DOI:
10.1039/C1PY00096A
(Paper)
Polym. Chem., 2011,
2, 1380-1388
Preparation of hyperbranched aromatic polyamide grafted nanoparticles for thermal properties reinforcement of epoxy composites
Received
3rd March 2011
, Accepted 22nd March 2011
First published on 2nd April 2011
Introduction
In the past decades, polymer/inorganic nanocomposites have emerged as a promising research area in the development of new materials for high added-value applications.1–6 These materials gain benefits by combining the merits of both organic (toughness, processability, etc.) and inorganic (rigidity, thermal stability, etc.) phases. The nature of the interface has been used to grossly divide these materials into two distinct classes.7,8 Firstly, only weak bonds (hydrogen, van der Waals or π–π bonds) between the matrix and filler are present. Secondly, the two phases are linked together through strong chemical bonds (covalent and ionic bonds). When polymer chains are tethered to the nanoparticle surface by covalent bond, a strong adhesion between nanoparticle and polymer matrix can be resulted. Generally, covalent attachment can be achieved by the so-called grafted procedure. Nanofillers, also at low concentrations, give nanocomposites an improvement of relevant properties such as increased modulus and strength, improved scratch, abrasion, solvent and heat resistance and decreased gas permeability. In particular, thermal properties are one of the most striking features of the nanocomposites. However, the claimed benefits of nanocomposites rely on a good dispersion of the nanoparticles. Previous studies demonstrated that hyperbranched polymer grafted nanoparticles could interfere with agglomeration of the nanomaterials and increase their surface affinities for organic solvent and polymer matrices.9–12 This is attributed to hyperbranched polymers with a highly branched structure and multiplicity of reactive end groups.13
This article describes the preparation of epoxy nanocomposites with hyperbranched aromatic polyamide grafted Al2O3 nanoparticles. Epoxy resins have long been applied in a variety of industrial applications, such as paints, coatings, inks, reactive diluents, vacuum pressure impregnation of coils, encapsulation of electronic circuit elements and printed circuit board coatings because of their good heat and chemical resistance, superior mechanical and electrical properties, as well as excellent processability.14–16 Despite many advantages of epoxy resin, certain properties, such as thermal and mechanical properties, electrical insulating properties as well as low thermal conductivity, should be improved to satisfy the demand of advanced microelectronic packaging.17 On the other hand, Al2O3 nanoparticles have good physical properties, such as abrasion resistance, corrosion resistance, thermal stability, electrical insulation, and relatively high thermal conductivity (about 30 W m−1 K−1), etc.18 Therefore, the inclusion of inorganic Al2O3 nanoparticles into an epoxy matrix for the formation of nanocomposites has proved to be an efficient way to overcome its drawbacks.19–24
By exploring the role of interfacial bonding in improving the performances of nanocomposites, this paper focuses on how compatibilization between the nanoparticles and matrix affects thermal properties. In order to generate strong interfacial interactions, the Al2O3 nanoparticles were grafted hyperbranched aromatic polyamide by two-step route with solution polymerization. Then the hyperbranched aromatic polyamide grafted Al2O3 nanoparticles were used to add in epoxy resin to afford the thermosets. The formation, structure and properties of nanoparticles and nanocomposites were addressed on the basis of Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance spectroscopy (NMR), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), thermal conductivity and dynamic mechanical analysis (DMA), etc. We probed their structure characteristics and thermal behavior of nanocomposites as a function of the hyperbranched aromatic polyamide grafted Al2O3 content with the aim of exploring the high performance materials, hoping this may help in the design not only of epoxy/Al2O3 nanocomposites but also of a wider range of polymer/inorganic nanocomposites.
Experimental
Materials
Al2O3
nanoparticles with an average diameter of 30 nm were obtained from Kaier Nano (Hefei, China). A cycloaliphatic epoxy resin (6105, DOW Chemicals, USA) along with hardener of methyl-hexahydrophthalic anhydride (MHHPA, Shanghai Li Yi Science & Technology Development Co. Ltd., China) was used in the present study. Neodymium(III) acetyulacetonate trihydrate (Nd(III)acac) purchased from Aldrich Chemicals was used as latent catalyst. γ-Aminopropyl-triethoxysilane (γ-APS) purchased from GE silicones was used as coupling agent. The grafting monomer, 3,5-diaminobenzoic acid (DABA, Tokyo Chemical Industry Co., Ltd., Japan) was purified by recrystallization from water and dried in a vacuum at 80 °C for 12 h. Lithium chloride (LiCl) was dried at 230 °C overnight before use. Other solvents, like triphenyl phosphate (TPP), N-methyl-2-pyrrolidone (NMP), N,N-dimethylacetamide (DMAc), N,N-dimethyform amide (DMF), methanol, acetone and pyridine were analytical grade, provided by Sinopharm Chemical Reagent co., Ltd, China, and were used without further purification.
