Phase behavior and self-assembly of PSn(P2VP-b-PAA)n multiarmed multisegmented star terpolymers with ampholytic arms
Zacharoula
Iatridi
a,
Yuri
Roiter
b,
Nikoletta
Stavrouli
ac,
Sergiy
Minko
b and
Constantinos
Tsitsilianis
*ac
aDepartment of Chemical Engineering, University of Patras, 26504, Patras, Greece
bDepartment of Chemistry and Biomolecular Science, Clarkson University, 8 Clarkson Ave, Potsdam, NY 13699-5810, USA
cInstitute of Chemical Engineering and High Temperature Chemical Processes ICE/HT-FORTH, P.O. Box 1414, 26504, Patras, Greece. E-mail: ct@chemeng.upatras.gr
First published on 29th June 2011
Abstract
We report on the phase behavior and self-assembly phenomena of (polystyrene)n(poly(2-vinylpyridine)-b-poly(acrylic acid))n, PSn(P2VP-b-PAA)n, heteroarm star block terpolymers, in DMF/water solvent mixture. The stars were prepared using a one-pot, four-step anionic polymerization method following the “in–out” approach. These multiarmed, multisegmented star terpolymers self-assemble in selective media exhibiting pH responsiveness owing to the ampholytic nature of their diblock copolymer arms. Two soluble and two insoluble states were observed depending upon the pH of the medium. At low pH (pH 1.0), core–shell unimolecular micelles, with a hydrophobic PS core and P2VP, PAA segments in the shell, were formed. At pH 3.0, H-bonding and electrostatic interactions between the P2VP and PAA segments led to compact spheres. Finally, at pH 8.0 the stars were transformed from a bis-hydrophilic state to bis-hydrophobic state, leading to a network-like intermicellar assembly.
1. Introduction
Numerous reports have been published on the ability of segmented polymers to self-assemble in selective media due to potential applications, for instance as nanocarriers in biomedicine and in the cosmetics industry. Block copolymers and terpolymers of various macromolecular architectures form a great variety of nanostructured morphologies that can be controlled by a number of factors, such as relative block lengths, functionalities, topology, ion content, and solvent selectivity.1–15 The use of proper building blocks with specific properties (i.e., hydrophobic, hydrophilic, pH responsive, thermo-sensitive, ionic strength sensitive, etc.) significantly affect the self-organization of the polymers.16–19 For example, thus far, spherical, cylindrical, disk-like, crew-cut, wormlike, Janus micelles, toroids, and vesicles have been observed. Multicompartment micelles, usually formed by block terpolymers bearing one hydrophilic and two incompatible hydrophobic segments,20–22 constitute a novel class of nanostructured materials.23It has been shown that the architecture of a block terpolymer is decisive on the micellar structures. Over the past few years, the synthesis and self-assembly of ABC miktoarm star terpolymers has been a very attractive topic for many research groups.24–28 Lodge et al. have widely studied the morphology of the self-assemblies formed by ABC miktoarm star terpolymers (μ-ABC). By controlling the copolymer composition, as well as solvent selectivity, they observed that these 3-armed star terpolymers can form a rich variety of nanostructured self-assemblies like spherical micelles, segmented or multicompartment worms, hamburger micelles, disk-like micelles, etc.29 S. Liu et al. synthesized well-defined μ-ABC bearing hydrophobic and thermo-responsive arms, as well as a pH-sensitive zwitterionic star with three hydrophilic segments, which exhibit diverse types of micellar associates in aqueous solutions.30
Other types of star terpolymer architecture, incorporating three kinds of blocks of different chemical nature, can be designed following the “in–out” synthetic route.31 This method leads mainly to multiarmed star-shaped topologies, called hetero-arm star block terpolymers of the type An(B-b-C)n, i.e., bearing equal number, n, of pure A and B-b-C diblock copolymer arms (Scheme 1). In fact this topology can be seen as a hybrid of the heteroarm star (AnBn) and the star-block copolymers (BC)n.32
 | ||
| Scheme 1 Schematic representation of the pH-dependent star terpolymer conformations. | ||
In this article, we explore the phase behavior and self-organization ability of novel multiarmed An(B-b-C)n star terpolymers bearing three kinds of segments, one hydrophobic and two hydrophilic, of oppositely charged weak polyelectrolyte, in dilute aqueous solutions. The star terpolymers were synthesized via “living” anionic polymerization and consisted of a poly(divinyl benzene) cross-linked core, bearing polystyrene (PS) and poly(2-vinyl pyridine)-b-poly(acrylic acid) (P2VP-b-PAA) block copolymer arms. These star terpolymers differ remarkably from the simplest three-armed μ-ABC stars explored so far. Thus, one of the motivations was to look for the first time what kind of self-assemblies could be formed from such multiarmed multisegmented stars. Due to the ampholytic character of the P2VP-b-PAA arms (Scheme 1), these star terpolymers were expected to respond to pH. Hence, another interest was to explore the pH responsive behavior of these star-shaped macromolecules, as well as to observe possible different behavior from that of linear topologies studied previously, like ABC triblock5,10 and/or ABCBA pentablock33 terpolymers, bearing P2VP and PAA segments.
