Photo-responsive, biocompatible polymeric micelles self-assembled from hyperbranched polyphosphate-based polymers
Chaojian
Chen
,
Gongyan
Liu
,
Xiangsheng
Liu
,
Shaopeng
Pang
,
Congshan
Zhu
,
Liping
Lv
and
Jian
Ji
*
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027, China. E-mail: jijian@zju.edu.cn; Fax: (+86)-571-87953729; Tel: (+86)-571-87953729
First published on 2nd April 2011
Abstract
Photo-responsive biocompatible micelles were constructed from amphiphilic hyperbranched polyphosphate based polymer (denoted as HPHEEP-DNQ) and applied in the controlled release of coumarin 102. HPHEEP-DNQ was designed and synthesized by modification of hydrophilic hyperbranched polyphosphate (HPHEEP) with hydrophobic, light-responsive 2-diazo-1,2-naphthoquinone (DNQ) molecules. Dynamic light scattering (DLS) and transmission electron microscopy (TEM) measurements revealed that the polymer can self-assemble into spherical micelles with an average diameter of about 186 nm in aqueous solution. The critical micelle concentration (CMC) of the micelles was 0.025 mg mL−1 determined by fluorescence spectroscopy using Nile Red as a fluorescence probe. By illuminating the micelles with 365 nm UV light, light triggered destabilization procedure was investigated by TEM and DLS. In addition, model drug coumarin 102 was successfully encapsulated into the micelles and the controlled release behavior under 365 nm UV light was investigated by fluorescence spectroscopy. Cell viability tests against two types of cells indicated that the micelles have excellent biocompatibility. These photo-responsive and biocompatible polymeric micelles self-assembled from hyperbranched polyphosphate based polymer might have great potential as smart carriers in the field of drug delivery.
Introduction
During the past two decades, stimuli-responsive polymeric self-assemblies, including micelles1–5 and polymersomes,6–9 have attracted considerable attention in the field of drug delivery, since the stimuli-responsive release may result in significantly enhanced therapeutic efficacy and minimal side effects. Various stimuli, such as temperature,10–12 pH,13–15redox,16–18 ultrasound19 and light,20–23 have been used to control the structures or functions of assemblies. Among all applied stimuli, light is especially appealing due to its non-contact mode, as well as utilizing the optimal precisely controlled wavelength, direction, illuminated area, and intensity.24After the first example of a photocontrollable copolymer micelle pioneered by Zhao's group,25 there has been rapidly growing interest in designing and developing photo-responsive polymer micelles. In order to control polymeric micelles using light, the amphiphilic polymers employed to construct micelles must be light-responsive. Light-responsive polymers can be obtained by introducing various photochromic groups including azobenzene,24–32spiropyran,33–35coumarin,36–38salicylideneaniline,39pyrene,40 and 2-diazo-1,2-naphthoquinone (DNQ)41–44 to their structure. Upon illumination of the polymer micelles with UV/visible or near-infrared (NIR) light, the incorporation of these chromophores can lead to reversible or irreversible morphological transition.
DNQ is an attractive chromophore that can be used to design light-responsive polymers, as its UV-induced Wolff rearrangement can result in the conversion of hydrophobic DNQ to hydrophilic 3-indenecarboxylic acid (IC).45–48 In addition, Urdabayev and Popik reported that DNQ can undergo the same reaction via a two-photon process under NIR light.49 Thus photoresponsive micelles composed of DNQ-containing polymers are more suitable for biomedical applications than UV/visible responsive systems, because NIR light with wavelengths in the range of about 700–1000 nm can penetrate the skin with less risk of damage to the irradiated area.50 Based on these principles, Fréchet and coworkers designed and synthesized two kinds of DNQ-containing molecules, a surfactant like amphiphile and a linear-dendritic copolymer, which can self-assemble into micelles and are sensitive to NIR light.41,42 Recently, Wang and coworkers also prepared photo-responsive random copolymers by incorporating DNQ groups into the polymers.43,44
For biomedical applications, the micelles should usually be constructed with materials that are nontoxic, biocompatible and biodegradable.51 The most commonly used biocompatible and biodegradable polymers are polyethylene glycol (PEG), polycaprolactone (PCL) and polylactic acid (PLA). Polyphosphates are another important class of eminent biomaterials due to their excellent biocompatibility, biodegradability and similarity to biomacromolecules such as nucleic and teichoic acids.52–56 Recently, Yan and coworkers first synthesized a hyperbranched polyphosphate (HPHEEP) from cyclic phosphate inimers by self-condensing ring-opening polymerization.57 Compared with linear polymers, hyperbranched polymers have some advantages for the construction of drug carriers, such as globular architectures, chain end functionalities, better solubility and lower viscosity. In their following work, a series of micelles were constructed from HPHEEP-based polymers,58–60 and all of the micelles possessed good biocompatibility and biodegradability.