Prior to silane modification, as received Al2O3 nanoparticles were dried in a vacuum oven at 180 °C for 24 h to remove moisture absorbed at the surface. 1 g of as received Al2O3 nanoparticles and an appropriate amount of the silane (0.5–1 wt% based on the weight of nanoparticles) were added into a 500 ml three-necked flask, equipped with a mechanical stirrer and a reflux condenser, and mixed in high purity dimethylbenzene by stirring at 110–120 °C for at least 4 h. After filtration, the γ-APS treated Al2O3 nanoparticles (named Al2O3-APS) were washed several times with dimethylbenzene to remove the unreacted siloxane moieties and dried in a vacuum oven at 120 °C for 2 h to remove the residual solvent.
Grafting of hyperbranched aromatic polyamide from Al2O3 nanoparticles surface
In a flask, 0.76 g Al2O3-APS and 0.76 g DABA were charged in 10 mL NMP and stirred until the DABA was dissolved completely. The 2.5 mL of pyridine and 2.6 mL of TPP were charged into the flask. The solution was heating to 100 °C and stirred under nitrogen for 3 h. After temperature was decrease to room temperature, the solution was poured into 50 mL of methanol to precipitate the polymer. The polymer was collected by filtration and purified by reprecipitaion from DMF solution into methanol containing 0.1% LiCl. The product was finally filtered and washed with cold methanol and dried in a vacuum at 90 °C to a constant weight. The Al2O3-APS grafted hyperbranched aromatic polyamide was denoted as Al2O3-HBP in the following text.
Preparation of epoxy/Al2O3-HBP nanocomposites
The epoxy/Al2O3-HBP nanocomposite was prepared as follows. Firstly, the required quantity of Nd(III)acac was added to the epoxy resin, stirred and degassed at 80 °C in a three-necked flask. The homogeneous solution was then cooling down to ambient temperature. Secondly, a desired amount of Al2O3-HBP was ultrasonically dispersed in acetone for 1 h at room temperature. The Al2O3-HBP/acetone suspension was then added to the pretreated epoxy resin step by step under stirring. Afterwards, the temperature was increased to 60 °C and the mixture was sonicated for 1 h, followed by intensive mechanical stirring for 1 h to ensure good homogeneity and remove the acetone entirely. Thirdly, MHHPA was added under vigorous mechanical stirring. Subsequently, the solution was degassed for 30 min. Finally, the mixture was poured onto preheated stainless steel molds, pre-cured in an oven at 135 °C for 2 h, followed by a post-cure at 165 °C for 14 h. The molds were left in the oven and allowed to cool gradually to room temperature. The experimental details of the process of formation of epoxy/Al2O3-HBP nanocomposites are shown in Scheme 1. Nanocomposites containing different weight fractions (5%, 10%, 15% and 20%) of the as received Al2O3 and Al2O3-HBP were prepared.
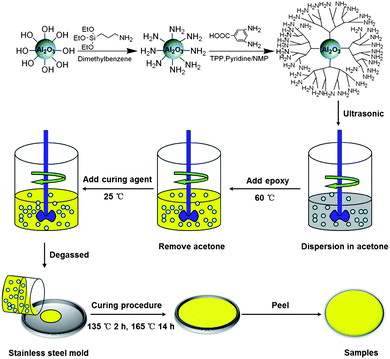 |
| | Scheme 1 Preparation process of the epoxy/Al2O3-HBP nanocomposites. | |
Characterization
Fourier-transform infrared (FT-IR) was conducted with a Perkin-Elmer Paragon 1000 instrument over the range of 4000–400 cm−1. Nuclear magnetic resonance (1H-NMR) measurement was conducted on a Varian mercury plus-400 spectrometer with DMSO-d6 as the solvent. The average size and size distribution of the nanoparticles were determined by dynamic light scattering method using a Nano-ZS90 ZetaSizer instrument (Malvern Instruments Ltd., UK). Transmission electron microscopy (TEM, JEM-2100, Japan) was used to observe the dispersion of the nanoparticles in the ethanol and in the composite. The specimens (about 70 nm in thickness) for TEM observation were trimmed using a microtome machine with a diamond knife. Field emission scanning electron microscope (FE-SEM, JEOL JEM-7401, Japan) was used to observe the dispersion of the nanoparticles in the composite samples. Samples were sputtered with thin layers of gold to avoid the accumulation of charge. Thermal gravimetric analyses (TGA) were performed with a Netzsch TG-209 F3 instrument (NETZSCH, Germany) at a heating rate of 20 °C min−1 in nitrogen atmosphere. Differential scanning calorimetry (DSC, 200 F3, NETZSCH, Germany) was performed at temperatures from 20 to 250 °C at a heating rate of 10 °C min−1 under a nitrogen atmosphere. Thermal conductivities of the nanocomposites were measured with LFA 447 Nanoflash (NETZSCH, Germany) according to ASTM E1461, using the measured heat capacity and thermal diffusivity, with separately entered density data. Samples were prepared in cylindrical shape of 12.7 mm in diameter and 1.0–2.0 mm in thickness. Dynamic mechanical analysis (DMA) was performed on a DMA Q800 dynamic mechanical analyzer (TA Instruments, USA), operating in the single-cantilever mode at an oscillation frequency of 1 Hz. Testing temperature was set as from room temperature to 250 °C at a heating rate of 3 °C min−1.