2. Experimental section
2.1. Polymer synthesis and characterization
PSn–PDVB–(P2VP-b-PtBA)n heteroarm star terpolymer precursors were prepared via a one-pot/four-step sequential “living” anionic polymerization procedure (an extended “in–out” method). In the first step, the PS arms were prepared in THF, using sec-BuLi as the initiator. After the consumption of styrene, the ‘living’ PS chains were used to polymerize a small quantity of DVB (the [DVB]/[living end] molar ratio was 3.4, 9.7 and 10.6), resulting in a ‘living’ star-shaped polystyrene precursor (PSn) bearing active sites in its PDVB core. The ‘living’ star polymer was used to initiate polymerization of 2VP, leading to the formation of a second generation of P2VP arms. Finally, the sites located at the end of the P2VP arms were used to polymerize tBA. In every step of the synthesis, sampling out was performed for the purpose of characterization. In Fig. 1, characteristic size-exclusion chromatography (SEC) traces of the star precursors corresponding to a one-pot synthetic procedure are presented, showing the expected shift to lower elution volumes upon adding the different segments. The final PSn–PDVB–(P2VP-b-PAA)n terpolymers were obtained after acidic hydrolysis of the PtBA blocks in 1,4-dioxane with a 6-fold excess of hydrochloric acid at 80 °C.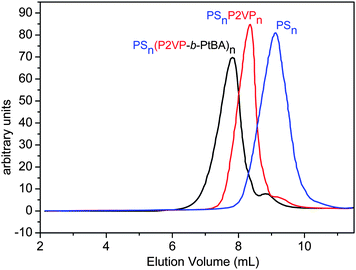 | ||
| Fig. 1 SEC traces of the raw precursors, PSn, PSnP2VPn and PSn(P2VP-b-PtBA)n of the (PS34)22(P2VP136-b-PAA119)22 star terpolymer. SEC was carried out by using two PLgel MiniMix columns “C” and “D” and tetrahydrofuran/triethylamine (THF/Et3N, 99/1, v/v) mixture as the mobile phase. | ||
The number of arms, n, was calculated from the molecular weight of the PSn star precursor and we consider that it remains the same for the second generation of arms as has been shown by AFM.34 The characterization results are presented in Table 1.
| Polymer | Number of arms | PS | P2VP | PAA | M w, tot | ||||
|---|---|---|---|---|---|---|---|---|---|
| n b | Total | M w a | N PS | M w c | N P2VP | M w d | N PAA | ||
| a By SEC. b By SLS. c Calculated by subtracting the Mw of the PSn from that of PSnP2VPn and dividing by the number of arms n. d Calculated by subtracting the Mw of the PSnP2VPn from that of PSn(P2VP-b-PtBA)n and dividing by n, considering quantitative hydrolysis of tBA to AA. | |||||||||
| PS9(P2VP-PAA)9 | 9 | 18 | 3400 | 33 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
126 | 8900 | 69 | 235![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| PS22(P2VP-PAA)22 | 22 | 44 | 3500 | 34 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
136 | 15250 | 119 | 717![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| PS28(P2VP-PAA)28 | 28 | 56 | 3000 | 29 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
152 | 11000 | 86 | 843![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.2. Sample preparation
The experiments were conducted in DMF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solvent mixture using distilled Millipore MILLI-Q water. The PSn–PDVB–(P2VP-b-PAA)n star terpolymers were first dissolved in DMF, followed by the slow addition of water. The pH of the solutions was regulated by adding 0.1 N HCl or 0.1 N NaOH. The samples were stirred overnight at room temperature.
1 v/v) solvent mixture using distilled Millipore MILLI-Q water. The PSn–PDVB–(P2VP-b-PAA)n star terpolymers were first dissolved in DMF, followed by the slow addition of water. The pH of the solutions was regulated by adding 0.1 N HCl or 0.1 N NaOH. The samples were stirred overnight at room temperature.
