Morphologies and deformation behavior of poly(vinylidene fluoride)/poly(butylene succinate) blends with variety of blend ratios and under different preparation conditions
Tianchang
Wang
a,
Huihui
Li
b,
Feng
Wang
b,
Jerold M.
Schultz
c and
Shouke
Yan
*b
aState Key Laboratory of Polymer Physics and Chemistry, Institute of Chemistry, The Chinese Academy of Sciences, Beijing, 100190, P. R. China
bState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing, 100029, P. R. China. E-mail: skyan@mail.buct.edu.cn; Fax: +86-10-64455928; Tel: +861064455928
cDepartment of Materials Science and Engineering, University of Delaware, Newark, DE 19716, United States
First published on 16th May 2011
Abstract
The morphological features and mechanical properties of PVDF/PBS blends with a variety of blend ratios and under different preparation conditions have been studied by optical and atomic force microscopy, as well as by tensile tests. It was found that, at high PVDF crystallization temperature, a small amount of PBS in the 70/30 PVDF/PBS blends has been expelled into the PVDF spherulite margin areas and interspherulitic regions due to the high diffusion ability of PBS and the slower crystal growth rate of the PVDF at high temperature. Nevertheless, the PBS affects the crystallization of PVDF significantly, which has been revealed by the increase in birefringence of PVDF spherulites of both α and γ types and the increase in band period of the α PVDF spherulites. With increasing PBS content, the increase in birefringence of PVDF spherulites and the increase in band period of the α PVDF spherulites get more evident, reflecting a more efficient influence of PBS on the crystallization of PVDF. In the PBS-rich blends, e.g., in the 40/60 and 30/70 PVDF/PBS blends, the PVDF forms isolated spherulites with large non-crystallizing PBS melt regions, which results in an interspherulitic phase separation with bigger interspherulitic PVDF areas. There is, however, PBS dispersed within the PVDF spherulites. During the crystallization of PBS at low temperature, it was found that the PBS in the interspherulitic and interaspherulitic regions growth in different manner with different growth rates. At lower PVDF crystallization temperatures, the PVDF crystallizes first and fills all the volume in regardless its content in the blends. This leads to the PBS being distributed in the interlamellar or interfibrillar regions of PVDF spherulites only. However, the growth of PBS in banded and non-banded PVDF matrix is different, reflecting the influence of pre-existing PVDF crystals on the crystallization of PBS. Tensile test shows that the deformation behavior depends remarkably on the blend ratio and the crystallization temperature of the PVDF. Such phenomena has been correlated and explained in view of the inner morphological change.
Introduction
Blending one polymer with another is a useful and economical way to produce high-value polymeric materials with a variety of properties without having to synthesize novel polymers. Among the large amount of polymeric mixtures, those involving semicrystalline polymers are of particular interest. This is not only because a certain degree of crystallinity is important in order to ensure satisfactory high-temperature strength and environmental resistance, but also because semicrystalline polymer blends offer another system for study of crystallization and morphology of polymers. In the past decades, most of the investigated systems represented mixtures in which only one of the components is crystalline.1–5 Blends in which both components are crystalline polymers have received relatively less attention compared with amorphous/crystalline systems.6–14 However, these kind blends open up new avenues for studying the relationships between phase behavior and structure development in polymeric mixtures. Consequently, this field has been a subject of continuing interest in both academia and industry.Miscible crystalline/crystalline polymer blends are more complicated and exhibit a wide variety of supermolecular structures and morphologies in comparison to the amorphous/crystalline blend systems.15,16 It was found that the most important fact influencing the morphologies of crystalline/crystalline polymer blends is the difference in the melting point (Tm) of the two components.17,18 When the difference in Tm is small enough, both constituents can crystallize simultaneously and form so-called interpenetrated spherulites.19–22 When the difference in Tm is relatively large, two-step crystallization usually occurs in which the high-Tm component crystallizes first during cooling from the melt. The situation in this case is twofold.23 On the one hand, the high-Tm component cannot fill the whole space after complete crystallization at a given temperature. The crystallization of the low-Tm component takes place at the second step and fills up the rest space. Spherulites of the two components then exist side-by-side. On the other hand, the high-Tm component fills the volume already in the first step with the low-Tm component all trapped within the spherulites of the high-Tm component as isolated lamellae or lamellar stacks. Blend ratio and crystallization temperature are the other factors affecting the crystallization behavior of the crystalline/crystalline polymer blends. They influence not only the growth and morphology of the high-Tm component, but also the diffusion and crystallization behaviors of the low-Tm component. Taking these complicated influences into account, the crystallization and morphology of binary miscible crystalline polymer blends are far away from being really understood until now. In particular, less attention has been paid to the crystallization and morphology of the low-Tm component, which are affected not only by blend composition and crystallization conditions, but also strongly by the pre-existing crystals of the high-Tm component in the blend. Therefore, sophisticated studies on the crystalline/crystalline polymer blends are needed.
PVDF is a representative material with a variety of crystal structures, and has attracted much attention due to its outstanding electroactive properties. PBS is a biodegradable polymer with environmental and ecological advantages. Both of them are crystalline polymers, with a melting point gap of about 60 K, and with good miscibility. Ikehara, et al.9 first reported the miscibility of the PVDF/PBS blend. Also, the crystallization behaviour of the blends was studied by DSC and optical microscopy. Later on, the structural changes on a lamellar scale of this miscible crystalline polymer blend system during melting and crystallization processes have been investigated by Kaito et al.24–26via real-time small-angle X-ray scattering measurements. In the present work, PVDF/PBS has been chosen again as a model system, and the structural evolution process of the blends under various conditions was visualized by optical microscopy as well as atomic force microscopy on a lamellar scale. Most importantly, the mechanical properties of the blends with various blend ratios and under different preparation conditions have been correlated directly to the fine structures formed.
Presented here in this paper are some new experimental results concerning the morphological features of the blends with various blend ratios and under different conditions, the location distribution of the PBS, particularly its crystal growth behavior. The different mechanical properties of the blends were then explained in view of the inner morphological change.
