.54 (d, 4H), 8.05 (d, 4H), 7.91 (t, 1H), 7.84 (m, 2H); 13C NMR (100 MHz, DMSO-d6) d = 169.78, 165.73, 135.91, 133.43, 132.87, 131.097, 130.64, 130.37, 130.05, 128.81, 116.12.Synthesis of polymer P-2
Compound 9 (0.1 mmol), compound 6 (0.1 mmol), Pd(PPh3)4 (120 mg, 0.1 mmol), and CuI (19.2 mg, 0.1 mmol) were combined in dry and degassed triethylamine (5 ml) and THF (5 ml). The mixture was heated at 60 °C for 24 h under argon atmosphere, and then cooled to room temperature and filtered. The filtrate was added dropwise to vigorously stirred methanol. The precipitate was centrifuged, dissolved in 5 mL of THF, and then precipitated in methanol again. The final product was dried under vacuum to give polymer P-2 as a yellow solid in 67% yield. Mn = 2420 PDI = 2.06 (GPC). 1H NMR (400 MHz, CDCl3) δ = 8.14–6.80 (17H), 3.96 (4H), 1.71–1.29 (16H), 0.91 (6H). (C45H43NO2)n (629.8)n: Calcd. C 85.82, H 6.88, N 2.22; Found C 85.07, H 6.84, N 2.18.Results and discussions
Synthesis and characterization
Syntheses of P-1 and P-2 were performed based on the Sonogashira reaction as illustrated in Scheme 1 and Scheme 2. Reaction of 2-acetyl thiophene 1 with benzaldehyde afforded compound 2 with a yield of 93%. We attempted to prepare compound 4 by the reaction of 1 with 2, but unfortunately it was unsuccessful. Therefore we used N-[1-oxo-2-(2-thienyl)ethyl]-pyridinium iodide 3 to react with compound 1 and obtained the target product. Subsequently, compound 4 was brominated by NBS to produce the monomer 5, 2,6-bis(5-bromo-2-thienyl)-4-phenylpyridine in a yield of 74%. Finally, the polymer P-1 was prepared by polymerization of monomer 5 and 2,5-dicyclohexyloxy-1,4-benzenediyne 6 in the presence of a catalytic amount of Pd(PPh3)4 and CuI with Et3N under Ar. The polymer P-2 was synthesized by the reaction of 6 and 9, which was derived from the reaction of chalcone 8 and 2-acetyl-4-bromobenzene 7. After purifying and drying, P-1 and P-2 were obtained as brown and yellow powders, respectively. Both of the polymers were prepared with satisfactory yields and showed good solubility in common organic solvents, such as THF, dichloromethane, and DMF. The number-average molecular weights (Mn) of P-1 and P-2 were determined by gel permeation chromatography (GPC) against the polystyrene standards to be 3560 and 2420 with the polydispersity index (PDI) of 2.25 and 2.06, respectively. The chemical structure of compounds was verified by 1H NMR and 13C NMR spectroscopy, seen in the ESI.†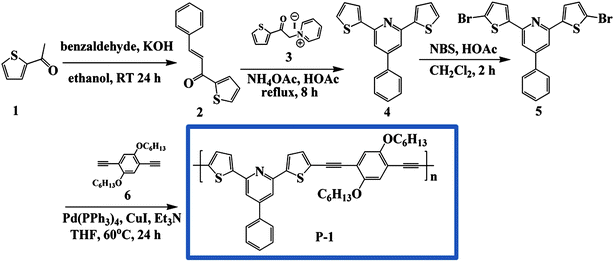 | ||
| Scheme 1 Synthesis of the polymers P-1. | ||
 | ||
| Scheme 2 Synthesis of the polymers P-2. | ||
Thermal properties
Thermal properties of the polymers were studied by thermogravimetric analysis (TGA) under nitrogen atmosphere. The comparison of thermal transitions between them revealed that P-2 is more thermally stable than P-1, shown in Fig. 2. Although meta-linkage polymers usually exhibit less stability than para-linkage polymers, the thermograms showed that both meta-linkage polymers exhibited good thermal stability with a weight loss of 5% above 170 °C. The high thermostability thus renders the polymers as good candidates for fluorescent sensors.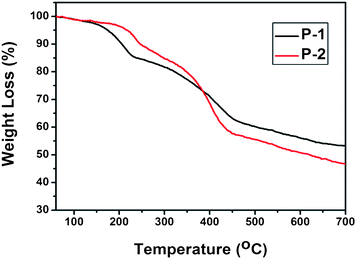 | ||
| Fig. 2 Thermogravimetric analysis of P-1 and P-2. | ||
Fluorescence properties