2.3. Turbidity measurements
The optical density, at 490 nm and at room temperature, was measured using a double beam HITACHI U-2001 UV-Vis spectrophotometer. The polymer solution was placed in a 10 mm path-length quartz cuvette containing a small magnetic bar set in motion with the aid of a miniature magnetic stirrer.2.4. Electrophoresis
Zeta-potentials were determined by electrophoretic measurements, carried out at 25 °C by means of a NanoZetasizer, Nano ZS Malvern apparatus. The incident light source was a 4 mW He–Ne laser at 633 nm and the intensity of the scattered light was measured at 173°.2.5. Static light scattering
Light scattering measurements were carried out using a thermally regulated (±0.1 °C) spectro-goniometer, Model BI-200SM (Brookhaven), equipped with a He–Ne laser (632.8 nm).2.6. Dynamic light scattering
Autocorrelation functions g(q,t) were measured with a full, multiple digital correlator (ALV-5000/FAST) equipped with 280 channels. The light source was a He–Ne laser operating at 632.8 nm. The correlation functions were analysed by the constrained regularized method (CONTIN) using CoVA-Jacek Gapinski 2001 software. The apparent diffusion coefficients, Dapp = Γ/q2, with q = (4πn/λ)sin(θ/2), where n is the refractive index of the solvent, were determined at the peak of the decay rate distributions and the apparent hydrodynamic radii were determined via the Stokes–Einstein equation:| Rh = kBT/6πηDapp |
2.7. Atomic force microscopy
Atomic force microscopy (AFM) images were taken using a multimode AFM instrument (Digital Instruments, Santa Barbara, CA, USA) operating in the tapping mode. Grade V-1 muscovite mica (Structure Probe, Inc., West Chester, PA, USA) was used as the substrate (disks of 12 mm diameter, freshly cleaved before each spin-coating). Silicon tips with a radius of 10–20 nm, a spring constant of 30 N m−1, and resonance frequency of 250–300 kHz were used, after they were calibrated with gold nanoparticles (diameter of 5 nm) to evaluate the tip radius. The structural dimensions from the AFM images were corrected using the tip radius. DMF-aqueous solutions of planned terpolymer content were prepared and spin-coated at 500 to 2000 rpm for 2 min. All atomic force microscopy (AFM) images were recorded using a MultiMode scanning probe microscope (Veeco Instruments Inc., Woodbury, NY, USA) operating in the tapping mode.Stock dimethylformamide (DMF) solutions of 2 mg ml−1polymer concentration were prepared by mixing 20 mg of the polymer with 10 ml of DMF followed by sonication, until clarity was achieved (about 2 h), and filtration, using the 0.45 μm polytetrafluoroethylene (PTFE) filters, was conducted. Aqueous media with adjusted pH values were used for the addition to the copolymer solutions in DMF. The volume ratio between the aqueous media and the DMF was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Filtered 0.1 N and 1 N HCl and 0.1 N and 1 N NaOH water solutions were utilized as pH regulators. Adjustment of the pH values was performed using a pH meter 345 (Corning Incorporated, Corning, NY, USA). Reproducible images were obtained, which were irrespective of the spin coating and scan conditions.
1. Filtered 0.1 N and 1 N HCl and 0.1 N and 1 N NaOH water solutions were utilized as pH regulators. Adjustment of the pH values was performed using a pH meter 345 (Corning Incorporated, Corning, NY, USA). Reproducible images were obtained, which were irrespective of the spin coating and scan conditions.
3. Results and discussion
The star terpolymers under investigation were not directly soluble in water either in acidic or in basic conditions. Thus, preliminary results were carried out in the presence of DMF which is a common good solvent of the different segments. We note here that in this case, the dielectric permittivity of the medium is lower than that in pure water which affects the charge density of the polyelectrolyte segments.The PSn(P2VP-b-PAA)n star terpolymers exhibit pH responsive behavior due to the protonation/deprotonation equilibria of the P2VP/PAA weak polyelectrolyte segments. The phase behavior and the charge state of the star terpolymers were explored using optical density and zeta-potential measurements. For the turbidity and electrophoresis experiments, a series of PSn(P2VP-b-PAA)n solutions, at different pH values ranging from 1 to 12 and at a concentration of 0.2 wt%, were prepared in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v DMF/H2O solvent mixture. The results are presented in Fig. 2.
1 v/v DMF/H2O solvent mixture. The results are presented in Fig. 2.
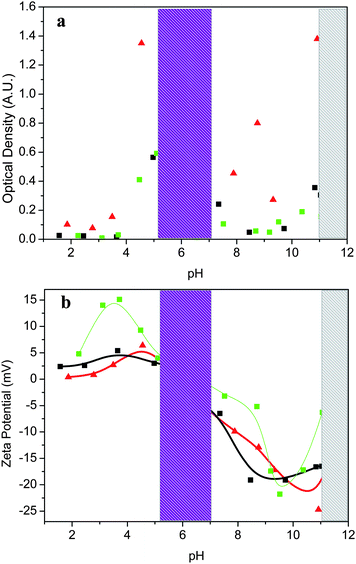 | ||
Fig. 2 pH-dependence of the optical density (a) and Z-potential (b) of (PS33)9(P2VP126-b-PAA69)9 (black coloured ■), (PS34)22(P2VP136-b-PAA119)22 (green coloured ■) and (PS29)28(P2VP152-b-PAA86)28 (▲) star terpolymer solutions in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v at a concentration of 0.2 wt%. The lines are a guide for the eyes. 1 v/v at a concentration of 0.2 wt%. The lines are a guide for the eyes. | ||
The optical density versus the pH plot revealed a four-phase behavior. At low pH values (pH < 5.1), the star terpolymers were completely dissolved in the DMF/H2O mixture and optically clear solutions were formed. It is well-known that, at low pH, the P2VP blocks are protonated, behaving as a cationic polyelectrolyte, while the PAA blocks are neutral (below pH ∼3.0). Above pH 3.0, the PAA segments become ionized (deprotonated) and electrostatic interactions of oppositely charged moieties in the P2VP and PAA segments become important, as revealed by the turbidity rise, leading to the formation of aggregates. As seen in Fig. 2b, the net charge of the polymer solution in the low pH regime is positive since the protonated P2VP moieties predominate.