Results and discussion
1. Morphologies of the blends formed by two step crystallization
Since the degree of supercooling and blend ratio of two components serve as key factors influencing the crystal structure and morphologies, we will first focus on the morphological features of the PVDF/PBS blends and their developing process. It should be mentioned ahead that the study is not only related to blend ratio and temperature dependence of the crystalline morphologies. Particular attention has been paid to the question of how the preexisting PVDF crystals affect the crystallization behavior of the PBS. To better understand the different crystallization behavior of the blend, pure PVDF crystallized at quite high temperature, e.g. 170 °C, was first presented in Fig. 1a. One sees that PVDF forms typical large γ form spherulites hundreds of microns in diameter with extraordinarily weak birefringence.27–29 In some cases, ring banded structures corresponding to α form crystals could be observed at the center part of the γ-PVDF spherulites (indicated by the arrows in Fig. 1a). It should be pointed out that the γ form of PVDF, which is associated with a low degree of supercooling, is a thermodynamically stable phase, while its α form is preferential in kinetics. When crystallizing PVDF at very high temperature, ring banded α form spherulites could start to grow first and subsequently more stable γ form crystals begin to form in succession. The weak birefringence of the γ form PVDF spherulites is associated with the spatial arrangement of the constructing lamellar units. As shown in Fig. 1b, closely packed lamelli-form crystals seen face-on are the predominant structures in the γ form of PVDF spherulites grown at 170 °C, such extraordinary crystallographic regularity together with the packing manner of the chains in its unit cell make the c axes (chain axes) of PVDF inclined at a small angle with respect to the incident light, which consequently lead to the sharp decrease in birefringence. | ||
| Fig. 1 (a) Optical micrograph and (b) AFM phase image of neat PVDF crystallized at 170 °C for 50 h. (c) Optical micrograph of PVDF in a 70/30 PVDF/PBS blend first crystallized completely at 165 °C for 120 h and (d) then cooled quickly to 80 °C for 5 min to enable the crystallization of PBS. The white arrows in parts (a) and (c) indicate the banded structures of PVDF. The white arrows in (d) indicate the banded structure of PBS, while the black arrow indicates the birefringence increment in the spherulite margins of PVDF. | ||
For the blend system a two step crystallization process, in which the PVDF crystallizes first and then followed by the crystallization of PBS, has been adopted. During this process, the diluting influence of PBS on the morphologies of PVDF and the influence of the preexisting PVDF framework on the crystallization behavior of the PBS were studied. Fig. 1c and 1d are the representative optical micrographs of a 70/30 PVDF/PBS blend under two step crystallization conditions. By holding the blend at 165 °C from homogeneous melt, crystallization of PVDF occurs while PBS remains in the molten state, as shown in Fig. 1c. The main morphological difference between Fig. 1a and 1c can be described as follows: first, the birefringence of the γ form PVDF spherulites in the blend (see Fig. 1c) is much stronger than that of pure PVDF, and they cannot fill the whole space even after prolonged crystallization time due to depletion of the material. Second, for the blend, the ring banded structures corresponding to α form PVDF crystals located at the central part of the spherulites (indicated by the arrow in Fig. 1c) have a larger band period, namely, a longer distance between the two equivalent rings than that shown in Fig. 1a. In fact, this kind of α form banded structures within the γ form spherulites have already transformed into the γ′ form through solid–solid phase transition under high temperature,30,31 regardless of whether or not the existence of PBS component. This has been verified by the increase of melting point for the central banded structures. Since the main aim of this work is not to reveal the effect of PBS on the phase transition behaviors of PVDF, related work will be reported in a separate paper. On decreasing the temperature quickly to 80 °C, as shown in Fig. 1d, one sees the emergence of PBS crystals which occupy the space left between PVDF spherulites. Even though regular PBS spherulite cannot be seen now, in some parts as denoted by two white arrows in Fig. 1d, the characteristic ring banded structures of PBS can still be recognized. This implies that almost all of the PVDF has crystallized completely at this time. Otherwise, the PBS might exhibit a different growth manner, which will be discussed in more detail later. With careful inspection, an apparent intensity increment around the margin areas of the γ form spherulites, which already exhibit birefringence before crystallization of PBS, can be identified (denoted by the black arrow in Fig. 1d), while the inner part of the spherulites remain almost unchanged. This results from the crystallization of PBS at the outboard of PVDF spherulites. Taking the above morphological features into account, it is clear that the high diffusion ability of PBS at high temperature together with the low growth rate of PVDF γ form spherulites make the PBS expelled into the growth front of PVDF spherulites with some of it in the margin regions of the spherulites.
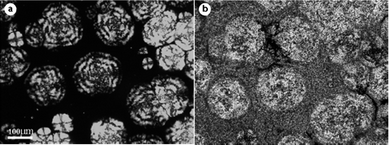
To disclose the exact location of PBS crystals in PVDF γ spherulites, an in situAFM experiment has been taken in the areas indicated by the black arrow of Fig. 1d. As shown in Fig. 2a, mixed randomly arranged flat-on and edge-on lamellae compose the γ form spherulites of PVDF in the blend. Selective melting of PBS at 125 °C (Fig. 2b), which is higher than the melting point of PBS, helps us to locate where PBS has been. Comparing the cycles in Fig. 2a with that in Fig. 2b, one can find that PBS disperses both between the edge-on interlamellar region and on the flat-on terraces of PVDF spherulite, which could account for the enhancement of birefringence in the periphery of γ spherulites. This kind of distribution of PBS in the γ form PVDF spherulites is the first time real space has been visualized.
 | ||
| Fig. 2 AFM phase images obtained in the area indicated by the black arrow in Fig. 1(d), scanned at (a) 25 °C and (b) 125 °C. The circles in parts (a) and (b) indicate the same scan areas before and after melting of PBS. Scan size is 4 μm. | ||
With the increase of PBS content up to 60%, the morphologies of PVDF spherulites change significantly. As shown in Fig. 3a, spherulites could seldom be seen when crystallized at low supercooling (e.g. at 165 °C) due to the lower nucleation ability of PVDF in the PBS-rich blend. In this case, only large α spherulites are observed, no γ spherulites could be found, indicating that the existence of large amount of PBS favors the formation of α PVDF spherulites. This phenomenon could be expected since the adding of PBS changes the stress on PVDF lamellar surface and results in the twisting of the lamellae. The melting point of these spherulites is found to be higher than PVDF α form crystals, indicating the occurrence of solid–solid phase transition and the formation of γ′ form crystal during the spherulites growing at high temperature. These spherulites are sort of loose and their growth rate becomes slower and slower with prolonging crystallization time because of the remarkable exclusion of PBS from the growing spherulites. Actually, this kind of PVDF spherulites contain almost no PBS as judged by further in situAFM scanning below and above the Tm,PBS. PBS, in this case, has been ejected into the rest melt upon PVDF spherulites growing, and inhibited the growth of them. Fig. 3b, 3c and 3d represent a time sequence of the crystallization of both PVDF and PBS when the sample was quickly cooled to 80 °C. It should be noted that non-crystalline PVDF in the rest melt of Fig. 3a always crystallized first and fill the left space during the cooling process before Tc of PBS was reached (see Fig. 3b). After 60 s at 80 °C, several PBS spherulites with relatively strong birefringence were found to nucleate and grow continuously within the preexisting PVDF matrix, as shown in Fig. 3c. They proceeded in spherulitic shape until impinging on others, and finally cover the whole remaining space (see Fig. 3d). Generally, observation of the growth of the low-Tm component is difficult in crystalline/crystalline polymer blend, due to the existence of the previously formed crystals of the high-Tm component. In this case, the nucleation density of PBS in PVDF matrix is relatively low and it is possible to observe and identify the nucleation and growth behavior of PBS in the PVDF matrix. One can see from Fig. 3c, the nucleation density of PBS around PVDF spherulites is much higher than that in other places in the PVDF matrix after cooling. This should be attributed to the enrichment of PBS melt around PVDF spherulite due to its strong diffusion from the growth front at high temperature. The increased concentration of PBS could enhance the PBS nucleation rate.