Results and discussion
Characteristics of the surface modified Al2O3 nanoparticles
In present work, the amino groups were firstly introduced onto the Al2O3 particles surface as an initiator site by the treatment of Al2O3 with γ-APS, and then hyperbranched polyamide was grafted from these amino groups. The FTIR spectra of as received Al2O3, Al2O3-APS and Al2O3-HBP nanoparticles are shown in Fig. 1. In as received Al2O3 nanoparticles, the strong absorption at 3468 cm−1 is attributed to hydroxyl group (–OH) peak stretching and peak at 1637 cm−1 is due to –OH bending. The spectra of silane-modified Al2O3 nanoparticles are very similar to those of as received Al2O3 nanoparticles, but some differences can still be detected. The peak appears at 2925 and 2854 cm−1 which are characteristics of asymmetric and symmetric –CH2 stretching vibrations respectively. The appearance of a band at 1490 cm−1 is due to –CH2 bending (scissoring) vibration. Bands at 1567 and 1120 cm−1 are due to N–H bending (scissoring) and C–N stretching respectively. For the spectrum of Al2O3-HBP nanoparticles, the peak at 1651 and 1549 cm−1 can be assigned to amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching. The peak at 1603 and 1446 cm−1 which are characteristics of aromatic structure. These changes of characteristic peaks indicate that silane and hyperbranched aromatic polyamide have already been grafted successfully on the surface of Al2O3 nanoparticles.
O stretching. The peak at 1603 and 1446 cm−1 which are characteristics of aromatic structure. These changes of characteristic peaks indicate that silane and hyperbranched aromatic polyamide have already been grafted successfully on the surface of Al2O3 nanoparticles.
 |
| | Fig. 1
FTIR spectra of as received Al2O3, Al2O3-APS and Al2O3-HBP. | |
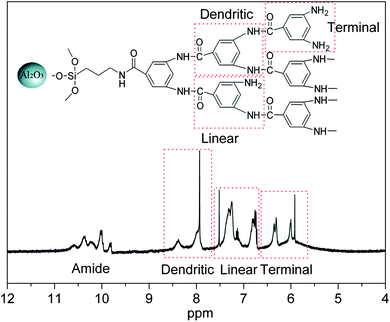
1H-NMR
spectra provide more detailed evidence to prove that hyperbranched aromatic polyamide is successfully grafted onto the surface of Al2O3 nanoparticles. As can be seen from Fig. 2, the characteristic peaks from the aromatic protons are clearly detected in the 1H-NMR spectra of Al2O3-HBP nanoparticles.12,25 The hyperbranched aromatic polyamide shows three of aromatic proton peaks, which can be attribute to linear, dendritic and terminal units. The peaks at 8.3 and 7.9 ppm are attributed to the aromatic protons of the dendritic units. Meanwhile the peaks at 7.3 and 6.8 ppm which are characteristics of the aromatic protons of linear units and those at 6.3 and 6.0 ppm can be assigned to the aromatic protons of terminal units. In addition, the proton peaks of the amide group (amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 10.6–9.8 ppm are also observed clearly, but the signals of terminal amine groups at 5.15 ppm are very weak.
O) at 10.6–9.8 ppm are also observed clearly, but the signals of terminal amine groups at 5.15 ppm are very weak.
The TGA data of as received Al2O3, Al2O3-APS and Al2O3-HBP are listed in Fig. 3. It can be seen from Fig. 3 that the weight loss of the nanoparticles show the order of as received Al2O3 < Al2O3-APS < Al2O3-HBP at 800 °C. The weight grafting ratios (Gr) can be calculated by using the following equation.26,27
| |  | (1) |
Where
WAl2O3-HBP,T and
WAl2O3,T are the percent weight loss of Al
2O
3-HBP and as received Al
2O
3 particles between 50 °C and temperature
T. On this basis, the value of Gr(%) for the Al
2O
3-HBP particles was 38.8% according to
eqn (1). Meanwhile the initial decomposition temperature of Al
2O
3-HBP particles was estimated to be 407 °C. These
TGA data present further evidence to prove the hyperbranched aromatic
polyamide have been grafted successfully on the surface of Al
2O
3 nanoparticles.