Upon further increasing pH, the optical density increased abruptly and a two-phase region (5.0 < pH < 7.0), in which the terpolymers precipitated, was observed. In this region, named the isoelectric point regime (denoted with a purple color in Fig. 2), the negatively ionized PAA segments interact with the oppositely charged P2VP segments and the zeta-potential passes through zero, which is consistent with the isoelectric point (iep) of the ampholytic star terpolymers.
In the third region (7.0 < pH < 11.0), the terpolymers become soluble again due to significant ionization of the PAA segments. As can be seen in Fig. 2b, at 7.0 < pH < 11.0 the net charge of the polymer solution switches to negative values, because of the ionized PAA segments. One can observe that the absolute zeta-potential exhibits remarkably higher values in the basic region than it does in the acidic region (pH < 5.0), although the opposite results should be expected, taking into consideration that the P2VP content is almost double that of the PAA. This could be rationalized mainly by the topology of the star terpolymer, where the PAA segments consist of the outer block in the P2VP-b-PAA arms. The upturn of the zeta-potential observed in this region, which is more pronounced for the star with the higher PAA content, could be attributed to the electrostatic screening arisen from the excess NaOH. Further increasing the pH (pH > 11.0), led to a second phase separation region.
Additional information on the association behavior of the star terpolymers was obtained by taking light scattering intensity measurements at the angle of 90°. I90 reflects the aggregation degree of the formed structures, since the intensity depends upon the mass of the particles. The size of the micelles formed is affected by the hydrophilic/hydrophobic balance of the polymer as well as by the interactions among the P2VP/PAA hydrophilic segments (H-bonding, electrostatic). As shown in Fig. 3, I90 rises above pH 2.5–3.0, which should be attributed to the interactions between the intermolecular hydrophilic segments, increasing therefore the mass of the formed associates. Finally, in the high pH region (pH > 7.0), I90 passes through a minimum in accordance with the turbidity results. At low pH, the protonated P2VP segments, are well soluble in water and the hydrophilic/hydrophobic balance of the terpolymer is shifted toward hydrophilic, resulting in a relatively weak light scattering intensity. At pH > 3.0, the abrupt augmentation of I90 implies that the interactions between the P2VP and the PAA segments become important, leading eventually to precipitation above pH 5.0. At pH > 7.0 the hydrophilic/hydrophobic balance of the star is significantly altered toward highly hydrophobic due to the entire deprotonation of P2VP. The star terpolymers are dissolved again, thanks to the PAA progressive ionization. The intensity still retains high values in the vicinity of the iep region, but it decreases upon pH enhancement, reaching a minimum value at pH 8.0–9.0, which is attributed to the maximum degree of ionization of the PAA segments. Finally, I90 rose again up to pH 11.0, above which a new phase separation was observed. It seems that further addition of NaOH, provokes partial electrostatic screening of the charged AA moieties, decreasing thus the solubility of the corona segments and inducing destabilization of the micellar structures, leading eventually to precipitation. This is corroborated from the high hydrophobic content of the stars.
 | ||
Fig. 3 pH-dependence of the light scattering intensity (at 90°) of 0.2 wt% (PS33)9(P2VP126-b-PAA69)9 (black coloured ■), (PS34)22(P2VP136-b-PAA119)22 (green coloured ■) and (PS29)28(P2VP152-b-PAA86)28 (▲) star terpolymer solutions in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v. The lines are a guide for the eyes. 1 v/v. The lines are a guide for the eyes. | ||
In order to characterize the formed micelles in each pH region, dynamic light scattering experiments were carried out in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v polymer solutions at pH 3.0 and 8.0. Fig. 4a depicts a characteristic autocorrelation function, along with the distribution of relaxation times (through CONTIN analysis) for a 0.06 wt% (PS33)9(P2VP126-b-PAA69)9 polymer concentration, at pH 3.0. Unimodal distribution of relaxation times was obtained, showing a rather low size distribution of the micelles. The hydrodynamic radius of the micelles was determined using the Stokes–Einstein equation. The dependence of Rh, on the polymer concentration is shown in Fig. 4b. From the extrapolation to infinite dilution, the hydrodynamic radius was found to be Rh = 33.9 nm.
1 v/v polymer solutions at pH 3.0 and 8.0. Fig. 4a depicts a characteristic autocorrelation function, along with the distribution of relaxation times (through CONTIN analysis) for a 0.06 wt% (PS33)9(P2VP126-b-PAA69)9 polymer concentration, at pH 3.0. Unimodal distribution of relaxation times was obtained, showing a rather low size distribution of the micelles. The hydrodynamic radius of the micelles was determined using the Stokes–Einstein equation. The dependence of Rh, on the polymer concentration is shown in Fig. 4b. From the extrapolation to infinite dilution, the hydrodynamic radius was found to be Rh = 33.9 nm.