 | ||
| Fig. 3 (a) Optical micrograph of a 40/60 blend first crystallized isothermally at 165 °C for 100 h. (b) subsequent crystallization of PVDF during cooling from 165 °C to 80 °C. (c) after 1 min at 80 °C. (d) after 3 min at 80 °C. | ||
For the 70/30 PVDF/PBS blend, when the crystallization temperature of PVDF was set at 164 °C, see Fig. 4a, ring banded α form spherulites (also see the inserted high magnification image), with some γ crystals dispersed sporadically between them with weaker birefringence, could be observed. Maltese-crosses could be clearly seen. Such morphological features are very common in the intermediate temperature range, the detailed information regarding the inner structures of ringed α spherulites has been given by AFM measurement, as shown in Fig. 4b. Alternate arrays of edge-on and flat-on lamellae coexist along the radial direction with their boundary clearly delineated, which means no gentle continuous twist of the lamellar orientation. This twisting habit with sudden change in c-axis orientation of the lamellae is somewhat different from that of other polymers with ringed structure, e.g. PEA,32 which is usually caused by changing of lamellar orientation gradually along radial direction. This result confirms the previous study on the ring patterns of PVDF by electron microscopy.33 Nevertheless, this kind of lamellar twisting is fairly regular along the radial direction on the whole, and therefore responsible for the formation of highly ordered banded structures shown in Fig. 4a. After blending with PBS, an apparent change in the morphology of the blend occurs. Fig. 4c shows the spherulitic morphologies of PVDF in the 70/30 PVDF/PBS blend crystallized completely at 160 °C. The banded α form spherulites, in their turn, get coarser compared with compact ones of neat PVDF, and become much more open and irregular as looking away from the center to the outer (see the dotted arrow in Fig. 4c). This results from the dilute effect after blending with PBS. With the place farther and farther away from the center of the spherulites, more and more PBS was accumulated due to the exclusion by growing PVDF spherulites. This leads to the formation of coarser fibrils along the radial direction of the spherulites. Moreover, an increase in the band period of α spherulites has been observed again and will be discussed later. The occurrence of none volume-filling spherulites of PVDF caused by material impoverishment also indicates the strong exclusion and enrichment of PBS component in the rest melt. As for the γ spherulites, a uniform increment of the birefringence happens in the blend with respect to the ones in neat PVDF, as seen in Fig. 4a. This is related to the inner structure changes of the γ form crystals in the blend, which has been confirmed by AFM observation (figure not shown since it looked very similar to that of Fig. 2a). Fig. 4d shows the corresponding morphological changes after completion of PBS crystallization. Just like the case in Fig. 1d, besides the interspherulitic suffusion of PBS crystals, a birefringence increment around the margin areas, resulting from the crystallization of PBS, has been observed for both α and γ form spherulites of PVDF. Similar margin-enrichment in the blend has been reported recently in the system of PVDF/PMMA,34 which was explained by the increase in the amount of excluded PMMA as the spherulite grows and the formation of large amorphous pockets of PVDF at the crystal growth front. The specific location of PBS, in this case, can be understood by the competition between displacement rate of crystal growth front (i.e., spherulites growth rate) and the diffusive displacement rate of the noncrystallizing melt. Just like crystallization of PVDF under quite low supercooling mentioned above, PBS melt diffuses away from the growth front of PVDF easily, whereas the growth rate of PVDF spherulites is relatively small at this temperature. As a result, PBS can go for a long distance toward the edge of PVDF spherulites, filling into the pockets of PVDF at the growth front and finally enriched both on the edge part of PVDF α and γ form spherulites and the interspherulitic regions between them after being fully crystallized.
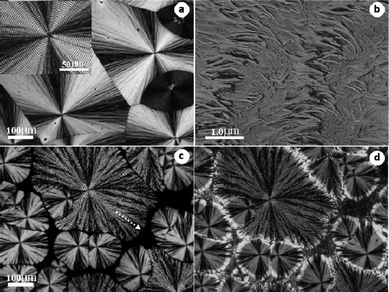 | ||
| Fig. 4 (a) Optical micrograph of neat PVDF crystallized at 164 °C. (b) Corresponding AFM phase image of banded α spherulites in (a). (c) 70/30 blend first crystallized completely at 160 °C and (d) then cooled quickly to 80 °C for 5 min. The insert in (a) is an enlarged part of the banded spherulite. The dotted arrow in (c) indicates that the spherulite gets more open with increasing distance from the center. | ||
When the PBS content is increased to 60%, i.e. a 40/60 PVDF/PBS blend, both α and γ form spherulites of PVDF become much coarser and more open than ever before, the bunch of fibrils in the γ spherulites could also be seen, as shown in Fig. 5a. The existence of crystal free space, which hardly changes or at most changes quite slowly with extending time, implies the existence of a large amount of PBS in the melt. Of particular interest is the growth behavior of subsequently crystallized PBS. Fig. 5b to 5e show the in situcrystallization process of PBS after quickly cooled to 80 °C. The birefringence increases significantly in the periphery of the α form PVDF spherulites after 30 s on reaching the temperature, which means that crystallization of PBS occurs (see Fig. 5b). This specific crystallization location is reasonable since the concentration of PBS is higher in the interspherulitic region of α spherulites caused by the exclusion effect. With careful inspection, a large amount of tiny PVDF crystals could also be observed filling the black background. These PVDF microcrystallites were generated by cooling to this lower temperature. As time goes, PBS crystals grow forward from the border of both α and γ form PVDF spherulites in the matrix with pre-existing small PVDF crystals, as denoted by the arrows in Fig. 5c. Unlike the case of Fig. 3c where the PBS crystals grow in a circular shape like spherulites, now the PBS nucleates mainly around the PVDF spherulites and no separated nucleation site could be seen in the crystalline PVDF matrix. Fig. 5d shows the crystallization of PBS after 120 s at 80 °C. The growth of PBS crystals in the matrix of tiny PVDF crystals has already finished. The growth of PBS in the existing larger α PVDF spherulites exhibits a different growth manner. Opposite to the growth direction in the left space, one can see that the PBS grows from the outboard of α spherulites backward to the center of them, and finally fills the whole spherulites (see Fig. 5e). They show a much slower growth rate and a nonlinear growth behavior. Generally speaking, the propagation of PBS crystallization across a PVDF spherulite depends on both the nucleation and growth of PBS lamellae. The inner structures for the bigger α spherulites should be more compact with a relatively high degree of crystallinity compared to the microcrystallites formed during the cooling process, i.e. the crystalline PVDF matrix in the left space. Therefore, the nucleation and growth of PBS lamellae in the narrow interfibrillar space of the compact ring-banded PVDF structure is very difficult, which slows down the growth rate of PBS. In addition, the nonlinear growth behavior clearly indicates the formation of the concentration gradient along the radius of PVDF spherulites due to the expelling of PBS. These different growth manners illustrate the influence of pre-existing PVDF crystals and their fine structure variation on the crystallization of PBS. The influence of PBS on the crystallization of PVDF is best demonstrated by the change in the band period of the PVDF α spherulites.