 |
| | Fig. 3
TGA curves of as received Al2O3, Al2O3-APS and Al2O3-HBP measured in N2. | |
Microscopy is very useful for the characterization of generated filler structures. The TEM images obtained for the as received Al2O3 and Al2O3-HBP particles dispersed in ethanol are compared in Fig. 4. The inset in Fig. 4(a) and 4(b) illustrates the identical selected area electron diffraction (SAED) patterns for both Al2O3 powders, which indicates that only the surface of the Al2O3 particles was modified by hyperbranched polyamide leaving the particle core structure intact. Comparison of TEM imaged Fig. 4(a) and 4(b) shows that the surface modification dramatically affects the aggregate particle size, which after grafted hyperbranched polyamide is reduced by more than 10 times from the micrometre to tens of nanometres scale. Fig. 4(c) depicts the HRTEM image of Al2O3-HBP particles. It can be obviously seen that a thin polymer layer was observed on the surface of Al2O3 particles. The success in preparing hyperbranched aromatic polyamide grafted on the surface of Al2O3 nanoparticles was further confirmed by the elemental signatures of C, Si, Al, and O in the EDS as shown in inset of Fig. 4(c). The peaks of Cu are from the copper grid. The aggregate particle size reduction by surface modification is further supported by the results of dynamic light scattering measurement. Fig. 5 shows that a peak with large particle size is observed for as received Al2O3 nanoparticle, indicating that the Al2O3-HBP nanoparticles have better dispersibility in ethanol.
 |
| | Fig. 4
TEM images of particles. (a) As received Al2O3 particle, inset: corresponding SAED patterns. (b) Al2O3-HBP, inset: corresponding SAED patterns. (c) High-resolution TEM image of Al2O3-HBP, inset: corresponding EDS patterns. | |
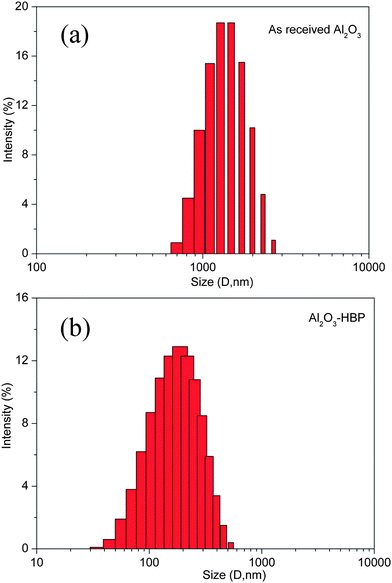 |
| | Fig. 5 The particle size and its distribution of Al2O3 nanoparticles in ethanol, (a) as received Al2O3 particle and (b) Al2O3-HBP particle. | |
Dispersion of Al2O3-HBP particles in epoxy matrix
The morphology of the fractured surfaces for the neat epoxy and 15 wt% epoxy/Al2O3-HBP nanocomposite are showed in Fig. 6, respectively. In the case of the neat epoxy, the fractured paths of river line patterns can be observed, indicating brittle fracture. The nanocomposite fail in brittle manner due to a crack deflection mechanism caused by the Al2O3-HBP particles. This crack deflection will increase the roughness of the fracture surfaces due to the tilting and twisting of the crack front. The inset in Fig. 6(b) shows that no obvious naked particles and inorganic clusters are present, suggesting that covalent bonding exists between epoxide groups in the epoxy matrix and amine groups on the Al2O3-HBP particles. The covalent bonding provided strong interfacial interaction between Al2O3-HBP particles and epoxy matrix. Consequently, the hyperbranched aromatic polyamide grafted Al2O3 nanoparticles can significantly improve the dispersion and interfacial bonding in composites.
 |
| | Fig. 6
SEM images of fractured surface of (a) neat epoxy and (b) 15 wt% epoxy/Al2O3-HBP nanocomposite. The inset in (b) is a magnification of the area indicated. | |
The good dispersion in the epoxy matrix is further supported by the TEM results. TEM can be used to observe the morphology of the nanocomposite, which indicates the dispersion abilities of 10 wt% as received Al2O3 particles and 10 wt% Al2O3-HBP particles in the epoxy matrix, as shown in Fig. 7. Fig. 7(a) shows the TEM images of the epoxy composites with 10 wt% as received Al2O3 particles, which show the form of large conglobations and agglomerates. The Al2O3-HBP particles exhibit better dispersion than as received Al2O3 particles in the epoxy matrix due to the existence of the amine groups on the surface of Al2O3 particles which results in reduced surface free energy and facilitates particle dispersion, as shown in Fig. 7(b). Though the Al2O3-HBP particles are not dispersed individually, it presents a high degree dispersion and the size of particle agglomerates is less or around 100 nm. No serious agglomerations, such as microsize agglomerates, are observed for the epoxy/Al2O3-HBP nanocomposite. As reported previously, the hyperbranched aromatic polyamide had a good solubility in organic solvents, such as DMF, DMAc, m-cresol, and methoxyethanol.25,28 The good solubility of the hyperbranched aromatic polyamide in organic solvents can possess good dispersion in epoxy matrix, and the better interfacial adhesion of Al2O3-HBP particles in epoxy monomer exhibits the higher viscosity than neat epoxy resin, which can prevent Al2O3-HBP particle reaggregation during the curing process. In summary, the results of SEM and TEM indicate that Al2O3-HBP particles are well dispersed and possess good compatibility with the epoxy matrix.