 | ||
| Fig. 4 (a) Autocorrelation function and distribution of relaxation times of 0.06 wt% (PS33)9(P2VP126-b-PAA69)9 solution at pH 3.0. (b) Concentration dependence of the apparent hydrodynamic radius, Rh, at pH 3.0. | ||
At pH 8.0, two peaks were observed in the distribution of relaxation times (Fig. 5), suggesting the formation of small and large associates. The corresponding hydrodynamic size of the two populations was determined to be 10.7 nm and 141.8 nm in radius, respectively. The (PS29)28(P2VP152-b-PAA86)28 terpolymer exhibits a similar behavior at both acidic and basic conditions. Rather broad unimodal distribution at pH 8.0 was observed in the case of (PS34)22(P2VP136-b-PAA119)22.
 | ||
| Fig. 5 (a) Autocorrelation function and distribution of relaxation times of 0.06 wt% (PS33)9(P2VP126-b-PAA69)9 solution at pH 8.0. (b) Concentration dependence of the apparent hydrodynamic radius, Rh, at pH 8.0. | ||
Table 2 presents the results for the apparent diffusion coefficients and hydrodynamic radii of all star terpolymers. As can be seen, the hydrodynamic radius of the associates at low pH, and the first population at high pH, increases with increasing number of arms. This trend could be attributed to two factors. On the one hand, denser structures could be present and, thus, more stretched conformations of the soluble segments would be formed in the corona, provided the hydrophobic content is about the same (comparable aggregation numbers). On the other hand, this increase in the micellar size could be attributed to the relative longer segment length of the P2VP and the PAA at low and high pH, respectively.
| Polymer | pH 3 | pH 8 | ||
|---|---|---|---|---|
| D × 10−12/m2s−1 | R h/nm | D × 10−12/m2s−1 | R h/nm | |
| PS9(P2VP–PAA)9 | 2.415 | 33.9 | 7.835 | 10.7 |
| 5.894 | 141.8 | |||
| PS22(P2VP–PAA)22 | 1.963 | 42.5 | 1.511 | 54.6 |
| PS28(P2VP–PAA)28 | 1.533 | 55.7 | 3.912 | 21.5 |
| 7.425 | 114.6 | |||
AFM was employed to explore the morphology and the structure of the self-assemblies formed in dilute solutions at different pH. Fig. 6 demonstrates the AFM topographies obtained from spin-coating of 0.06 wt% polymer solutions in DMF/H2O, pH 1.0.
 | ||
Fig. 6
AFM images recorded on a mica surface after spin-coating of (PS33)9(P2VP126-b-PAA69)9 (a), (PS34)22(P2VP136-b-PAA119)22 (b) and (PS29)28(P2VP152-b-PAA86)28 (c) solutions in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v at pH 1.0. 1 v/v at pH 1.0. | ||
At pH 1.0, core–shell micelles were revealed by AFM (see height profiles in the inset of Fig. 6b) in all the stars examined. If the contour length of the soluble diblock arms is much higher than the micellar radius, it seems that the star terpolymers primarily form unimolecular micelles (Fig. 6b inset). This is in agreement with previous findings dealing with PSnP2VPn heteroarm stars, which constitute the precursor stars of the terpolymer.34,35 As we have suggested, the amphiphilic multiarm hetero-arm stars adopt unimolecular structures by collapsing the PS hydrophobic arms in the star center, thereby avoiding inter-star association at concentrations significantly higher than the cmc of their linear diblock counterparts. In the case of the PSn(P2VP-b-PAA)n star terpolymers, the addition of the PAA segments, sequentially, to the P2VP arms confers new properties on these stars, because the resulting micelles in aqueous media exhibit interactive coronas in a certain pH window. Consequently, the P2VP and PAA segments of the corona may interact intra- and/or inter-molecularly through H-bonding and/or electrostatic interactions, which affect the association of these stars.
At pH 1.0, the P2VP segments display maximum ionization, while the PAA segments are in the non-ionized state. In this case, the driving force of self-assembling primarily arises from the hydrophobic attractions between the PS segments. Thanks to the star architecture and the topology of the various segments, the hydrophobic PS blocks collapse close to the PDVB nodule forming the core of the micelles, while the shell consists of the P2VP and the PAA. Since the zeta-potential displays positive values, it is suggested that some positively charged P2VP moieties should be located on the surface of the micelles. It seems that the topology of the P2VP-b-PAA segments (a P2VP inner block close to the star nodule), and the better solubility of the P2VP does not favor segregation of the soluble blocks at pH 1.0.
Upon increasing pH, two effects were elaborated, simultaneously, i.e., decrease of P2VP ionization and increase of PAA ionization. Thus, the different soluble segments in the corona interact with each other through H-bonding and possibly through electrostatic interactions among oppositely charged moieties. This leads to the formation of compact spherical nanoparticles at pH 3.0 as observed by AFM (Fig. 7). These interactions reduce the solubility of the stars, inducing intermolecular association.
 | ||
Fig. 7
AFM images recorded on a mica surface after spin-coating of (PS33)9(P2VP126-b-PAA69)9 (a and c) and (PS29)28(P2VP152-b-PAA86)28 (b and d) solutions in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v at pH 3.0. The copolymer concentration was 0.04 mg ml−1 (a and b) and 0.2 mg ml−1 (c and d). 1 v/v at pH 3.0. The copolymer concentration was 0.04 mg ml−1 (a and b) and 0.2 mg ml−1 (c and d). | ||
At low concentrations, the star with the greatest number of arms and the longest length of the P2VP segments, (PS29)28(P2VP152-b-PAA86)28, displays a narrow corona, as can be seen in Fig. 7b. Furthermore, these micelles tend to interact with each other through coronal interactions forming twin micelles.