 | ||
| Fig. 5 Optical micrographs of 40/60 blend (a) crystallized at 160 °C for 100 h and then cooled quickly to 80 °C for (b) 30 s, (c) 60 s, (d) 120 s and (e) 240 s. The arrows in (c) indicate the growth of PBS in the PVDF microcrystallite matrix formed during cooling. | ||
It was found that the band periods of PVDF α spherulites in the PBS-rich blends are all larger than those in the PVDF-rich blends at a given supercooling. Fig. 6 shows the changes of the band periodicity of PVDF α spherulites as a function of blend ratio and crystallization temperature. It is evident that the band period increases with crystallization temperature as well as the amount of PBS content. The increase of band periodicity with temperature is expected as frequently reported in many other polymer systems with extinction feature.35–38 However, the increase of band periodicity with increase of the second component is quite different from the results of other blend systems, where a reduction in band spacing is usually obtained,39,40 although with a few exceptions.32,41 Considering that the band periodicity depends generally on the chain mobility, crystal growth rate and surface free energy,37 the much lower melting point of PBS and its full miscibility with PVDF leads to the PBS acting as solvent for PVDF. This results in an increment in chain mobility of PVDF, which may contribute to the increment of band periodicity in PVDF spherulites. Moreover, a significant decrease in crystal growth rate of PVDF after blending with PBS is also in favor of lamellar surface stress relaxation. This will also unambiguously increase the band period when the stress at the lamellar fold surface is considered as the cause of lamellar twisting.
 | ||
| Fig. 6 Variation of band periodicity of PVDF banded spherulites in PVDF/PBS blends as a function of crystallization temperature and blend ratio. | ||
When crystallizing a 70/30 PVDF/PBS blend first at 155 °C for 60 min, see Fig. 7a, α PVDF spherulites with evident ring-banded structures and an average diameter of 200 μm filling the whole space have been seen. Now no γ PVDF spherulites have been observed at all. In comparison with neat PVDF crystallized at the same temperature, the spherulites shown in Fig. 7a exhibit relatively loose structures with coarse fibril bundles and nonlinear boundaries. The typical Maltese-crosses also become weaker. Following the crystallization of PVDF component, the blend is cooled rapidly to 80 °C, allowing the crystallization of PBS component. As shown in Fig. 7b, the brightness of PVDF spherulites increases to some extent. Also tiny bright dots are seen dispersed within the PVDF spherulites. All these indicate the occurrence of crystallization of PBS. From these results, it is concluded that under these conditions no interspherulitic phase separation takes place. Also the existence of a well-defined spherulite boundary of PVDF spherulites implies that most of the PBS was ejected into the interlamellar and interfibrillar regions of the PVDF spherulites, instead of the interspherulitic regions as in the case of relatively lower supercooling mentioned above. Nevertheless, with careful inspection, an uneven birefringence increment of the PVDF spherulites could be identified. The increment of birefringence of PVDF spherulites around the periphery is somewhat more significant than at the center part. This is caused by the fact that the PBS content in the amorphous pockets of PVDF gets larger with increasing distance from the center of the PVDF spherulites. Therefore, the larger amount of PBS crystals formed after cooling to 80 °C contribute more to the birefringence. For a better understanding of the observed phenomenon, the location of PBS in banded PVDF spherulites on lamellar scale was monitored by AFM. Fig. 7c shows a representative AFM phase image of the 70/30 blend prepared as in Fig. 7a. The AFM scan was taken at 125 °C (above the melting temperature of PBS). It can be seen that alternative arrays of edge-on and flat-on lamellae coexist in this scanning area, which is typical lamellar organization model for banded spherulites. Compared with banded structures in neat PVDF (Fig. 4b), the boundary between edge-on and flat-on lamellae are now not sharply delineated. With careful inspection, continuous twist of some single lamellar sheets from flat-on into edge-on orientation, or vice versa, is discernable as indicated by the arrows in Fig. 7c. There exist numerous crevices in the interlamellar regions as well as uncrystallized pockets located in the interfibrillar area. After cooling to enable the crystallization of PBS, as shown in Fig. 7d, crystalline PBS filled the pockets and the interlamellar region of PVDF, as comparing the areas indicated by the circles and rectangles in Fig. 7c and 7d. This clearly indicates the occurrence of interlamellar and interfibrillar phase separation in PVDF/PBS blends under such condition, which consequently results in the intensity increase in the banded spherulites.