In our work, stimuli-responsiveness, biodegradability and biocompatibility were combined by synthesizing an amphiphilic hyperbranched polyphosphate-based polymer HPHEEP-DNQ. The synthesis and detail structure of the polymer are shown in Scheme 1. This polymer can self-assemble into micelles in aqueous solution. Cell viability assay was performed to determine biocompatibility of the micelles. It is expected that the introduction of photo-responsive DNQ groups will lead to light triggered disruption and controlled release effects of the micelles. Model drug coumarin 102 was encapsulated into the micelles and the light triggered release behaviour was also investigated by fluorescence spectroscopy (Scheme 2).
 | ||
| Scheme 1 Synthesis of amphiphilic hyperbranched polymer HPHEEP-DNQ. | ||
 | ||
| Scheme 2 Wolff rearrangement of HPHEEP-DNQ and schematic illustration of the self-assembly and light triggered drug release behaviour of HPHEEP-DNQ micelles. | ||
Experimental
Materials
Hydrophilic hyperbranched polyphosphate (HPHEEP) was kindly provided by Professor Deyue Yan of Shanghai Jiao Tong University. The polymer was dissolved in deionized water, enclosed in a dialysis membrane (MWCO 1.0 kDa), and then purified by dialyzing in deionized water before use. 2-diazo-1,2-naphthoquinone-5-sulfonyl chloride (sc-DNQ, 99%) was purchased from Benxi Rst Chemical Co., Ltd. and was stored in the dark. Trimethylamine hydrochloride (Aladdin), Nile Red (98%, TCI) and coumarin 102 (97%, Fluka) were used as received without further purification. N,N-Dimethyl formamide (DMF) was purified by stirring with drying agent, calcium hydride, over night, followed by vacuum distillation. Triethylamine was purchased from Sinopharm Chemical Regent Co., Ltd. and was distilled before use. 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Sigma and used as received. Clear polystyrene tissue culture treated 96-well plates were obtained from Corning Costar. All other reagents and solvents were purchased from the domestic suppliers and used as received.Synthesis of HPHEEP-DNQ
Amphiphilic hyperbranched polymer (HPHEEP-DNQ) was synthesized by modification of hydrophilic hyperbranched polyphosphates with hydrophobic, light-responsive sc-DNQ molecules. To begin with, 0.55 g (0.1 mmol) HPHEEP and 10 mL DMF were added to a 100 mL three-neck round-bottom flask equipped with a magnetic stirrer and a condenser. The solution was stirred until the polymer was totally dissolved. Then, 0.668 g (6.6 mmol) triethylamine and 0.063 g (0.66 mmol) trimethylamine hydrochloride were added dropwise, followed by dropwise addition of 1.77 g (6.6 mmol) sc-DNQ in 8 mL DMF. After 72 h of reaction in the dark, the resulting solution was enclosed in dialysis membrane (MWCO 1.0 kDa), and then dialyzed in 500 mL deionized water at 4 °C for 3 days. After dialysis, the product was frozen and then dried by a freeze-dryer system at −50 °C for 5 days. Some brown viscous HPHEEP-DNQ was obtained. All experiments in this study were conducted under yellow light or in the dark.Micelles preparation and their encapsulation of Nile Red and coumarin 102
Three types of micelles were prepared: the first one without loading for TEM and DLS measurements, the second loaded with Nile Red for the determination of critical micelle concentration (CMC), and the third loaded with coumarin 102 to investigate light triggered release behavior of the micelles. All the three types of micelles were obtained in a similar way. For the micelles without loading, 21 mg HPHEEP-DNQ was first dissolved in 7 mL DMF at 25 °C, 7 mL water was then added dropwise into the stirred solution. After stirring for 6 h, the solution was dialyzed against deionized water in dialysis membrane (MWCO 1.0 kDa) for 3 days to remove the solvent DMF. The final polymer concentration was adjusted to 1.0 mg mL−1. For the micelles loaded with Nile Red, 21 mg HPHEEP-DNQ and 0.21 mg Nile Red were first dissolved in 7 mL DMF, followed by addition of 7 mL water dropwise into the solution. The following steps were the same with the corresponding steps of the first one. The hydrophobic dye, Nile Red was encapsulated into hydrophobic core of the micelles. The final concentration of HPHEEP-DNQ/DMF solution and Nile Red/DMF solution was adjusted to 1.0 mg mL−1 and 0.01 mg mL−1, respectively. For the preparation of micelles loaded with coumarin 102, all steps were the same with the second, the only difference was that Nile Red was replaced with coumarin 102.Characterization