 |
| | Fig. 7
TEM images of (a) 10 wt% epoxy/Al2O3 nanocomposite and (b) 10 wt% epoxy/Al2O3-HBP nanocomposite. The insets in (a) and (b) are magnifications of the areas indicated. | |
Glass transition temperatures of nanocomposites
The DSC thermograms of cured neat epoxy and epoxy/Al2O3-HBP nanocomposites with different Al2O3-HBP contents are shown in Fig. 8. It is known that the glass transition temperature increases, keeping a good correlation with the crosslinking density.29 Since the degree of crosslinking in the epoxy matrix is not uniform, the glass temperature transition occurs in a wide temperature range. Therefore, three characteristic temperatures, namely the onset temperature, the intermediate temperature, and the end temperature, are observed from the DSC spectra. Here the intermediate temperature is denoted as the glass transition temperature. As compared to the neat epoxy, an obvious increase of Tg is observed for epoxy/Al2O3-HBP nanocomposites, indicating that the improvement in crosslinking density in the epoxy matrix caused by the amino groups on the surface of Al2O3-HBP nanoparticles involving in a ring-opening etherification reaction. This effect can be understood in terms of decreasing the free volume.30 Meanwhile, the Tgs of the epoxy/Al2O3-HBP nanocomposites were decreased with increasing the content of Al2O3-HBP nanoparticles. There could be the main reason that Al2O3-HBP nanoparticles agglomerate in the epoxy matrix. Once nanoparticles agglomerate, the interactions are more between Al2O3-HBP nanoparticles rather than Al2O3-HBP nanoparticles and epoxy matrix. The agglomerated Al2O3-HBP nanoparticles cannot impose any restrictions on the mobility of epoxy, and therefor higher loading of Al2O3-HBP nanoparticles results in the lower Tg.
 |
| | Fig. 8
DSC curves of neat epoxy and epoxy/Al2O3-HBP nanocomposites with different Al2O3-HBP contents. | |
Thermal stability of nanocomposites
Thermal stability is very important for polymeric materials. In the present study, the thermal stability of the epoxy/Al2O3-HBP nanocomposites has been investigated by TGA by using heating rate of 20 °C min−1. Fig. 9 shows the TG and DTG thermograms of the epoxy/Al2O3-HBP nanocomposites. The weight loss of the nanocomposites due to degradation is monitored as a function of temperature. The characteristic thermal parameters selected were the temperature for 5% weight loss and 50% weight loss and maximum degradation temperature, which is the highest thermal degradation rate temperature. The results are summarized in the Table 1. When the temperature was raised to above 250 °C, the neat epoxy matrix began to decompose and the decomposition temperature became higher when the Al2O3-HBP nanoparticles were added into the epoxy matrix. At same temperature, the TGA curves of the nanocomposites indicated that the weight loss of the nanocomposites was less than that of the neat of epoxy matrix. In other words, the epoxy/Al2O3-HBP nanocomposites have a higher decomposition temperature when the neat epoxy matrix loses the same weight. For instance, incorporation of 5 wt% Al2O3-HBP nanoparticles into the epoxy matrix significantly improves the thermal stability because it increases the temperature at 5% weight loss by 23.3 °C. The maximum degradation temperature (Tmax) was also increased by addition of the Al2O3-HBP nanoparticles. It is worth pointing out that the thermal stability of epoxy/Al2O3-HBP nanocomposites was decreased with the increasing Al2O3-HBP contents. The epoxy/Al2O3-HBP nanocomposites exhibit step weight loss and, as expected, the thermal degradation of epoxy/Al2O3-HBP nanocomposites is two stages between 250 and 550 °C. The significant weight loss occurring in the first stage, between 250 and 400 °C, is attributed to the decomposition reaction of epoxy network.31 In the second stage, from 400 to 550 °C, is attributed to the degradation of hyperbranched aromatic polyamide. In addition, the amount of Al2O3 nanoparticles in the nanocomposites could be caculated accurately in the TGA plots. The experimental results show that the residual weight percent at 700 °C of the epoxy/Al2O3-HBP nanocomposites increases upon addition of increasing amount of Al2O3-HBP fillers.