The spectroscopic properties of compounds 4, monomers 5 and 9 as well as the polymer P-1, P-2 were measured in dilute THF solution at room temperature. The UV-Vis absorption and fluorescence spectra of P-1 (Fig. S1 ESI†) and P-2 (Fig. S2 ESI†) are similar due to the similar conjugated structure of the polymer backbone consisting of meta-linkage pyridine and p-phenylene ethynylene. It can be seen that a strong and broad absorption appears in a region from 350 to 450 nm, which is attributed to the effective π–π* transition of the conjugated structure of the polymers. In comparison of P-2, the UV-Vis absorption maxima λmax of P-1 shows about a 25 nm red shift. This can be ascribed to more effective electron transfer transition of thiophene group than benzene group, which also affects the fluorescent maxima of P-1 and P-2.Fig. 3a shows the fluorescence spectra of compounds 4, monomer 5, and polymer P-1 in THF at excitation wavelength 345 nm, 350 nm, and 423 nm. 4 and 5 show weak fluorescent maxima at 385 nm and 390 nm, however, P-1 reveals intense fluorescent maximum at 472 nm, which exhibits characteristic features of the poly(p-phenylene ethynylene) (PPE). Fig. 3b represents the fluorescence spectra of monomers 9 and polymer P-2 in THF at excitation wavelength 327 nm and 400 nm. Similarly, monomer 9 and polymer P-2 have fluorescent maxima at 365 nm and 467 nm. Both of the two fluorescent polymers P-1 and P-2 can emit blue (467 nm and 472 nm) light due to the extended ρ-electronic structure in the main chain backbone. Compared with P-2, the fluorescent maximum of P-1 shows a little red shift (about 5 nm). The results suggest that electron-rich moieties such as electron-rich thiophene group could increase effective conjugation length. Moreover, to estimate PL efficiencies of the polymers P-1 and P-2, their emission spectra was calculated with quinine bisulfate in 0.1 M H2SO4 as the standard.11 The fluorescence quantum yield (QY) of P-1 and P-2 are estimated to be 24% and 22%. The high QY is considered as a necessary condition for fluorescent sensors.
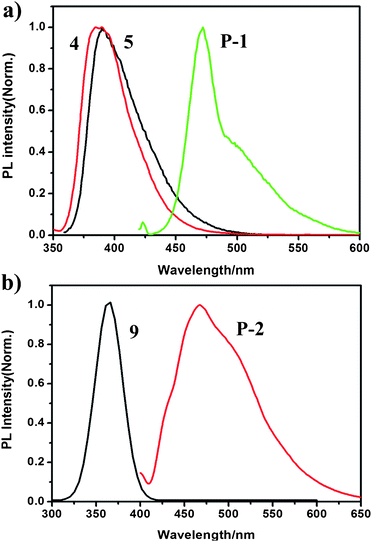 | ||
| Fig. 3 (a) Normalized emission spectra of compound 4, 5 and polymer P-1 in THF. (b) Normalized emission spectra of compound 9, and polymer P-2 in THF. | ||
Metal ion sensing properties
Metal ion responsive properties of P-1 and P-2 were examined by fluorescence emission spectroscopy in THF solution at a repeating unit concentration of 4 × 10−6 M, and shown in Fig. 4. The metal ions examined include Ag+, Ca2+, Cd2+, Co2+, Cu2+, Fe3+, Hg2+, Ni2+, Pd2+, Zn2+ and Pt4+. It was found that the fluorescence intensity of P-1 obviously decreased upon the addition of 25 equiv. Co2+, Cu2+, Fe3+, Ni2+, Pd2+, and Pt4+. To estimate selectivity for different ions, the fluorescent quenching degree of P-1 was calculated and plotted in Fig. 4c. Some “soft” metal ions such as Hg2+ and Ag+, which are normally considered as competitive species of the target analyte, almost don't cause any fluorescence quenching of the polymer. Moreover, an extensive survey of metal ions indicates that the polymer P-1 is also, to a much lesser extent, responsive to Pt4+, but the extent of fluorescence quenching by Pd2+ is about three times as effective as that by Pt4+. These results demonstrate that the sensor possesses an excellent selectivity for palladium ions.![Normalized emission spectra of P-1 (a) and P-2 (b), fluorescence quenching degree (c) of P-1 and P-2 upon addition of different metal cations (25 equiv. of Ag+, Ca2+, Cd2+, Co2+, Cu2+, Fe3+, Hg2+, Ni2+, Pd2+, Zn2+ and Pt4+) in THF solution. F0 and F denote fluorescence intensity of polymer before and after adding different cations. ([polymer] = 4 × 10−6 M in repeat units).](/image/article/2011/PY/c1py00149c/c1py00149c-f4.gif) | ||
| Fig. 4 Normalized emission spectra of P-1 (a) and P-2 (b), fluorescence quenching degree (c) of P-1 and P-2 upon addition of different metal cations (25 equiv. of Ag+, Ca2+, Cd2+, Co2+, Cu2+, Fe3+, Hg2+, Ni2+, Pd2+, Zn2+ and Pt4+) in THF solution. F0 and F denote fluorescence intensity of polymer before and after adding different cations. ([polymer] = 4 × 10−6 M in repeat units). | ||