Cell culture and treatment
Two types of cells, HepG2 (a human liver carcinoma cell line) and HUVEC (Human Umbilical Vein Endothelial Cells), were used to determine cytotoxicity of the micelles in this study. They were cultivated in different media, Dulbecco's modified eagle's medium (DMEM) and RPMI-1640 culture medium, and both with 10% fetal bovine serum (FBS), antibiotics penicillin (100 IU mL−1), and streptomycin (100 μg mL−1) at 37 °C under a humidified atmosphere containing 5% CO2.In vitro cytotoxicity assay
The cytotoxic effects of HPHEEP-DNQ micelles were determined by the MTT assay. Before determination, the cells were first counted and seeded in 96-well plates at 8 × 103cells per well in 200 μL of corresponding medium. After 24 h culture, the culture medium was removed and replaced with 200 μL of medium containing different concentrations of HPHEEP-DNQ micelles (10—200 mg L−1). Phosphate buffered saline (PBS) was chosen as the control. The cells were incubated for another 24 h. Following this, the culture medium was removed and wells were washed with PBS. Then, 100 μL culture medium and 20 μL of 5 mg mL−1 MTT assay stock solution in PBS were added to each well. After incubating the cells for 4 h, the medium containing unreacted MTT was removed carefully. The obtained blue formazan crystals generated by live cells were dissolved in 150 μL DMSO, and the absorbance at a wavelength of 570 nm of each well was measured using a microplate reader. The relative cell viability (%) was determined by comparing the absorbance at 570 nm with control wells. Cytotoxic effects of the micelles against the two types of cells were tested by the same method.Light triggered destabilization of HPHEEP-DNQ micelles
DLS measurements were conducted and the average count rate change of HPHEEP-DNQ micelles (1.0 mg mL−1) in response to 365 nm UV light was followed. After irradiation for 5 h, TEM measurement of the micelles was performed and the obtained image was compared with that before irradiation. The light source for this study was a 200 W high-pressure mercury lamp (SB-100P Series Long Wave UV Lamps, Spectronics Corporation).Light triggered release of coumarin 102 from HPHEEP-DNQ micelles
The micelles loaded with coumarin 102 were prepared to investigate light triggered release behavior of the micelles. The concentration of HPHEEP-DNQ/DMF solution and coumarin 102/DMF solution was 1.0 mg mL−1 and 0.01 mg mL−1, respectively. After the preparation, the fluorescence spectrum of the micelles was recorded immediately. Then the micelles were exposed to 365 nm UV light and the corresponding data at different times were collected. Fluorescence measurements were taken at an excitation wavelength of 420 nm and the emission monitored from 450 to 700 nm. Excitation and emission slit widths were both maintained at 5.0 nm and spectra were accumulated with a scan speed of 200 nm min−1.Results and discussion
Synthesis and characterization of amphiphilic hyperbranched polymer HPHEEP-DNQ
Amphiphilic hyperbranched polymer (HPHEEP-DNQ) was readily synthesized by modification of hydrophilic hyperbranched polyphosphate with hydrophobic, light-responsive sc-DNQ molecules. The synthetic approach is shown in Scheme 1. The resulting product was purified by dialysis for 3 days to totally remove the residual sc-DNQ molecules, and then was characterized by SEC, FTIR, 1H NMR and UV-vis spectroscopy. The characterizations of the polymers before and after modification are summarized in Table 1.| Polymer | M n a | M w/Mna | Mass contents of DNQ moieties (wt%) | Degree of grafting (%) | ||
|---|---|---|---|---|---|---|
| (1H NMR)b | (UV-vis)c | (1H NMR)b | (UV-vis)c | |||
| a The number-average molecular weight (Mn) and molecular weight distributions (Mw/Mn) were determined by SEC, DMF containing 0.01 mol L−1LiBr was used as eluent. b The data were calculated from 1H NMR knowing the molecular weight of HPHEEP. c The data were calculated from UV-vis spectra knowing the linear relationship between the UV absorbance at 403 nm and the concentration of sc-DNQ in DMF. | ||||||
| HPHEEP | 5500 | 1.08 | — | — | — | — |
| HPHEEP-DNQ | 6270 | 1.07 | 4.03 | 4.42 | 4.13 | 4.55 |
As shown in Fig. 1, FTIR spectra of sc-DNQ, HPHEEP and HPHEEP-DNQ are compared. The characteristic absorption peaks of DNQ group at around 2200 cm−1 can be observed in the spectrum of HPHEEP-DNQ but are not visible in the spectrum of HPHEEP, which was evidence of the successful attachment of DNQ groups on the terminal hydroxyl groups of HPHEEP.