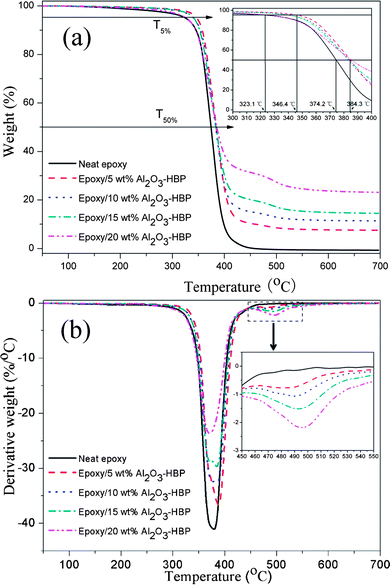 |
| | Fig. 9 (a) TGA and (b) DTG curves of the epoxy/Al2O3-HBP nanocomposites. | |
Table 1 thermal properties of neat epoxy and epoxy/Al2O3-HBP nanocomposites
|
Al2O3-HBP (wt%) |
Weight loss temperature/°C |
Glass transition temperature/°C |
|
T
5%
|
T
50%
|
T
max
|
T
g (DSC) |
ΔTg (DSC) |
T
g (DMA) |
ΔTg (DMA) |
| 0 |
323.1 |
374.2 |
379.8 |
191.7 |
— |
204.9 |
— |
| 5 |
346.4 |
384.3 |
388.1 |
205.6 |
13.9 |
218.1 |
13.2 |
| 10 |
338.3 |
385.2 |
385.2 |
200.6 |
8.9 |
216.0 |
11.1 |
| 15 |
341.1 |
383.8 |
384.7 |
198.3 |
6.6 |
208.7 |
3.8 |
| 20 |
326.2 |
383.7 |
370.7 |
197.2 |
5.5 |
208.1 |
3.2 |
Thermal conductivity of nanocomposites
The thermal conductivity of epoxy nanocomposites was measured by the laser flash method, as shown in Fig. 10. Fig. 10(a) shows the variation of thermal conductivity of epoxy/Al2O3-HBP nanocomposite as a function of temperature.
 |
| | Fig. 10 (a) Thermal conductivity of epoxy/Al2O3-HBP nanocomposite as a function of test temperature. (b) Thermal conductivity and thermal conductivity enhancement of nanocomposites with various contents at 25 °C. | |
The effective thermal conductivity for neat epoxy was found to be 0.236 W m−1 K−1 at 25 °C and increases with temperature over the temperature range investigated. The thermal conductivity of epoxy/Al2O3-HBP nanocomposites exhibits temperature dependences similar to the neat epoxy, although the values of 5 wt% epoxy/Al2O3-HBP nanocomposite and 20 wt% epoxy/Al2O3-HBP nanocomposite deviates from this trend at 100 °C. Meanwhile, the thermal conductivity of epoxy/Al2O3-HBP nanocomposites increases with increasing Al2O3-HBP content for all samples. The results are consistent with the general trend for highly disordered dielectric materials.32
Fig. 10(b) compares the thermal conductivity and thermal conductivity enhancement of epoxy-based nanocomposites prepared with the as received Al2O3 particles and Al2O3-HBP particles at 25 °C. Here, λ enhancement (%) = (λ1 − λ0)/λ0, λ0 is the thermal conductivity of the neat epoxy matrix (0.236 W m−1 K−1) and λ1 is the thermal conductivity of the nanocomposites due to the addition of fillers at 25 °C. The results show that there are a 69% enhancement in thermal conductivity for epoxy/Al2O3-HBP nanocomposite and only a 21% enhancement in thermal conductivity for epoxy/Al2O3 nanocomposite by adding the same quantity (20 wt%). Clearly, the Al2O3-HBP fillers provide substantially greater thermal conductivity enhancement when embedded into epoxy as compared to as received Al2O3 fillers. The experimental measurements of thermal conductivity for epoxy/Al2O3-HBP nanocomposites show higher thermal conductivity than epoxy/Al2O3 nanocomposites, which may be attributed to two reasons. First, the rigid linkage between Al2O3 and epoxy matrix which provides good interface compatibility that may reduce interfacial thermal resistance. Second, the good interface compatibility allows Al2O3-HBP particles to disperse well in the epoxy matrix. The Al2O3-HBP particles are well dispersed and possess good compatibility in epoxy matrix confirmed by the above SEM and TEM results. A model was proposed to present the efficient network for heat flow in the nanocomposite is shown in Fig. 11. The model of heat flow for nanocomposites shows the three phenomena as follows: (1) the hyperbranched aromatic polyamide layer on the surface of Al2O3 particles; (2) good dispersion leading to the higher contact area between Al2O3-HBP particles and epoxy; (3) the strong covalent bonding formation between Al2O3-HBP particles and epoxy matrix during the process of the epoxy curing reaction. As reported previously, the polymer layer on the surface of particles will decrease the scattering for the phonon scattering.33 Thus, this can explain that better Al2O3-HBP particle dispersion causes greater enhancement of thermal conductivity in the epoxy nanocomposites. Moreover, the strong covalent bonding between Al2O3-HBP particles and epoxy matrix forms a heat flow network that can reduce the large number of interfacial thermal resistance throughout the epoxy/Al2O3-HBP nanocomposites. As a result, the epoxy/Al2O3-HBP nanocomposites exhibit higher thermal conductivities than the epoxy/Al2O3 nanocomposites.