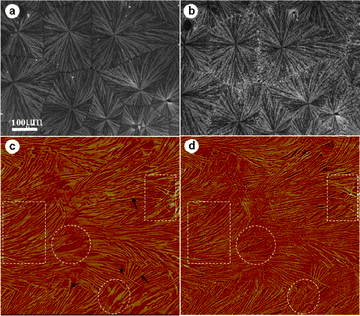 | ||
| Fig. 7 (a) Optical micrograph of a 70/30 blend crystallized at 155 °C and (b) then cooled quickly to 80 °C for 5min. (c) AFM phase image of the sample prepared as in (a) scanned at 125 °C. (d) The same scanning area of (c) after cooling to 80 °C for 5 min. The rectangles and the circles indicate the edge-on and flat-on lamellar regions, respectively. The black arrows in (c) indicate the twisted single lamellar sheets. | ||
As mentioned above, at a given blend ratio, the final morphology, especially the crystallization induced phase separation of the system is quite different under different condition. For instance, the 70/30 blend exhibits mainly interspherulitic phase separation at high crystallization temperature of PVDF (see Fig. 4d), while a dominated interlamellar or interfibrillar phase separation is found at low temperature, e.g. at 155 °C. Herein we discuss the morphologic development in this system as resulting from kinetic and mass balance inputs. In the present case, both the PVDF and PBS components are capable of crystallization over a wide range of composition. Since the two components cannot co-crystallize and the crystallization of each component occurs in well-separated temperature regions, the rejection of PBS by the crystallizing PVDF affects the kinetics and morphology of both PVDF and PBS, and thereby controls also the space available for subsequent crystallization of PBS. The phase separation behavior in crystalline blends during the crystallization of high-Tm component can be discussed in terms of the diffusion length, δ = D/G, where D is the diffusion coefficient of the noncrystallizable species (i.e.PBS in the present case) in the blend and G is the radial growth rate of the crystalline polymer spherulites (PVDF in the present case). The diffusion length is a quantitative measure of the distance a noncrystallizable polymer chain can move in the melt ahead of the crystallization front. If δ is sufficiently small, the noncrystallizable component can diffuse no farther than into the interlamellar region of the crystalline component. For an intermediate range of δ, the noncrystallizable component should locate between the fibrils, while at large δ, between spherulites.42–45 Therefore, the morphology formation of the blend under different conditions can be explained qualitatively on the basis of the diffusion length δ. Let's take 70/30 blend as an example. To estimate δ, the crystal growth rate G of PVDF at varying crystallization temperatures was measured. It is found that the growth rate of PVDF spherulites at 155 °C is about 2 orders of magnitude higher than that grown at 164 °C. At the same time, the diffusivity (D) of PBS melt in the front of growing PVDF crystals should decrease with decreasing temperature. Therefore, diffusion length (δ) could be increased by more than a factor of 100 as the crystallization temperature increased from 155 °C to 164 °C. If this is true, the PBS component will build up more and more ahead of growing PVDF spherulite and slow down its growth at higher crystallization temperature. In this case, a R ∝ t1/2 relationship between the spherulite radius and time should be observed.44–47 Otherwise, a steady state (R ∝ t) could be established due to the inclusion of PBS within the PVDF spherulites at lower crystallization temperature. These scenarios can be seen from the growth kinetics curves shown in Fig. 8. There we see that the PVDF spherulite radius increases with the square root of time at 162 °C, while the radius increases linearly with time at 150 °C.
 | ||
| Fig. 8 Spherulitic radius of PVDF versuscrystallization time in 70/30 blend crystallized at (a) 162 °C and (b) 150 °C. The line in (a) is the least squares parabolic fit and the line in (b) is the least squares linear fit. | ||
Fig. 9a shows the spherulitic morphology in a PBS-rich blend, i.e. 40/60 PVDF/PBS blend, under intermediate supercooling. Large ring banded spherulites with an even larger band period could be seen compared with that in PVDF-rich blend (Fig. 7a). Their ringed structures get coarse and much looser, and the boundaries become curved as well due to the dilute effect. Following crystallization of PVDF component, the blend is cooled rapidly to 80 °C, where the PBS component crystallizes isothermally. Several crystallized PBS domains were found to nucleate and grow continuously within the pre-existing PVDF spherulites until the domains impinge on each other (see Fig. 9b to 9d). These small domains were confirmed to be PBS as they melted at about 120 °C, while darker banded PVDF spherulites still existed. The brightness of the PVDF spherulites increases in the region where PBS has crystallized, and remains unchanged in the area where the growth front of PBS crystals has not yet reached. This implies that PBS crystallizes inside the PVDF spherulites and that the PBS crystals have taken the same orientation as the PVDF crystals; i.e., the PVDF crystals template the growth of PBS crystals. This case is quite different from that of Fig. 3c, since PBS growth confined in anisotropic PVDF banded spherulites instead of isotropic PVDF matrix shown in Fig. 3c. In fact, PBS exhibits an ellipsoidal spherulitic growth outline whose long axis coincides with the radial direction of the PVDF spherulites as seen under high-magnification view in the present case. Since the PBS grow outward in all directions simultaneously in the PVDF matrix, the PBS must have a larger growth rate along the direction parallel to the radial of PVDF spherulite than that perpendicular to it due to the differences in topological hindrance along different directions of the extend lamellae. This non-spherical growth habit also helps to confirm that the PBS grows in the matrix of PVDF instead of forming a layered structure where the spherulites of both components are merely superimposed on each other as two separate layers. In the later case, PBS spherulites should grow with circular boundaries because the subjacent PVDF lamellae do not influence the growth of PBS spherulites.
 | ||
| Fig. 9 (a) Optical micrographs of a 40/60 blend crystallized at 155 °C. (b), (c) and (d) present the nucleation and growth process of PBS in PVDF matrix after quenching to 80 °C. The crystallization time of PBS is (b) 3 min, (c) 5 min, and (d) 15 min. | ||
When the PVDF was crystallized at 140 °C, Fig. 10a, compact spherulites with clear Maltese-crosses were obtained. They have linear boundaries and completely lose their ring banded feature under such a high supercooling. On the other hand, when the PVDF in the 40/60 PVDF/PBS blend was first crystallized at the same temperature, i.e. 140 °C, coarser spherulites developed (Fig. 10b). These coarse spherulites consisting of lamellar bundles with a large cross section and the Maltese-crosses are completely lost, suggesting a random arrangement of lamellar bundles caused by the exclusion of PBS from the lamellar bundles of PVDF. This kind of exclusion of the noncrystalline component from the lamellar bundles has been confirmed by small-angle X-ray scattering experiments on the system of PMMA/PVDF.1
 | ||
| Fig. 10 Optical micrographs show (a) neat PVDF and (b) 40/60 PVDF/PBS blend crystallized at 140 °C. (c) 40/60 blend first crystallized at 120 °C and subsequently cooled to 80 °C for 2 min. (d) After 4 min at 80 °C. | ||
When the PVDF in a 40/60 PVDF/PBS blend was first crystallized at 120 °C, see the background of Fig. 10c and 10d, densely nucleated spherulites of PVDF with a final size of approximately 10 μm were created. After crystallization of PBS at 80 °C for 2 min (Fig. 10c) and 4 min (Fig. 10d), strong birefringence against the darker PVDF matrix was observed. One can see that the PBS nucleates randomly in this case. Their growth occurs with a circular front. Since the nucleation frequency of PBS is low enough, the PBS domains grow through many PVDF spherulites. Therefore, growth velocity of PBS is averaged over many PVDF intraspherulitic directions and the envelopes of the domains are circular.