Another ion-sensing experiment was performed by adding transition metals to P-2 in THF solvent. No fluorescence change of P-2 was observed when the metal ions were added. This can be attributed to the steric hindrance effect of two benzene rings on the two ortho positions of pyridine of 2, 6-diphenyl-4-phenylpyridine (PPP), which might form an improper spatial matching structure for binding metal ions. If smaller groups such as acetylene group take the place of benzene groups linked on the two ortho positions of pyridine, the structure becomes an efficient ligand for binding metal ions.10f
Fluorescent titration experiments of 4, 5 and P-1 were carried out in THF, with different concentration of palladium ions as shown in Fig. 5. In comparison of the polymer P-1, the fluorescence spectra of the compounds 4 and 5, exhibit much lower fluorescence response toward palladium ions. The slight fluorescent quenching of 4 and 5 could be attributed to formation of TPP-Pd2+ complex, which could be prepared via C–H activation reaction.12 We further calculated the Stern–Volmer data of 4, 5, and P-1 in order to estimate palladium ions binding capability of these three compounds. We notice that the Stern–Volmer data of 4 (KSV = 8.81 × 102 L mol−1) and 5 (6.63 × 102 L mol−1) is much lower than polymer P-1 (KSV = 2.46 × 104 L mol−1) (Fig. 5d, 5e and 5f). These results demonstrate that the single TPP unit is an inefficient ligand for a palladium ion, which has also been proved in previous literature.13 However, it is noteworthy that the fluorescence quenching degree of P-1 containing TPP units reach about 60%. It could be attributed to the extended conjugated length of the polymer playing a particularly important role in the amplified sensitivity of the sensor.
![Fluorescent emission spectra of 4 (a), 5 (b) and P-1 (c) in the presence of different concentrations of Pd2+ ions in THF ([4] or [5] = 4 × 10−6 M; [P-1] = 4 × 10−6 M in repeat units, the concentration of Pd2+ is from 0 to 1.0 × 10−4 M). Fluorescence quenching of compound 4 (d), 5 (e) and P-1 (f) by various concentration of palladium, in which F0 and F denote the intensity of the fluorescence signal of the sensing materials in the absence and presence of the palladium ions, respectively. KSV = (F0/F − 1)/[Pd2+]. The emission wavelength is 385 nm, 390 nm and 472 nm, respectively.](/image/article/2011/PY/c1py00149c/c1py00149c-f5.gif) | ||
| Fig. 5 Fluorescent emission spectra of 4 (a), 5 (b) and P-1 (c) in the presence of different concentrations of Pd2+ ions in THF ([4] or [5] = 4 × 10−6 M; [P-1] = 4 × 10−6 M in repeat units, the concentration of Pd2+ is from 0 to 1.0 × 10−4 M). Fluorescence quenching of compound 4 (d), 5 (e) and P-1 (f) by various concentration of palladium, in which F0 and F denote the intensity of the fluorescence signal of the sensing materials in the absence and presence of the palladium ions, respectively. KSV = (F0/F − 1)/[Pd2+]. The emission wavelength is 385 nm, 390 nm and 472 nm, respectively. | ||
According to the usual mechanism of fluorescent conjugated sensors, we first ascribed fluorescence quenching to the interchain TPP-Pd2+ binding-induced aggregation, shown in Scheme 3. This interpolymer interaction may facilitate formation of multiple binding sites structures similar to macrocyclic ligands, and acquire cation-binding properties. Macrocycles containing two and more pyridine rings have been proved to be excellent ligands for divalent transition metals.13 As a result, the analogous macrocycles structure via the coordination of two or more TPP units as crosslinking point may cause the aggregation of polymer chains, which leads to the fluorescence quenching. To confirm the binding mode between the palladium ions and TPP units of P-1, we attempted to examine the NMR shift of the polymer P-1 binding with Pd2+. Unfortunately, the solubility of the complex is too low to display an NMR signal.