 |
| | Fig. 11 The model of heat flow for nanocomposites (a) epoxy/Al2O3 and (b) epoxy/Al2O3-HBP. | |
Thermomechanical properties of nanocomposites
Dynamic mechanical analysis (DMA) was performed to obtain the temperature dependent properties of materials such as the storage modulus and the tanδ, as shown in Fig. 12. The dynamic properties reflect the amount of the energy in the composite stored as elastic energy and the amount of energy dissipated during the strain process. These properties are highly dependent on the existence of fillers: dispersion within the matrix, volume fraction, geometrical characteristics, and load transfer from the filler to the matrix.34Fig. 12(a) shows the storage modulus of the neat epoxy and its nanocomposites. The addition of Al2O3-HBP fillers to epoxy resin showed influence irregularly on storage modulus in the glass region. In contrast, there was a stronger effect of Al2O3-HBP fillers in the rubbery region at elevated temperatures where the improvement in the elastic properties of nanocomposites was clearly observed. This phenomenon can be explained in terms of interfacial interactions between the Al2O3-HBP fillers and epoxy. The covalent bonding formation between Al2O3-HBP particles and epoxy matrix reduced the mobility of the local matrix material around the Al2O3-HBP particles, increasing the thermal stability at elevated temperatures.
 |
| | Fig. 12 (a) Storage modulus and (b) loss factors of epoxy/Al2O3-HBP nanocomposite as a function of test temperature. | |
The glass-transition temperature (defined as the temperature at which maximum tanδ is reached) is an indicator of the degree of crosslinking in the epoxy/Al2O3-HBP nanocomposites. The higher glass-transition temperature reflects a higher crosslinking density. As shown in Fig. 12(b), it is interesting to note the maximum value of tanδ for the epoxy/Al2O3-HBP nanocomposites is higher than that of the neat epoxy. Moreover, the magnitude of tanδ is lower when Al2O3-HBP fillers are incorporated, confirming the interaction between Al2O3-HBP particles and epoxy matrix again. The Tg of 5 wt% epoxy/Al2O3-HBP nanocomposite is 218.1 °C, about a 13.2 °C increase compared to that of neat epoxy. A comparison of the Tg values from the DMA and DSC data is obtained in Table 1. While the absolute magnitudes of Tg measured by these different methods differ, the trend in terms of changes of Tg from the neat epoxy are quite similar. It should also be noted that the glass transition is a diffuse thermodynamic transition and measurement method and sample preparation always affect the absolute value of Tg obtained.
Conclusions
In order to obtain uniform dispersion of Al2O3 nanoparticles in the epoxy matrix and combine the design of Al2O3-epoxy interfacial interaction with the demand for the improvement of the thermal properties of the nanocomposites, we carried out hyperbranched aromatic polyamide grafting of Al2O3 nanoparticles. In the grafting procedure, the Al2O3 nanoparticles were firstly treated with a silane coupling agent to generate amine groups on their surface, and then grafting of the hyperbranched aromatic polyamide started from the modified surface by solution polymerization. The results of FTIR, NMR and TGA showed hyperbranched aromatic polyamide grafted Al2O3 nanoparticles were successfully prepared by solution polymerization. TEM showed that there was a thin polymer layer on the Al2O3 nanoparticles surface, which contributes to the uniform dispersion of Al2O3 nanoparticles in epoxy matrix and the improvement of the interfacial interaction between Al2O3 nanoparticles and epoxy matrix. Thus, the Tgs, thermal stability and dynamic mechanical properties of the epoxy/Al2O3-HBP nanocomposites showed an obvious increase compared to the neat epoxy. In addition, the epoxy nanocomposites containing Al2O3-HBP nanoparticles exhibited higher thermal conductivity than those containing as received Al2O3 nanoparticles.
References
- C. M. Chan, J. Wu, J. X. Li and Y. K. Cheung, Polymer, 2002, 43, 2981–2992 CrossRef CAS.
- G. Kickelbick, Prog. Polym. Sci., 2003, 28, 83–114 CrossRef CAS.
- M. J. MacLachlan, I. Manners and G. A. Ozin, Adv. Mater., 2000, 12, 675–681 CrossRef CAS.
- J. Pyun, T. Kowalewski and K. Matyjaszewski, Macromol. Rapid Commun., 2003, 24, 1043–1059 CrossRef CAS.
- C. Sanchez, B. Lebeau, F. Chaput and J. P. Boilot, Adv. Mater., 2003, 15, 1969–1994 CrossRef CAS.
- C. Sanchez, G. J.d. A. A. Soler-Illia, F. Ribot, T. Lalot, C. R. Mayer and V. Cabuil, Chem. Mater., 2001, 13, 3061–3083 CrossRef CAS.
- S. Barus, M. Zanetti, M. Lazzari and L. Costa, Polymer, 2009, 50, 2595–2600 CrossRef CAS.
- C. Sanchez, B. Julian, P. Belleville and M. Popall, J. Mater. Chem., 2005, 15, 3559–3592 RSC.