The growth process of PBS crystals within the existing PVDF spherulites under present conditions has been followed by in situatomic force microscopy. To better monitor the growth process of PBS in lamellar resolved scale, a relatively high temperature, e.g. 95 °C, is chosen for the crystallization of PBS. Fig. 11 presents the typical and representative AFM images, showing the growth of PBS in a 40/60 blend with the PVDF matrix pre-crystallized at 120 °C. Fig. 11a is scanned at 120 °C, at which the PBS is still in the molten state. An admixture of edge-on and flat-on lamellae could be seen coexisting in the scan area. One can also find branched lamellar bundles, i.e. the fibrils with many interfibrillar molten pockets in the lower right quadrant. After quickly cooled to 95 °C, PBS starts to crystallize first in the interfibrillar molten zones of PVDF scaffold (Fig. 11b). As time goes on, PBS grows forward, and the rest molten space is gradually filled with PBS lamellae (Fig. 11c). The interfibrillar spaces in Fig. 11a are well interconnected and could serve as thoroughfare for PBS growing through. However, no sharply delineated growth front can be seen in present case; PBS lamellae are dispersed over the field of view. However, the polarized optical microscopic images, at a much lower magnification, show that a coherent front propagates. This front must be diffused at the scale of a few microns. It is by no means evident how the crystallization is sensed and triggered across these distances. The results given above do not permit us to distinguish among possible reasons.
 | ||
| Fig. 11 AFM phase images of a 40/60 blend with PVDF matrix first crystallized at 120 °C. (a) scanning at 120 °C. (b) and (c) are the growth process of PBS after cooling to 95 °C. The scanning time for each image is about 3 min and the interval between two sequential scans is about 9 min. | ||
With further increase of the PBS content up to 70%, i.e. a 30/70 PVDF/PBS blend, similar phase separation behavior was observed. At high PVDF crystallization temperature, e.g., at 150 °C see Fig. 12a, the PVDF grows in isolated spherulites with large interspherulitic space filled by the PBS melt. The PBS crystallizes then in these free spaces connecting the isolated PVDF spherulites (see Fig. 12b). At lower PVDF crystallization temperature, e.g., 100 °C, a situation essentially the same as that shown in Fig. 11 was observed.
 | ||
| Fig. 12 (a) optical micrographs of a 30/70 blend crystallized first at 150 °C for sufficient times and (b) then cooled quickly to 80 °C for 5 min to enable the crystallization of PBS. | ||
2. Mechanical properties
These affluent morphological changes observed in the above blends are expected to have significant meaning with regard to the mechanical properties of the system. Therefore, it is of great interest to follow the mechanical properties of this system under different conditions, which could correlate to different final morphologies of the blends. It should be mentioned first that we make no attempt to extend the practical usability of either of the components, but accentuate the correlation between the mechanical properties and morphologies of the blends formed under various conditions. Fig. 13 shows the tensile stress-strain curves of neat PVDF and PBS crystallized at varying temperatures (denoted by the short lines). It can be seen that the tensile behaviors of both pure PVDF and PBS are not sensitive to the crystallization temperatures. The PVDF (Fig. 13a) is somewhat more ductile than the PBS (Fig. 13b). Moreover, from Fig. 13a, homogeneous deformation is found for PVDF and there is no evident necking during the whole tensile process.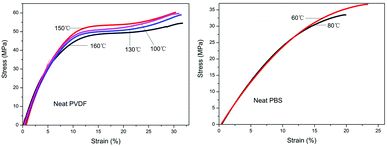 | ||
| Fig. 13 Stress-strain curves of (a) neat PVDF and (b) neat PBS crystallized completely at various temperatures indicated by the short lines. Curves obtained at room temperature. | ||
After blending with different amounts of PBS under different conditions, the tensile behaviors of the PVDF/PBS blends change significantly. Herein, we choose the PVDF-rich blend, e.g. 70/30 blend, and the PBS-rich blend, e.g. 30/70 blend, as models to illustrate the effect of PBS, and particularly the thermal treatment conditions on the mechanical properties of the systems. Fig. 14a shows the tensile stress-strain curves of the 70/30 PVDF/PBS blends crystallized first at varying temperatures as denoted aside each curve for sufficient time, and then cooled to 80 °C to enable the crystallization of PBS. One can see that by blending 30% PBS with PVDF, except for a tremendous increment in elongation at break (ca. 7 times larger than neat PVDF), different tensile behaviors depending on the crystallization temperature of PVDF are observed. This reflects the influence of morphology on the deformation behavior of the blends. From Fig. 14a, when the PVDF was crystallized at 160 °C and 155 °C followed by crystallization of PBS at 80 °C, the tensile stress-strain curves manifest a definite upper yield point after the elastic deformation, followed by a quick stress drop. Then clear necking and cold-drawing phenomena are observed; On the other hand, when the PVDF was crystallized at relatively low temperature, e.g. 130 °C and 100 °C, the stress continue to drop slowly during yielding, while the strain continue to increase substantially.
 | ||
| Fig. 14 Stress-strain curves of (a) 70/30 and (b) 30/70 PVDF/PBS blends pretreated at various temperatures for sufficient times followed by quickly cooled to 80 °C to enable complete crystallization of PBS. Curves were obtained at room temperature. | ||
As the strain reaches a certain point cold-drawing occurs. Without distinct yield point and necking, the materials start to behave as a macroscopically homogenous deformation. One may correlate these different mechanical behaviors to the crystallinity change of PVDF in the blend, since the yield and necking behaviors are greatly influenced by the crystallinity of the material. In the present case, the crystallinity of PVDF crystallized at 160 °C and 155 °C is lower than that at 130 °C and 100 °C. Taking into account that the heterogeneous deformation, which is preferential for the materials with high crystallinity, is observed for the blends prepared at high temperatures, the influence of crystallinity could be ruled out. Therefore, different mechanical behavior should relate to the morphological differences of the blends pretreated at different temperatures. For the blends pretreated at high temperatures, the PBS component mainly dispersed in the small interspherulitic space between the PVDF spherulites and the less ordered growth frontier of PVDF spherulites owning to the relatively large diffusion length. The PBS located at the small intersphrulitic regions and the spherulite margins of PVDF could act as glues connecting different spherulites of PVDF, and most likely be responsible for the distinct yielding and the sharp stress drop of the blends. As for the blends pretreated at relatively low temperatures (e.g. 130 °C and 100 °C), the PBS component distributes mainly within the PVDF spherulites, namely the interlamellar or interfibril regions, which does not influence the tensile behavior of the PVDF. Therefore, similar deformation behavior of the blends as the neat PVDF is observed. In this way, the temperature dependent interspherulitic-intraspherulitic transition of the distribution of PBS may cause the macroscopic heterogeneous–homogeneous transition of the deformation upon temperature. Fig. 14b shows the tensile behavior of 30/70 PVDF/PBS blends under different preparation conditions. Now the shapes of the stress–strain curves are similar for blends pretreated at different temperatures. However, the elongation at break increases with decreasing PVDF crystallization temperature. This can be explained in the follow way. Owing to the limited amount of PVDF in the blends, when crystallizing the PVDF at higher temperature, e.g. 150 °C, isolated PVDF spherulites are formed with the expelled PBS filling the large interspherulitic regions. These mechanically weak PBS regions are essentially responsible for the deformation behavior of the whole material. Therefore, an elongation at break similar to the pure PBS is obtained. On the contrary, with decreasing crystallization temperature of PVDF, loosely arranged PVDF small spherulites fill the whole space and form scaffold for the later crystallized PBS at lower temperature. This leads to the formation of an interlaced PVDF/PBS structure, which is responsible for an improvement of the elongation at break through a synergetic effect. The synergetic effect should be enhanced by fine dispersion of PBS in the PVDF at lower PVDF crystallization temperature, e.g. 100 °C, which results in a further enhancement of elongation at break of ca. 2 times.