 | ||
| Fig. 1 FTIR spectra of (A) sc-DNQ, (B) HPHEEP, and (C) HPHEEP-DNQ, confirming the successful attachment of DNQ groups on the terminal hydroxyl groups of HPHEEP. | ||
In addition, 1H NMR measurements were conducted using DMSO-d6 as solvent and the spectra are shown in Fig. 2. In the spectrum of HPHEEP-DNQ, the newly emerged characteristic resonance signals at 8.60 ppm, 8.13 ppm, 7.72 ppm, 7.63 ppm and 5.3 ppm are attributed to DNQ groups in the polymer. These results also confirmed the successful synthesis of DNQ-containing hyperbranched polymer HPHEEP-DNQ. Moreover, mass contents of DNQ moieties in HPHEEP-DNQ and the degree of grafting were further calculated from 1H NMR (Table 1). The degree of grafting was defined as the molar ratio of DNQ groups in HPHEEP-DNQ to terminal hydroxyl groups in HPHEEP. Specifically, the ratio of integral areas of methylene protons (δ 3.11–4.29) to DNQ protons (δ 5.27–5.46, 7.55–8.65) was first calculated. Because the number of methylene protons and terminal hydroxyl groups in each HPHEEP-DNQ can be counted based on the molecular weight and structure of HPHEEP, the molar content of DNQ groups in HPHEEP-DNQ was easily obtained. Finally, the degree of grafting was calculated as 4.13%. Mass contents of DNQ moieties were calculated by multiplying MDNQ/MHPHEEP-DNQ and the molar content of DNQ groups in HPHEEP-DNQ, in which MDNQ/MHPHEEP-DNQ is the ratio of molecular weights of DNQ and HPHEEP-DNQ.
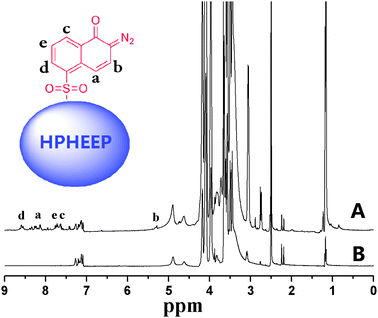 | ||
| Fig. 2 1H NMR spectra of (A) HPHEEP-DNQ, and (B) HPHEEP in DMSO-d6. | ||
UV-vis spectroscopy was also employed to further characterize the structure of HPHEEP-DNQ and to investigate its light sensitivity to UV light. Firstly, HPHEEP-DNQ sample was dissolved in DMF with a concentration of 1 mg mL−1. Then UV-vis spectrometry of the sample was carried out and the results are shown in Fig. 3 (the top curve of 0 s). From this curve, an obvious peak can be easily found at the wavelength around 403 nm which was the characteristic absorption peak of DNQ. This was further evidence of successful synthesis of the DNQ-modified polymer.
 | ||
| Fig. 3 UV-vis spectra of HPHEEP-DNQ in DMF (1 mg mL−1) after irradiation at different time intervals. | ||
Since HPHEEP does not have UV absorbance at the wavelength around 403 nm, the peak which appeared in this region was totally attributed to the DNQ moieties in HPHEEP-DNQ, thus the mass contents of DNQ moieties in HPHEEP-DNQ and the degree of grafting can also be calculated knowing the linear relationship between the UV absorbance of sc-DNQ and the concentration. UV-vis spectrometry of sc-DNQ samples with different concentrations in DMF were carried out and the absorbance at 403 nm versus the concentration of sc-DNQ in DMF was plotted by a linear fitting method. Then we got the following equation:
| y = 32.086x − 0.001 |
Besides, the photochemical reaction of the hyperbranched polymer HPHEEP-DNQ was confirmed by irradiation with 365 nm UV light, and the change in the UV-vis spectra was followed during irradiation at various time intervals. As shown in Fig. 3, with increasing of irradiation time, the characteristic absorption peaks of DNQ moieties decreased gradually, showing that DNQ moieties underwent the Wolff rearrangement reaction. After irradiation for 5 min, the absorbance did not decrease anymore and the bubbling stopped, indicating the complete conversion of DNQ to 3-indenecarboxylic acid.
Self-assembly of HPHEEP-DNQ and determination of CMC
It is known that amphiphilic polymers can self-assemble into micelles, polymersomes or other assemblies in selected solvents. In this study, we synthesized an amphiphilic polymer HPHEEP-DNQ which contains a hydrophilic hyperbranched polyphosphate headgroup and some hydrophobic DNQ tails. Self-assemblies of HPHEEP-DNQ were prepared by dialysis method with a concentration of 1.0 mg mL−1 and were then characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS).TEM was used to investigate the morphology of the assemblies formed by amphiphilic hyperbranched polymer. Fig. 4A revealed that micelles were formed with a spherical morphology. It was reported by Discher and Eisenberg that phospholipid-like molecules with a ratio of hydrophilic to total mass of fhydrophilic > 45% can be expected to form micelles.61 Our result was in good accordance with their rule as the mass contents of hydrophobic DNQ moieties were less than 5% determined by 1H NMR and UV-vis. From TEM images, over 200 micelles were counted and the average size was about 200 nm.
 | ||
| Fig. 4 Size distribution profiles for HPHEEP-DNQ micelles (1.0 mg mL−1) determined by TEM (A) and DLS (B). | ||
DLS was further conducted and the results showed that HPHEEP-DNQ formed micelles with a number-average hydrodynamic diameter (Dh) of 186 nm (Fig. 4B), which was consistent with that determined by TEM. The polydispersity obtained from DLS was only 0.127, indicating that the micelles had a narrow size distribution.