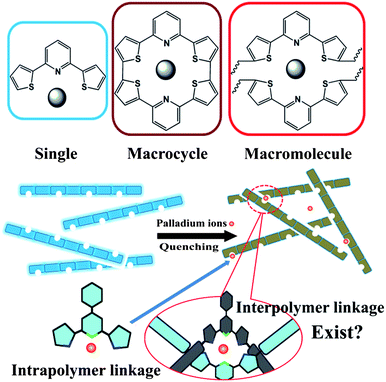 | ||
| Scheme 3 Schematic representation of the proposed sensing mechanism. | ||
In order to further unravel the binding mode, the absorption and emission spectrum of P-1 were further measured. The absorption spectrum (Fig. 6a) shows no obvious red shift upon addition of palladium ions. This result suggests that the polymer chains are on average conjugated with a similar length, which reveals the interchain linkage maybe not the major mode of linkage between ions and TPP units. Moreover, no obvious shift of emission spectra (Fig. 6c) may demonstrate that the facile energy transfer can occur along the different segments of the backbone. In other words, the effective conjugation length along the polymer backbone doesn't markedly extend. As a result, fluorescence quenching of P-1 is mostly ascribed to the intrachain binding, the fluorescence response signal of which is amplified along conjugated polymer chains, instead of interchain Pd2+-induced aggregation. There isn't any other direct evidence to prove whether the interchain binding-induced aggregation exists, so we considered that the intrachain linkage between Pd2+ and TPP units is possibly the major reason for the fluorescence quenching.
![UV-Vis absorption spectra (a), the plot of the absorbance at 426 nm, (b) fluorescent emission spectra (c) and the plot of the fluorescence intensity I472 (d) of P-1 in the presence of different concentrations of Pd2+ ions in THF ([P-1] = 4 × 10−6 M in repeat units, [Pd2+] = 0, 2, 4, 8, 12, 16, 20, 40, 60, 80, 120 × 10−6 M); excitation wavelength was 423 nm.](/image/article/2011/PY/c1py00149c/c1py00149c-f6.gif) | ||
| Fig. 6 UV-Vis absorption spectra (a), the plot of the absorbance at 426 nm, (b) fluorescent emission spectra (c) and the plot of the fluorescence intensity I472 (d) of P-1 in the presence of different concentrations of Pd2+ ions in THF ([P-1] = 4 × 10−6 M in repeat units, [Pd2+] = 0, 2, 4, 8, 12, 16, 20, 40, 60, 80, 120 × 10−6 M); excitation wavelength was 423 nm. | ||
The absorption spectrum of P-1 was measured with a series of concentrations of Pd2+ ions in THF solution. The polymer sensor displays a chromogenic behavior toward palladium ion with a color change from yellow green to brown, which can be easily observed by the naked eye, as shown in Fig. 1. Note that the absorption (Fig. 6a) centered at 426 nm is enhanced about 2-fold with the addition of palladium ions to the solution of P-1, meanwhile, a new peak of absorption appears at 330 nm. The absorbance at 426 nm is linearly proportional to the amount of Pd2+ in the range of 1 μM–100 μM (Fig. 6b). It is proved that the detection limit of polymer P-1 is less than 1 × 10−6 M. Fig. 6c displays the changes of emission spectra of P-1 with Pd2+ concentrations and it can be seen that with the increase of Pd2+ concentration, the fluorescence intensity of the emission maximum at 472 nm is decreased dramatically with the QY from 0.24 to 0.1. Fig. 6d is the plot of fluorescence intensity I472vs. Pd2+ concentration and it reveals that the polymer P-1 with the detection limit of 1 ppm below palladium ions threshold 5–10 ppm, could be used as an excellent sensor for palladium ions detection.