- Y. Kaneko, Y. Imai, K. Shirai, T. Yamauchi and N. Tsubokawa, Colloids Surf., A, 2006, 289, 212–218 CrossRef CAS.
- B. Mu, T. Wang and P. Liu, Ind. Eng. Chem. Res., 2007, 46, 3069–3072 CrossRef CAS.
- Y. Taniguchi, K. Shirai, H. Saitoh, T. Yamauchi and N. Tsubokawa, Polymer, 2005, 46, 2541–2547 CrossRef CAS.
- Y. Yu, M. Z. Rong and M. Q. Zhang, Polymer, 2010, 51, 492–499 CrossRef CAS.
- Y. Zhou, W. Huang, J. Liu, X. Zhu and D. Yan, Adv. Mater., 2010, 22, 4567–4590 CrossRef CAS.
-
N. Kinjo, M. Ogata, K. Nishi and A. Kaneda, Speciality Polymers/Polymer Physics, Springer Berlin/Heidelberg, 1989, vol. 88, pp. 1–48 Search PubMed.
- M. Xie and Z. Wang, Macromol. Rapid Commun., 2001, 22, 620–623 CrossRef CAS.
- M. Xie, Z. Wang and Y. Zhao, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 2799–2804 CrossRef CAS.
- Z. Tao, S. Yang, J. Chen and L. Fan, Eur. Polym. J., 2007, 43, 1470–1479 CrossRef CAS.
- C. H. Chen, J. Y. Jian and F. S. Yen, Composites, Part A, 2009, 40, 463–468 CrossRef.
- Z. Guo, T. Pereira, O. Choi, Y. Wang and H. T. Hahn, J. Mater. Chem., 2006, 16, 2800–2808 RSC.
- S. Gupta, P. C. Ramamurthy and G. Madras, Polym. Chem., 2011, 2, 221–228 RSC.
- M. Kurimoto, H. Okubo, K. Kato, M. Hanai, Y. Hoshina, M. Takei and N. Hayakawa, IEEE Trans. Dielectr. Electr. Insul., 2010, 17, 1268–1275 CrossRef CAS.
- W. Z. Jiao, Y. Z. Liu and G. S. Qi, Compos. Sci. Technol., 2009, 69, 391–395 CrossRef CAS.
- W. Naous, X. Y. Yu, Q. X. Zhang, K. Naito and Y. Kagawa, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 1466–1473 CrossRef CAS.
- S. Singha and M. J. Thomas, IEEE Trans. Compon. Packag. Technol., 2010, 33, 373–385 CrossRef CAS.
- Y. Ishida, A. C. F. Sun, M. Jikei and M.-a. Kakimoto, Macromolecules, 2000, 33, 2832–2838 CrossRef CAS.
- Y. Zhao and S. Perrier, Macromolecules, 2006, 39, 8603–8608 CrossRef CAS.
- Y. Zhao and S. Perrier, Macromolecules, 2007, 40, 9116–9124 CrossRef CAS.
- M. Jikei, K. Fujii, G. Yang and M.-a. Kakimoto, Macromolecules, 2000, 33, 6228–6234 CrossRef CAS.
- N. Tagami, M. Hyuga, Y. Ohki, T. Tanaka, T. Imai, M. Harada and M. Ochi, IEEE Trans. Dielectr. Electr. Insul., 2010, 17, 214–220 CrossRef CAS.
- T. Zhou, X. Wang, X. Liu and D. Xiong, Carbon, 2009, 47, 1112–1118 CrossRef CAS.
- J. H. Yu, J. K. Duan, W. Y. Peng, L. C. Wang, P. Peng and P. K. Jiang, eXPRESS Polym. Lett., 2011, 5, 132–141 Search PubMed.
- M. T. Hung, O. Choi, Y. S. Ju and H. T. Hahn, Appl. Phys. Lett., 2006, 89, 023117 CrossRef.
- S. Y. Yang, C. C. M. Ma, C. C. Teng, Y. W. Huang, S. H. Liao, Y. L. Huang, H. W. Tien, T. M. Lee and K. C. Chiou, Carbon, 2010, 48, 592–603 CrossRef CAS.
- Y. Zhao and E. V. Barrera, Adv. Funct. Mater., 2010, 20, 3039–3044 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching. The peak at 1603 and 1446 cm−1 which are characteristics of aromatic structure. These changes of characteristic peaks indicate that silane and hyperbranched aromatic polyamide have already been grafted successfully on the surface of Al2O3 nanoparticles.
O stretching. The peak at 1603 and 1446 cm−1 which are characteristics of aromatic structure. These changes of characteristic peaks indicate that silane and hyperbranched aromatic polyamide have already been grafted successfully on the surface of Al2O3 nanoparticles.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 10.6–9.8 ppm are also observed clearly, but the signals of terminal amine groups at 5.15 ppm are very weak.
O) at 10.6–9.8 ppm are also observed clearly, but the signals of terminal amine groups at 5.15 ppm are very weak.