The self-assembly behavior of HPHEEP-DNQ micelles was also investigated by fluorescence spectroscopy study according to the literature.41,42 Nile Red, an important fluorescence probe in studies of self-assemblies, was used to determine the micellization process of hyperbranched polymer in water and the critical micelle concentration (CMC) can be calculated by tracking the fluorescence intensity of Nile Red as a function of the sample concentration.
As shown in Fig. 5A, Nile Red exhibits a relatively low fluorescence intensity at low concentrations (e.g., 0.005 mg mL−1), indicating that the Nile Red was in water, and few micelles were present. With increasing concentration of micellar solution, the fluorescence intensity increased dramatically, showing the Nile Red was encapsulated in a hydrophobic environment, i.e., the interior of the micelles.
 | ||
| Fig. 5 (A) Fluorescence emission spectra of Nile Red in HPHEEP-DNQ micelles of varying concentrations. (B) Plot of the emission intensity at 656 nm versus the log of concentration (mg mL−1) of HPHEEP-DNQ micelles. | ||
CMC is a key parameter to describe the physical properties of micelles. At the CMC, hydrophobic portion start to associate to reduce the interaction with water molecules, leading to the micelle formation. The relative emission intensity at 656 nm versus the log of concentration was plotted and is shown in Fig. 5B. It was easily noticed that the curve was almost flat at low concentration but rapidly increased at high concentration. The CMC was obtained from the intersection of two straight lines: the base line and the tangent of the rapid rising curve. The value of the CMC was about 0.025 mg mL−1, which was comparable to that of amphiphilic diblock copolymers.62 All the results above also suggested the formation of the micelles.
In vitro cytotoxicity measurements
In order to be acceptable as drug carrier systems, polymeric materials have to fulfil the requirement of biocompatibility, so it is of great importance to evaluate the potential toxicity of HPHEEP-DNQ micelles. As a class of eminent biomaterials, polyphosphates have been deeply studied in the field of drug delivery and shown nontoxicity to various cultural cell lines.53–60In our study, two types of cells, HepG2 and HUVEC were cultured and used to determine the in vitro cytotoxicity of the micelles. The viability of the two cells in corresponding culture medium after 24 h incubation with different concentrations of micelles was determined by MTT method and the results are shown in Fig. 6. It was found that HPHEEP-DNQ micelles had low cytotoxicity, as over 90% cells were viable at micelle concentrations ranging from 10 mg L−1 to 200 mg L−1. These results can be mainly attributed to the excellent biocompatibility of polyphosphates. It is believed that these micelles with good biocompatibility have great potential for biomedical applications.
 | ||
| Fig. 6 Cytotoxicity of HPHEEP-DNQ micelles with different concentrations after incubating for 24 h. Two types of cells, HepG2 and HUVEC, were used and the cell viability was determined by MTT assay. | ||
Light triggered destabilization of HPHEEP-DNQ micelles
To investigate the light responsiveness of the HPHEEP-DNQ micelles, the micellar solution (1.0 mg mL−1) without loading was irradiated under 365 nm UV light and DLS measurements were followed at different times. The light triggered destabilization process was monitored by measuring the average count rate and the values are plotted as a function of irradiation time (Fig. 7A). The average count rate, a measurement of light scattering intensity with units of kilo counts per second (kcps), can be used to investigate the self-assembly, disintegration and degradation behaviours of aggregates, complexes and nanoparticles.63–66 The light scattering intensity of spherical particles is mainly dependent on the size and number of particles. In our experiments, the size of the micelles did not change obviously (Fig. 7B), thus the number of the micelles here can be reflected by the average count rate determined by DLS. As shown in Fig. 7A, with the increase of irradiation time, the average count rate gradually decreased from 329.6 kcps at 0 min to 119.6 kcps at 300 min, indicating that the number of the micelles was decreasing in the process. These results were not surprising and revealed the dissociation of the micelles under 365 nm UV light. It is believed that the results happened because of the Wolff rearrangement of DNQ moieties in HPHEEP-DNQ molecules. The reaction changed the amphiphilic properties of HPHEEP-DNQ and then destroyed the structure of the micelles. | ||
| Fig. 7 (A) The average count rate change of HPHEEP-DNQ micelles (1.0 mg mL−1) in response to 365 nm UV light followed by DLS measurement. (B) DLS size distributions of HPHEEP-DNQ micelles (1.0 mg mL−1) before and after irradiation for 5 h. | ||
After irradiating the micellar solution for 5 h, TEM measurement was also performed to further confirm the light triggered destabilization behaviour of the micelles. From the TEM image shown in Fig. 8, we can see the micellar structure of HPHEEP-DNQ was partly converted into irregular aggregates after 5 h UV irradiation. The morphology of the irregular aggregates was different compared with the micelles' morphology shown in Fig. 4A, indicating that some of the micelles were disassembled. It was inferred that the irregular aggregates might be the residues of micelle dissociation. This morphological change was also caused by the light-triggered conversion of hydrophobic DNQ moieties to hydrophilic IC. Both the DLS and TEM results above proved that the micelles possessed the ability to respond to UV light.