Moreover, the Pd2+ ion sensing properties of P-1 in aqueous solution were measured both in THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution and DMF–H2O (1
1, v/v) solution and DMF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution with HEPES buffer (10 mM), seen in Fig. 7. After adding 25 equiv. Pd2+ ions, the fluorescence of P-1 is 99% quenched in THF–H2O (1
1, v/v) solution with HEPES buffer (10 mM), seen in Fig. 7. After adding 25 equiv. Pd2+ ions, the fluorescence of P-1 is 99% quenched in THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution and 91% quenched in DMF–H2O (1
1, v/v) solution and 91% quenched in DMF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), which is more effective than in pure THF solution (59%). This may be due to the different metal binding capabilities of polymer P-1 in different polar solvents. These results reveal that P-1 can be used as a Pd2+ sensor both in organic and aqueous solution, which is particularly important for detection in drinking water and biological systems.
1, v/v), which is more effective than in pure THF solution (59%). This may be due to the different metal binding capabilities of polymer P-1 in different polar solvents. These results reveal that P-1 can be used as a Pd2+ sensor both in organic and aqueous solution, which is particularly important for detection in drinking water and biological systems.
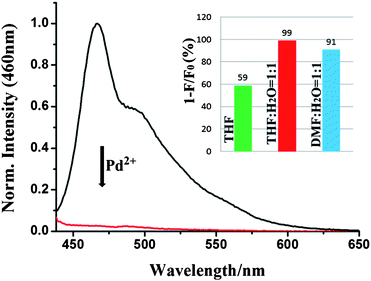 | ||
Fig. 7 Normalized emission spectra of P-1 upon addition of 25 equiv. Pd2+ ions in THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution with HEPES buffer (10mM). Inset: Fluorescence quenching degree of P-1. F0 and F denote fluorescence intensity of polymer before and after adding 25 equiv. Pd2+ ions in different solution. 1, v/v) solution with HEPES buffer (10mM). Inset: Fluorescence quenching degree of P-1. F0 and F denote fluorescence intensity of polymer before and after adding 25 equiv. Pd2+ ions in different solution. | ||
Conclusions
Two conjugated polymers P-1 and P-2 have been successfully synthesized by polymerization of monomers 2,6-bis(5-bromo-2-thienyl)pyridine (5) or 2,6-bis(4-bromophenyl)-4-phenylpyridine (9) with 1,4-bis(ethynyl)-2,5-bis(hexyloxy)-benzene via Sonogashira coupling reaction. Polymer formation was confirmed both by GPC, NMR spectrum, and elemental analyses. The thermal properties of the two polymers were measured and the polymers revealed high thermal stability. The UV-Vis absorbance and fluorescence spectra of the monomers and polymers have been investigated in THF. It is found that P-2 exhibits almost no response to all transitional metal ions due to the steric hindrance effect. On the contrary, for P-1, a selective chromogenic behavior towards Pd2+ can be observed with the naked eye and meanwhile, Pd2+ ion can effectively quench the fluorescence of the polymer with an excellent selectivity. The mechanism of fluorescent quenching and the linkage mode between Pd2+ ions and TPP units of polymer P-1 chain have been discussed. There isn't direct evidence to prove that interchain binding-induced aggregation is the major reason causing the fluorescence quenching. The simple intrachain binding which is amplified through conjugated polymer backbone plays an important role for much enhanced sensitivity of polymer than its monomer. All the results demonstrate that polymer P-1 can be used as an efficient sensor with a high sensitivity and an excellent selectivity for Pd2+ detection both in organic and aqueous solution, whose detection limit is below 1 ppm. This work demonstrates the feasibility of varying the polymer structures to match the molecular spatial dimension for selective binding of specific metal ions.Acknowledgements
The authors acknowledge financial support from the Ministry of Science and Technology of China (NO. 2007CB936401). Mr H. Wang's assistance with the TGA measurements is appreciated.Notes and references
- (a) R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419 CrossRef CAS; (b) J. Liu, Z. Cao and Y. Lu, Chem. Rev., 2009, 109, 1948 CrossRef CAS; (c) J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780 CrossRef CAS; (d) E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517 CrossRef CAS; (e) P. Buhlmann, E. Pretsch and E. Bakker, Chem. Rev., 1998, 98, 1593 CrossRef; (f) Y. Qu, J. Hua, Y. Jiang and H. Tian, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1544 CrossRef CAS; (g) V. D. B. Bonifacio, J. Morgado and U. Scherf, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2878 CrossRef CAS.
- (a) T. M. Swager, Acc. Chem. Res., 1998, 31, 201 CrossRef CAS; (b) D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS; (c) S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef CAS.
- L. Chen, D. W. Mcbranch, H. L. Wang, R. Helgeson, F. Wudl and D. G. Whitten, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 12287 CrossRef CAS.