Conclusions
In summary, we have systematically investigated the morphological features of PVDF/PBS blends by optical and atomic force microscopy under various conditions. It was found that neat PVDF forms large γ form spherulites with extraordinarily weak birefringence under extremely low supercooling. These spherulites are composed of face-on lamellae. Adding 30% PBS leads to a significant increase of the birefringence of γ PVDF spherulites, owing to the formation of some edge-on PVDF lamellae. After cooling to 80 °C, PBS crystals mainly distribute in the margin regions and the interspherulitic areas of PVDF spherulites due to its high diffusion ability at high temperature. In situAFM observation shows that the PBS disperses both between the edge-on lamellae and on the flat-on terraces which are arranged randomly in γ PVDF spherulites. Such irregular arrangement of the lamellae of γ spherulites in the blend causes the increment of the birefringence. Further increase of the PBS content to 60% favors the formation of sparsely dispersed ring banded PVDF spherulites at high temperature. While decreasing the crystallization temperature of PVDF a little bit, well organized ring banded α form spherulites with some sporadically dispersed γ crystals could be observed in neat PVDF. Blending with PBS results in the spherulites of both types (α and γ) being much coarser. They become more and more open as the distance from the center to the boundary increase. Subsequently crystallized PBS crystals were found to be expelled between the spherulites of PVDF, as well as in the less ordered edges of both kinds of spherulites. For the 40/60 blend, the large amount of PBS hinders the growth of PVDF spherulites significantly. Subsequent growth of PBS in the PVDF microcrystalline matrix was found different from that growing in the PVDF α spherulites. In the latter case, PBS grows much more slowly from the PVDF spherulite border to the center, and exhibits a nonlinear growth behavior duo to the formation of concentration gradient. The band period of α PVDF spherulites increases with crystallization temperature as well as the amount of PBS content. At relatively low PVDF crystallization temperature, PVDF in the 70/30 blend only forms volume-filling ring banded α spherulites with nonlinear boundaries. At this time, PBS distributes mainly in the interlamellar and interfibrillar regions of PVDF spherulites, and no interspherulitic phase separation was observed. The difference in the phase separation behavior of this blend was discussed in terms of the diffusion length. At very low PVDF crystallization temperature, neat PVDF completely loses its ring banded feature while coarse spherulites consisting of evident lamellar bundles could be found in the 40/60 blend. The growth of PBS confined in the non-banded PVDF matrix shows a circular growth front due to its velocity of growth is averaged over many PVDF intraspherulitic directions.Mechanical properties tests show that the deformation processes of neat PVDF and PBS are homogeneous, and their tensile behaviors are much less sensitive to the pretreated temperatures. However, the PVDF-rich blends pretreated at high temperatures undergo clearly necking and cold-drawing phenomena after yielding, while those pretreated at low temperatures have no distinct yield points, and behave as macroscopically homogenous deformation. The temperature dependent interspherulitic-intraspherulitic transition of the distribution of PBS causes the macroscopic heterogeneous–homogeneous transition under tensile. For the 30/70 blends, the stress–strain curves resemble that of neat PBS, showing homogeneous deformation without yielding or necking region. But the modulus and elongation at break increase with decreasing PVDF crystallization temperature. This originates from the resultant different morphologies of the blends.
Experimental
1. Materials
PVDF and PBS used in this work were purchased from Sigma-Aldrich Company and have a weight-average molecular weight of about 2.7 × 105 g mol−1 and 6.3 × 104 g mol−1, determined by gel permeation chromatography (GPC) with eluants of N,N-dimethyl formamide (DMF) and chloroform, respectively. The melting points were measured to be 186 °C for PVDF and 120 °C for PBS. Both of them were used as received.2. Samples preparation
Blends of PVDF and PBS were prepared by solution blending. Both of them were dissolved in DMF, which serve as a common solvent, with desired mass proportions (total polymer concentration was 20 mg mL−1). Thin films for optical microscopy (OM) and atomic force microscopy (AFM) observations were prepared by solution casting and spin-coating on glass slides, respectively. These films were allowed to dry under vacuum at 50 °C for 3 days. The thicknesses of the resultant films were estimated to be 10 μm for OM and 600 nm for AFM observations. The obtained films were heat-treated to 200 °C for 3 min to erase the thermal history of the samples and subsequently cooled to predetermined isothermal crystallization temperatures. Melt blending of PVDF/PBS blends for injection moulding was performed with a co-rotating twin screw extruder (TSE-30A). The diameter of the screw is 30 mm and the length-to-diameter ratio is 40/1. Temperatures along the barrel were 200, 210, 220, 230, 230, 230, 230, 220, 200 and 195 °C in sequence. The screw rotating and feeding speeds were set as 250 and 25 rpm, respectively. The dog-bone shape specimens with length of 20 mm, width of 4 mm and 2 mm at the neck position were made by using HAAKE MiniJet. The melt of the blends were first injected into the hot mould with preset temperature for sufficient times, and then quickly transferred to an environmental chamber which preset at 80 °C for the crystallization of PBS.3. Characterizations
An Olympus BH-2 microscope equipped with a Linkam LK-600PM temperature controller was used in this study to observe crystalline morphology. All of the optical micrographs shown in this paper were taken under crossed polarizers. Tapping-mode AFM images were obtained in the repulsive force region using a NanoScope III MultiMode AFM (Digital Instruments) equipped with a high-temperature heater accessory (Digital Instruments). Si cantilever tips (TESP) with a resonance frequency of approximately 300 kHz and a spring constant of about 40 Nm−1 were used. The scan rate varied from 0.7 to 1.2 Hz. The scanning density was 512 lines/frame. The set-point amplitude ratio, Asp/Ao, was adjusted to 0.6–0.9, where Asp is the set-point amplitude and Ao is the amplitude of the free oscillation. Mechanical tensile tests were performed at room temperature by using an Instron Universal Testing Machine (3365). The strain rate of 5mm min−1 was chosen for the tests.Acknowledgements
The financial support of the National Natural Science Foundations of China (No. 50833006, 20974011 and 50973008) and the program of Introducing Talents of Discipline to Universities (B08003) are gratefully acknowledged.References
- H. Saito and B. Stuhn, Macromolecules, 1994, 27, 216 Search PubMed.