 | ||
| Fig. 8 TEM image of HPHEEP-DNQ micellar solution after 5 h UV irradiation. | ||
Loading and light controlled release of coumarin 102
Stimuli-responsive micelles are ideal carriers for controlled release of hydrophobic molecules and widely used in the field of drug delivery.1–5 In this work, coumarin 102, a hydrophobic fluorescence dye, was chosen as model drug to be encapsulated into HPHEEP-DNQ micelles since it will exhibit a relatively high fluorescence intensity in a hydrophobic environment such as the core of micelles.33,34Fluorescence spectroscopy was used to investigate the loading and controlled release behaviours of the micelles. The micelles encapsulated with coumarin 102 were prepared by dialysis method. After the preparation, the fluorescence spectrum of the samples was recorded immediately. As can be seen in Fig. 9A, the characteristic emission peak of coumarin 102 at the wavelength around 488 nm appeared, indicating that the hydrophobic dye was successfully encapsulated into the micelles. | ||
| Fig. 9 (A) Fluorescence emission spectra of HPHEEP-DNQ micelles with encapsulated coumarin 102 at various times. (B) Light triggered release of coumarin 102 from HPHEEP-DNQ micelles, emission intensity at 488 nm was used for drawing this stepped curve. | ||
To verify the effect of light on the release process, the experiments of drug release were carried out by alternatively turning on and off the 365 nm UV lamp. During this process, fluorescence emission spectra were recorded at various times (Fig. 9A). The actual status of the lamp and the controlled release characteristics of the micelles are shown in Fig. 9B. It was found that the line was almost flat when the lamp was turned off. And in comparison, the emission intensity of coumarin 102 decreased obviously when the light was open. These results suggested that coumarin 102 was released into water, which was attributed to the dissociation of the micelles under UV light. All the results showed that hydrophobic coumarin 102 can be loaded into the micelles and released into the aqueous solution in a light controlled manner.
Conclusions
In summary, amphiphilic hyperbranched polymer HPHEEP-DNQ was successfully prepared by modification of hydrophilic hyperbranched polyphosphate with light-responsive, hydrophobic 2-diazo-1,2-naphthoquinone-5-sulfonyl chloride molecules. The polymer can self-assemble into micelles in water with an average size of 186 nm and a critical micelle concentration of 0.025 mg mL−1. In vitro cytotoxicity assay showed that the micelles have excellent biocompatibility. The photochemical reaction of DNQ moieties of the polymer under UV irradiation can result in destabilization of the micelles. Furthermore, hydrophobic model drug coumarin 102 was successfully encapsulated into the micelles and then released into water in a controlled manner under 365 nm UV light. These photo-responsive, biocompatible polymeric micelles are highly promising as smart carriers for triggered delivery of hydrophobic biopharmaceutics such as anticancer drugs.Acknowledgements
Financial support from the Natural Science Foundation of China (NSFC-50830106) and China National Funds for Distinguished Young Scientists (51025312) is gratefully acknowledged. The authors thank Prof. Yan (Shanghai Jiao Tong University) very much for providing HPHEEP.References
- V. P. Torchilin, Pharm. Res., 2007, 24, 1–16 CAS.
- S. Ganta, H. Devalapally, A. Shahiwala and M. Amiji, J. Controlled Release, 2008, 126, 187–204 CrossRef CAS.
- A. Klaikherd, C. Nagamani and S. Thayumanavan, J. Am. Chem. Soc., 2009, 131, 4830–4838 CrossRef CAS.
- C. J. F. Rijcken, O. Soga, W. E. Hennink and C. F. van Nostrum, J. Controlled Release, 2007, 120, 131–148 CrossRef CAS.
- N. Rapoport, Prog. Polym. Sci., 2007, 32, 962–990 CrossRef CAS.
- J. Z. Du and R. K. O'Reilly, Soft Matter, 2009, 5, 3544–3561 RSC.
- M. H. Li and P. Keller, Soft Matter, 2009, 5, 927–937 RSC.
- F. H. Meng, Z. Y. Zhong and J. Feijen, Biomacromolecules, 2009, 10, 197–209 CrossRef CAS.