In the basic one-phase regime, where entire deprotonation of the P2VP segments occurs, the star terpolymers were transformed to a highly hydrophobic state, since their hydrophobic/hydrophilic balance was drastically altered, e.g., for (PS29)28(P2VP152-b-PAA86)28 the hydrophobic content increased from 0.10 to 0.67. Thus, the stability of the star associates arises from the ionization of the outer PAA segments.
In Fig. 8, AFM topographies obtained from (PS29)28(P2VP152-b-PAA86)28 star terpolymer solutions at pH 8.0 demonstrate the formation of interparticle aggregates, which leads, eventually, to dendron-like giant assemblies as a result of increasing concentration (Fig. 8c). It is suggested that spherical nanoparticles, with a negatively charged PAA corona and a PS/P2VP hydrophobic core initially, were formed. This corresponds to the first population of small-size particles observed by dynamic light scattering (Table 2).
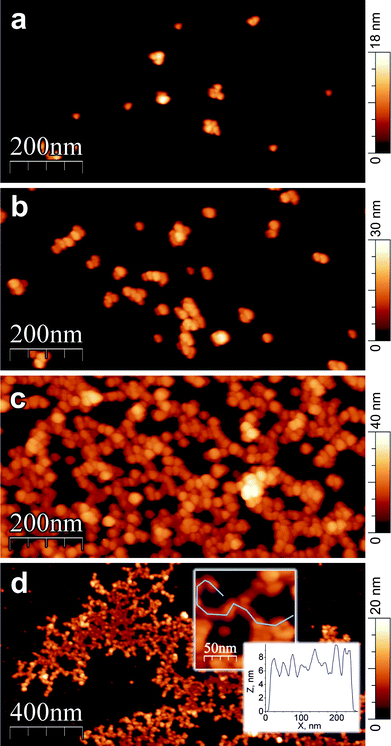 | ||
Fig. 8
AFM images recorded on a mica surface after spin-coating of (PS29)28(P2VP152-b-PAA86)28 (a–c) and (PS33)9(P2VP126-b-PAA69)9 (d) solutions in DMF/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v at pH 8.0. The copolymer concentration was 0.2 mg ml−1 (a and d) and 1.0 mg ml−1 (b and c). 1 v/v at pH 8.0. The copolymer concentration was 0.2 mg ml−1 (a and d) and 1.0 mg ml−1 (b and c). | ||
Further association of the particles was observed by AFM, which corresponds to the second population of larger-size particles as seen in Table 2. This can be clearly seen in Fig. 8a and b, where the polymer concentration increases from 0.2 mg ml−1 to 1.0 mg ml−1, respectively. The mechanism of this interparticle association seems to be similar to that reported for spherical polyelectrolyte brushes in the osmotic limit.36
The star cores of neighboring stars can approach each other by retraction of the stretched arms, driving the nanoparticles to self-assemble in a second level of hierarchy, leading to dendron-like super structures stabilized by interparticle hydrophobic attractions. In Fig. 8d, the AFM image corresponding to (PS33)9(P2VP126-b-PAA69)9, shows a behavior similar to that of (PS29)28(P2VP152-b-PAA86)28.
At this point, we compare the self-assembly morphologies observed in this work with those reported for linear block terpolymers bearing P2VP and PAA segments. At basic conditions, a similar morphology was observed from the P2VP–PMMA–PAA triblock terpolymer, which was attributed to the formation, in a first stage, of heteroarm star-like micelles composed from a PMMA frozen hydrophobic core bearing P2VP and PAA segments in the corona.5 On the other hand, the P2VP–PAA–PnBMA triblock, with the hydrophobic segment at the one end, formed different morphologies, i.e. flower like micelles and toroids.10 Thus, it seems that the heteroarm star topology (chemically or physically formed) is the factor which influences significantly the morphology in the alkaline media. However, in the acidic conditions and at pH 3.0, where the interactions among the P2VP and PAA segments are remarkable, compact spherical micelles were observed in all cases. Finally, at pH 1.0 only the PSn(P2VP-b-PAA)n terpolymers formed unimolecular micelles thanks to the star topology.
4. Conclusions
The phase behavior and self-organization of multiarmed PSn(P2VP-b-PAA)n heteroarm star block terpolymers with hydrophobic (PS) and ampholytic (P2VP-b-PAA) arms were explored in DMF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solvent mixture by varying the pH of the medium. Thanks to the pH-dependent properties of the two P2VP and PAA blocks, and specific interactions among them, these stars respond to pH changes, altering the mechanisms of self-assembling. Turbidimetry, electrophoresis, and static light scattering experiments demonstrated the existence of a rich phase behavior in the DMF/water solutions constituted of four different pH regimes.
1, v/v) solvent mixture by varying the pH of the medium. Thanks to the pH-dependent properties of the two P2VP and PAA blocks, and specific interactions among them, these stars respond to pH changes, altering the mechanisms of self-assembling. Turbidimetry, electrophoresis, and static light scattering experiments demonstrated the existence of a rich phase behavior in the DMF/water solutions constituted of four different pH regimes.