- J. P. Runt, X. Zhang, D. M. Miley, K. P. Gallagher and A. Zhang, Macromolecules, 1992, 25, 3902 Search PubMed.
- I. S. Zemel and C. M. Roland, Polymer, 1992, 33, 3427 Search PubMed.
- D. J. Lohse and G. E. Wissler, J. Mater. Sci., 1991, 26, 743 Search PubMed.
- G. Crevecoeur and G. Groeninckx, Macromolecules, 1991, 24, 1190 Search PubMed.
- H. Marand and M. Gollins, ACS Polym. Prepr., 1990, 31, 552 Search PubMed.
- E. Blümm and A. Owen, Polymer, 1995, 36, 4077 Search PubMed.
- J. P. Penning and R. St. J. Manley, Macromolecules, 1996, 29, 84 Search PubMed.
- J. C. Lee, H. Tazawa, T. Ikehara and T. Nishi, Polym. J., 1998, 30, 327 CrossRef CAS.
- S. Hirano, Y. Nishikawa, Y. Terada, T. Ikehara and T. Nishi, Polym. J., 2002, 34, 85 Search PubMed.
- Z. Qiu, T. Ikehara and T. Nishi, Polymer, 2003, 44, 2799 CrossRef CAS.
- T. Ikehara, Y. Nishikawa and T. Nishi, Polymer, 2003, 44, 6657 Search PubMed.
- Z. Qiu, S. Fujinami, M. Komura, K. Nakajima, T. Ikehara and T. Nishi, Polymer, 2004, 45, 4515 CrossRef CAS.
- T. Ikehara, H. Kimura and Z. Qiu, Macromolecules, 2005, 38, 5104 Search PubMed.
- B.-J. Jungnickel, in Polymer Crystallization: Observations, Concepts and Interpretations, Springer Lecture Notes in Physics, ed. J.-U. Sommer and G. Reiter, Springer-Verlag, Berlin, 2002, ch. 12, pp. 207–238 Search PubMed.
- Polymer Blends Handbook, ed. L. A. Utracki, Springer, Heidelberg, 2003, vol. 1 and 2 Search PubMed.
- Z. Qiu, C. Z. Yan, J. M. Lu, W. T. Yang, T. Ikehara and T. Nish, J. Phys. Chem. B, 2007, 111, 2783 Search PubMed.
- Z. Qiu, C. Z. Yan, J. M. Lu and W. T. Yang, Macromolecules, 2007, 40, 5047 Search PubMed.
- M. Avella, E. Martuscelli and P. Greco, Polymer, 1991, 32, 1647 Search PubMed.
- L.-Z. Liu, B. Chu, J. P. Penning and R. St. Manley, Macromolecules, 1997, 30, 4398 Search PubMed.
- J. C. Lee, H. Tazawa, T. Ikehara and T. Nishi, Polym. J., 1998, 30, 780 CrossRef CAS.
- T. Ikehara and T. Nishi, Polym. J., 2000, 32, 683 Search PubMed.
- J. P. Liu and B.-J. Jungnickel, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 1921 Search PubMed.
- A. Kaito, Y. Iwakura, K. Hatakeyama and Y. Li, Macromolecules, 2007, 40, 2715–2759 Search PubMed.
- Y. Li, A. Kaito and S. Horiuchi, Macromolecules, 2004, 37, 2119 Search PubMed.
- A. Kaito, M. Shimomura, M. Akaba and S. Nojima, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 1959 Search PubMed.
- A. J. Lovinger, J. Polym. Sci. Part B: Polym. Phys., 1980, 18, 793–809 Search PubMed.
- B. S. Morra and R. S. Stein, Polym. Eng. Sci., 1984, 24, 311 Search PubMed.
- R. Gregorio and M. Cestari, J. Polym. Sci., Part B: Polym. Phys., 1994, 32, 859 Search PubMed.
- A. Lovinger, Polymer, 1980, 21, 1317 Search PubMed.
- D. Braun, M. Jacobs and G. P. Hellmann, Polymer, 1994, 35, 706 Search PubMed.
- T. Wang and S. Yan, Phys. Chem. Chem. Phys., 2009, 11, 1622 Search PubMed.
- D. C. Bassett, Proc. R. Soc. London, Ser. A, 1961, 377, 61 Search PubMed.
- Y. Okabe, H. Saito and T. Inoue, Polymer, 2010, 51, 1500 Search PubMed.
- Z. G. Wang and B. Z. Jiang, Macromolecules, 1997, 30, 6223 CrossRef CAS.
- C. Su and J. H. Lin, Colloid Polym. Sci., 2004, 283, 188.
- J. Xu, B. Guo, E. Chen, J. Zhou, L. Li and J. Wu, Polymer, 2005, 46, 9176 CrossRef CAS.
- H. J. Wang, Z. H. Gan, J. M. Schultz and S. K. Yan, Polymer, 2008, 49, 2342 CrossRef CAS.
- Z. G. Wang, X. H. Wang, D. H. Yu and B. Z. Jiang, Polymer, 1997, 38, 5899.
- Q. Xiao, S. Yan, K. D. Rogausch, J. Petermann and Y. J. Huang, Appl. Polym. Sci., 2001, 80, 1684 Search PubMed.
- J. P. Penning and R. St. J. Manley, Macromolecules, 1996, 29, 77 Search PubMed.
- H. D. Keith and F. J. Padden, J. Appl. Phys., 1964, 35, 1270 Search PubMed.
- S. D. Hudson, D. D. Davis and A. J. Lovinger, Macromolecules, 1992, 25, 1759 Search PubMed.
- H. Tanaka and T. Nishi, Phys. Rev. A: At., Mol., Opt. Phys., 1989, 39, 783 Search PubMed.
- J. M. Schultz, Polymer, 1991, 32, 3268 CrossRef CAS.
- T. Okada, H. Saito and T. Inoue, Macromolecules, 1990, 23, 3865 Search PubMed.
- J. M. Schultz, Polymer Crystallization, Oxford University Press, 2001, ch. 10 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