- O. Onaca, R. Enea, D. W. Hughes and W. Meier, Macromol. Biosci., 2009, 9, 129–139 CrossRef CAS.
- J. E. Chung, M. Yokoyama, M. Yamato, T. Aoyagi, Y. Sakurai and T. Okano, J. Controlled Release, 1999, 62, 115–127 CrossRef CAS.
- M. Nakayama, T. Okano, T. Miyazaki, F. Kohori, K. Sakai and M. Yokoyama, J. Controlled Release, 2006, 115, 46–56 CrossRef CAS.
- Y. Li, B. S. Lokitz and C. L. McCormick, Angew. Chem., Int. Ed., 2006, 45, 5792–5795 CrossRef CAS.
- S. Y. Liu, J. V. M. Weaver, Y. Q. Tang, N. C. Billingham and S. P. Armes, Macromolecules, 2002, 35, 6121–6131 CrossRef CAS.
- J. Z. Du and S. P. Armes, J. Am. Chem. Soc., 2005, 127, 12800–12801 CrossRef CAS.
- M. Oishi, K. Kataoka and Y. Nagasaki, Bioconjugate Chem., 2006, 17, 677–688 CrossRef CAS.
- W. F. Dong, A. Kishimura, Y. Anraku, S. Chuanoi and K. Kataoka, J. Am. Chem. Soc., 2009, 131, 3804–3805 CrossRef CAS.
- C. Wang, Y. S. Guo, Y. P. Wang, H. P. Xu and X. Zhang, Chem. Commun., 2009, 5380–5382 RSC.
- N. Ma, Y. Li, H. P. Xu, Z. Q. Wang and X. Zhang, J. Am. Chem. Soc., 2010, 132, 442–443 CrossRef CAS.
- Y. W. Li, R. Tong, H. S. Xia, H. J. Zhang and J. Xuan, Chem. Commun., 2010, 46, 7739–7741 RSC.
- Y. Zhao, J. Mater. Chem., 2009, 19, 4887–4895 RSC.
- J. S. Katz and J. A. Burdick, Macromol. Biosci., 2010, 10, 339–348 CrossRef CAS.
- J. M. Schumers, C. A. Fustin and J. F. Gohy, Macromol. Rapid Commun., 2010, 31, 1588–1607 CrossRef CAS.
- F. Ercole, T. P. Davis and R. A. Evans, Polym. Chem., 2010, 1, 37–54 RSC.
- Q. Jin, G. Y. Liu, X. S. Liu and J. Ji, Soft Matter, 2010, 6, 5589–5595 RSC.
- G. Wang, X. Tong and Y. Zhao, Macromolecules, 2004, 37, 8911–8917 CrossRef CAS.
- F. D. Jochum and P. Theato, Chem. Commun., 2010, 46, 6717–6719 RSC.
- J. Ruchmann, S. Fouilloux and C. Tribet, Soft Matter, 2008, 4, 2098–2108 RSC.
- S. Khoukh, R. Oda, T. Labrot, P. Perrin and C. Tribet, Langmuir, 2007, 23, 94–104 CrossRef CAS.
- Y. P. Wang, M. Zhang, C. Moers, S. L. Chen, H. P. Xu, Z. Q. Wang, X. Zhang and Z. B. Li, Polymer, 2009, 50, 4821–4828 CrossRef CAS.
- X. Tong, G. Wang, A. Soldera and Y. Zhao, J. Phys. Chem. B, 2005, 109, 20281–20287 CrossRef CAS.
- X. K. Liu and M. Jiang, Angew. Chem., Int. Ed., 2006, 45, 3846–3850 CrossRef CAS.
- C. Alvarez-Lorenzo, S. Deshmukh, L. Bromberg, T. A. Hatton, I. Sández-Macho and A. Concheiro, Langmuir, 2007, 23, 11475–11481 CrossRef.
- H.i. Lee, W. Wu, J. K. Oh, L. Mueller, G. Sherwood, L. Peteanu, T. Kowalewski and K. Matyjaszewski, Angew. Chem., Int. Ed., 2007, 46, 2453–2457 CrossRef CAS.
- Q. Jin, G. Y. Liu and J. Ji, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 2855–2861 CrossRef CAS.
- R. Byrne, C. Ventura, F. B. Lopez, A. Walther, A. Heise and D. Diamond, Biosens. Bioelectron., 2010, 26, 1392–1398 CrossRef CAS.
- J. Babin, M. Pelletier, M. Lepage, J. F. Allard, D. Morris and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 3329–3332 CrossRef CAS.
- Q. Jin, G. Y. Liu and J. Ji, Eur. Polym. J., 2010, 46, 2120–2128 CrossRef CAS.