Dynamic light scattering and AFM investigation revealed the formation of micellar self-assemblies. At pH 1.0, positively charged unimolecular core–shell micelles comprised of a hydrophobic PS core, and P2VP and PAA segments in the shell were determined. As pH increases from 1.0 to 3.0, compact spherical micelles were formed, primarily due to H-bonding and possibly electrostatic interactions among the opposite charges of the P2VP and PAA segments. Finally, at even higher pH values, ca. pH 8.0, dendron-like intermicellar large assemblies were observed, as the cores of neighboring micelles interacted by the retraction of the stretched PAA arms and were stabilized through core–core hydrophobic interactions.
We would like to underline that these multifunctional ampholytic star terpolymers can use their different segments as receptors to bind various charged molecules like for instance m-RNA and/or proteins. A first attempt was accomplished by using surfactants that were selectively complexed with the oppositely charged blocks under specific pH conditions, inducing self-organization, the morphology of which depends on which block had been complexed.37
Further work is in progress aiming to explore these multifunctional stars as nanocarriers in pure aqueous environment for controlled multimodal delivery of small and large molecules. Provided that P2VP can be coordinated with a variety of inorganic nanoparticles (e.g., Fe3O4, gold, and quantum dots), novel organic/inorganic smart hybrids can also emerge.
Acknowledgements
The authors would like to thank Mr Michalis Gotsopoulos for his assistance to light scattering experiments.References
- K. Yu and A. Eisenberg, Macromolecules, 1996, 29, 6359 CrossRef CAS.
- K. Yu and A. Eisenberg, Macromolecules, 1998, 31, 3509 CrossRef CAS.
- S. Forster and T. Plantenberg, Angew. Chem., Int. Ed., 2002, 41, 688 CrossRef CAS.
- G. Riess, Prog. Polym. Sci., 2003, 28, 1107 CrossRef CAS.
- V. Sfika, C. Tsitsilianis, A. Kiriy, G. Gorodyska and M. Stamm, Macromolecules, 2004, 37, 9551 CrossRef CAS.
- N. Hadjichristidis, H. Iatrou, M. pitsikalis, S. Pispas and A. Avgeropoulos, Prog. Polym. Sci., 2005, 30, 725 CrossRef CAS.
- J.-F. Gohy, Adv. Polym. Sci., 2005, 190, 65 CrossRef CAS.
- A. Walther, A. S. Goldmann, R. S. Yelamanchili, M. Drechsler, H. Schmalz, A. Eisenberg and A. H. E. Muller, Macromolecules, 2008, 41, 3254 CrossRef CAS.
- H. Cui, Z. Chen, K. L. Wooley and D. J. Pochan, Macromolecules, 2006, 39, 6599 CrossRef CAS.
- C. Tsitsilianis, Y. Roiter, I. Katsampas and S. Minko, Macromolecules, 2008, 41, 925 CrossRef CAS.
- M. Uchman, M. Stepanek, K. Prochazka, G. Mountrichas, S. Pispas, I. K. Voets and A. Walther, Macromolecules, 2009, 42, 5605 CrossRef CAS.
- R. C. Hayward and D. J. Pochan, Macromolecules, 2010, 43, 3577 CrossRef CAS.
- M. Zhang, M. Wang, S. He, J. Qian, A. Saffari, A. Lee, S. Kumar, Y. Hassan, A. Guenther, G. Scholes and M. A. Winnik, Macromolecules, 2010, 43, 5066 CrossRef CAS.
- K. Skrabania, H. V. Berlepsch, C. Bottcher and A. Laschewsky, Macromolecules, 2010, 43, 271 CrossRef CAS.
- J. Dupont and G. Liu, Soft Matter, 2010, 6, 3654 RSC.
- F. Liu and A. Eisenberg, J. Am. Chem. Soc., 2003, 125, 15059 CrossRef CAS.
- A. Walther, P.-E. Millard, A. S. Goldmann, T. M. Lovestead, F. Schacher, C. Barner-Kowollik and A. H. E. Muller, Macromolecules, 2008, 41, 8608 CrossRef CAS.
- A. Walther, C. Barner-Kowollik and A. H. E. Muller, Langmuir, 2010, 26, 12237 CrossRef CAS.
- X. Li, H. Yang, L. Xu, X. Fu, H. Guo and X. Zhang, Macromol. Chem. Phys., 2010, 211, 297 CrossRef CAS.
- S. Kubowicz, J.-F. Baussard, J.-F. Lutz, A. F. Thunemann, H. von Berlepsch and A. Laschewsky, Angew. Chem., Int. Ed., 2005, 44, 5262 CrossRef CAS.
- B. Fang, A. Walther, A. Wolf, Y. Xu, J. Yuan and A. H. E. Muller, Angew. Chem., Int. Ed., 2009, 48, 2877 CrossRef CAS.
- A. Walther, C. Barner-Kowollik and A. H. E. Muller, Langmuir, 2010, 26, 12237 CrossRef CAS.