- (a) J. Le Bars, U. Specht, J. S. Bradley and D. G. Blackmond, Langmuir, 1999, 15, 7621 CrossRef; (b) T. Iwasawa, M. Tokunaga, Y. Obora and Y. Tsuji, J. Am. Chem. Soc., 2004, 126, 6554 CrossRef CAS; (c) M. Lafrance and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 16496 CrossRef CAS.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337 RSC.
- (a) J. C. Wataha and C. T. Hanks, J. Oral Rehabil., 1996, 23, 309 CrossRef CAS; (b) International Programme on Chemical Safety, Palladium, Environmental Health Criteria Series 226, World Health Organization, Geneva, 2002 Search PubMed; (c) J. Kielhorn, C. Melber, D. Keller and I. Mangelsdorf, Int. J. Hyg. Environ. Health, 2002, 205, 417 CrossRef CAS.
- C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889 CrossRef CAS.
- R. N. Rao and M. V. N. K. Talluri, J. Pharm. Biomed. Anal., 2007, 43, 1 CrossRef CAS.
- (a) D. Beauchemin, Anal. Chem., 2008, 80, 4455 CrossRef CAS; (b) E. H. Evans and J. J. Giglio, J. Anal. At. Spectrom., 1993, 8, 1 RSC; (c) B. Godlewska-Zylkiewicz, Microchim. Acta, 2004, 147, 189 CAS.
- (a) E. Unterreitmaier and M. Schuster, Anal. Chim. Acta, 1995, 309, 339 CrossRef CAS; (b) K. Kubo, Y. Miyazaki, K. Akutso and T. Sakurai, Heterocycles, 1999, 51, 965 CrossRef CAS; (c) B. K. Pal and M. S. Rahman, Microchim. Acta, 1999, 131, 139 CrossRef CAS; (d) Y. J. Fang, H. Chen, Z. X. Gao and X. Y. Jin, Indian J. Chem., Sect. A, 2002, 41, 521; (e) B. Tang, H. Zhang and Y. Wang, Anal. Lett., 2004, 37, 1219 CrossRef CAS; (f) H. Huang, K. Wang, W. Tan, D. An, X. Yang, S. Huang, Q. Zhai, L. Zhou and Y. Jin, Angew. Chem., Int. Ed., 2004, 43, 5635 CrossRef CAS; (g) T. Schwarze, H. Müller, C. Dosche, T. Klamroth, W. Mickler, A. Kelling, H. G. Löhmannsröben, P. Saalfrank and H. J. Holdt, Angew. Chem., Int. Ed., 2007, 46, 1671 CrossRef CAS; (h) F. Song, A. L. Garner and K. Koide, J. Am. Chem. Soc., 2007, 129, 12354 CrossRef CAS; (i) L. P. Duan, Y. F. Xu and X. H. Qian, Chem. Commun., 2008, 44, 6339 Search PubMed; (j) R. J. T. Houk, K. J. Wallace, H. S. Hewage and E. V. Anslyn, Tetrahedron, 2008, 64, 8271 CrossRef CAS; (k) A. L. Garner and K. Koide, Chem. Commun., 2009, 45, 86 Search PubMed; (l) A. L. Garner, F. Song and K. Koide, J. Am. Chem. Soc., 2009, 131, 5163 CrossRef CAS; (m) H. Li, J. Fan, J. Du, K. Guo, S. Sun, X. Liu and X. Peng, Chem. Commun., 2010, 46, 1079 RSC; (n) F. L. Song, E. J. Carder, C. C. Kohler and K. Koide, Chem.–Eur. J., 2010, 16, 13500 Search PubMed; (o) H. Li, J. Fan, F. Song, H. Zhu, J. Du, S. Sun and X. Peng, Chem.–Eur. J., 2010, 16, 12349 Search PubMed; (p) B. Liu, Y. Y. Bao, F. F. Du, H. Wang, J. Tian and R. K. Bai, Chem. Commun., 2011, 47, 1731 RSC.
- W. R. Dawson and M. W. Windsor, J. Phys. Chem., 1968, 72, 3251 CrossRef CAS.
- E. C. Constable, R. P. G. Henney and D. A. Tocher, J. Chem. Soc., Dalton Trans., 1992, 2467 RSC.
- O. Meth-Cohn and H. Jiang, J. Chem. Soc., Perkin Trans. 1, 1998, 3737 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data of the monomer, polymer and additional spectroscopic data. See DOI: 10.1039/c1py00149c |
| This journal is © The Royal Society of Chemistry 2011 |