- Q. Jin, X. S. Liu, G. Y. Liu and J. Ji, Polymer, 2010, 51, 1311–1319 CrossRef CAS.
- F. D. Jochum and P. Theato, Macromolecules, 2009, 42, 5941–5945 CrossRef CAS.
- J. Q. Jiang, X. Tong and Y. Zhao, J. Am. Chem. Soc., 2005, 127, 8290–8291 CrossRef CAS.
- A. P. Goodwin, J. L. Mynar, Y. Z. Ma, G. R. Fleming and J. M. J. Fréchet, J. Am. Chem. Soc., 2005, 127, 9952–9953 CrossRef CAS.
- J. L. Mynar, A. P. Goodwin, J. A. Cohen, Y. Z. Ma, G. R. Fleming and J. M. J. Fréchet, Chem. Commun., 2007, 2081–2082 RSC.
- F. Tian, Y. Y. Yu, C. C. Wang and S. Yang, Macromolecules, 2008, 41, 3385–3388 CrossRef.
- Y. Y. Yu, F. Tian, C. Wei and C. C. Wang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2763–2773 CrossRef CAS.
- W. Kirmse, Eur. J. Org. Chem., 2002, 14, 2193–2256 CrossRef.
- J. I. K. Almstead, B. Urwyler and J. Wirz, J. Am. Chem. Soc., 1994, 116, 954–960 CrossRef.
- J. Andraos, A. J. Kresge and V. V. Popik, J. Am. Chem. Soc., 1994, 116, 961–967 CrossRef CAS.
- D. Bratton, R. Ayothi, H. Deng, H. B. Cao and C. K. Ober, Chem. Mater., 2007, 19, 3780–3786 CrossRef CAS.
- N. K. Urdabayev and V. V. Popik, J. Am. Chem. Soc., 2004, 126, 4058–4059 CrossRef CAS.
- W. G. Fisher, W. P. Partridge, C. Dees and E. A. Wachter, Photochem. Photobiol., 1997, 66, 141–155 CrossRef CAS.
- M. C. Branco and J. P. Schneider, Acta Biomater., 2009, 5, 817–831 CrossRef CAS.
- J. Libiszowski, K. Kaluzynski and S. Penczek, J. Polym. Sci., Part A, 1978, 16, 1275–1283 Search PubMed.
- S. Wang, A. C. A. Wan, X. Xu, S. Gao, H. Q. Mao, K. W. Leong and H. Yu, Biomaterials, 2001, 22, 1157–1169 CrossRef CAS.
- J. Wang, P. C. Zhang, H. F. Lu, N. Ma, S. Wang, H. Q. Mao and K. W. Leong, J. Controlled Release, 2002, 83, 157–168 CrossRef CAS.
- Y. C. Wang, X. Q. Liu, T. M. Sun, M. H. Xiong and J. Wang, J. Controlled Release, 2008, 128, 32–40 CrossRef CAS.
- Y. C. Wang, Y. Y. Yuan, J. Z. Du, X. Z. Yang and J. Wang, Macromol. Biosci., 2009, 9, 1154–1164 CrossRef CAS.
- J. Y. Liu, W. Huang, Y. F. Zhou and D. Y. Yan, Macromolecules, 2009, 42, 4394–4399 CrossRef CAS.
- J. Y. Liu, W. Huang, Y. Pang, X. Y. Zhu, Y. F. Zhou and D. Y. Yan, Biomacromolecules, 2010, 11, 1564–1570 CrossRef CAS.
- J. Y. Liu, Y. Pang, W. Huang, X. Y. Zhu, Y. F. Zhou and D. Y. Yan, Biomaterials, 2010, 31, 1334–1341 CrossRef CAS.
- J. Y. Liu, W. Huang, Y. Pang, X. Y. Zhu, Y. F. Zhou and D. Y. Yan, Biomaterials, 2010, 31, 5643–5651 CrossRef CAS.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967–973 CrossRef CAS.
- M. L. Adams, A. Lavasanifar and G. S. Kwon, J. Pharm. Sci., 2003, 92, 1343–1355 CrossRef CAS.
- Z. Gu, M. Yan, B. Hu, K. I. Joo, A. Biswas, Y. Huang, Y. Lu, P. Wang and Y. Tang, Nano Lett., 2009, 9, 4533–4538 CrossRef CAS.
- D. Tong, J. Yao, Q. Wang, T. Zhai, H. Li and S. Han, J. Appl. Polym. Sci., 2009, 114, 1551–1556 CrossRef CAS.
- Q. Jin, L. P. Lv, G. Y. Liu, J. P. Xu and J. Ji, Polymer, 2010, 51, 3068–3074 CrossRef CAS.
- J. Oishi, K. Kawamura, J. H. Kang, K. Kodama, T. Sonoda, M. Murata, T. Niidome and Y. Katayama, J. Controlled Release, 2006, 110, 431–436 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