- H. Huang, B. Chung, J. Jung, H.-W. Park and T. Chang, Angew. Chem., Int. Ed., 2009, 48, 4594 CrossRef CAS.
- H. Huckstadt, A. Gopfert and V. Abetz, Macromol. Chem. Phys., 2000, 201, 296 CrossRef CAS.
- C. Li, Z. Ge, H. Liu and S. Liu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4001 CrossRef CAS.
- W. Zhang, W. Zhang, N. Zhou, J. Zhu, Z. Cheng and X. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6304 CrossRef CAS.
- W. Kong, B. Li, Q. Jin, D. Ding and A.-C. Shi, J. Am. Chem. Soc., 2009, 131, 8503 CrossRef CAS.
- K. Van Butsele, C. A. Fustin, J. F. Gohy, R. Jerome and C. Jerome, Langmuir, 2009, 25, 107 CrossRef CAS.
- (a) Z. Zhou, Z. Li, Y. Ren, M. A. Hilmyer and T. Lodge, J. Am. Chem. Soc., 2003, 125, 10182 CrossRef CAS; (b) Z. Li, M. A. Hilmyer and T. P. Lodge, Macromolecules, 2004, 37, 8933 CrossRef CAS; (c) Z. Li, E. Kesselman, Y. Talmon, M. A. Hilmyer and T. P. Lodge, Science, 2004, 306, 98 CrossRef CAS; (d) T. P. Lodge, A. Rasdal, Z. Li and M. A. Hilmyer, J. Am. Chem. Soc., 2005, 127, 17608 CrossRef CAS; (e) Z. Li, M. A. Hilmyer and T. P. Lodge, Macromolecules, 2006, 39, 765 CrossRef CAS; (f) Z. Li, M. A. Hilmyer and T. P. Lodge, Langmuir, 2006, 22, 9409 CrossRef CAS; (g) N. Saito, C. Liu, T. P. Lodge and M. A. Hilmyer, Macromolecules, 2008, 41, 8815 CrossRef CAS; (h) C. Liu, M. A. Hilmyer and T. P. Lodge, Langmuir, 2009, 25, 13718 CrossRef CAS; (i) N. Saito, C. Liu, T. P. Lodge and M. A. Hilmyer, ACS Nano, 2010, 4, 1907 CrossRef CAS.
- (a) H. Liu, C. Li, H. Liu and S. Liu, Langmuir, 2009, 25, 4724 CrossRef CAS; (b) C. Li, Z. Ge, H. Liu and S. Liu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4001 CrossRef CAS; (c) Y. Zhang, H. Liu, H. Dong, C. Li and S. Liu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1636 CrossRef CAS.
- (a) C. Tsitsilianis, P. Chaumont and P. Rempp, Makromol. Chem., 1990, 191, 2309 CrossRef CAS; (b) C. Tsitsilianis, S. Graff and P. Rempp, Eur. Polym. J., 1991, 27, 243 CrossRef CAS; (c) J. P. Rein, J. P. Lamps, P. Rempp, P. Lutz, D. Papanagopoulos and C. Tsitsilianis, Acta Polym., 1993, 44, 225 CrossRef; (d) C. Tsitsilianis, D. Papanagopoulos and P. Lutz, Polymer, 1995, 36, 3745 CrossRef CAS; (e) C. Tsitsilianis and D. Voulgaris, J. Macromol. Sci., Part A: Pure Appl.Chem., 1995, 32(Suppl. 5 & 6), 569 Search PubMed; (f) C. Tsitsilianis and D. Voulgaris, Macromol. Chem. Phys., 1997, 198, 997 CrossRef CAS.
- G. Linardatos, G. Tsoukleri, J. Parthenios, C. Galiotis, O. Monticelli, S. Russo and C. Tsitsilianis, Macromol. Rapid Commun., 2011, 32, 371 CrossRef CAS.
- C. Tsitsilianis, N. Stavrouli, V. Bocharova, S. Angelopoulos, A. Kiriy, I. Katsampas and M. Stamm, Polymer, 2008, 49, 2996 CrossRef CAS.
- A. Kiriy, G. Gorodyska, S. Minko, M. Stamm and C. Tsitsilianis, Macromolecules, 2003, 36, 8704 CrossRef CAS.
- (a) A. Gorodyska, A. Kiriy, S. Minko, C. Tsitsilianis and M. Stamm, Nano Lett., 2003, 3, 365 CrossRef; (b) A. Kiriy, G. Gorodyska, S. Minko, C. Tsitsilianis, W. Jaeger and M. Stamm, J. Am. Chem. Soc., 2003, 125, 11202 CrossRef CAS.
- (a) A. Jusufi, C. N. Likos and M. Ballauff, Colloid Polym. Sci., 2004, 282, 910 CrossRef CAS; (b) A. Wittermann, M. Drechsler, Y. Talmon and M. Ballauff, J. Am. Chem. Soc., 2005, 127, 9688 CrossRef.
- M. R. Hammond, C. Li, C. Tsitsilianis and R. Mezzenga, Soft Matter, 2009, 5, 2371 RSC.
| This journal is © The Royal Society of Chemistry 2011 |
