Menthol-based chiral copolymers for polymer optical fibres (POF)†
Eun Hee
Min
*a,
Kok Hou
Wong
a,
Eki
Setijadi
a,
François
Ladouceur
a,
Mark
Straton
b and
Alexander
Argyros
*b
aPhotonics Group, School of Electrical Engineering and Telecommunications, The University of New South Wales, Sydney, NSW 2052, Australia. E-mail: eunhee.min@unsw.edu.au; Fax: +61 2 93855388; Tel: +61 2 93854892
bInstitute of Photonics and Optical Science, School of Physics A28, The University of Sydney, NSW 2006, Australia. E-mail: alexander.argyros@sydney.edu.au; Fax: +61 2 93517726; Tel: +61 2 91140872
First published on 5th July 2011
Abstract
This study investigates the fabrication of menthol-based copolymers for use in polymer optical fibres (POF). A chiral monomer, (−)-menthyl methacrylate ((−)-MnMA) derived from (−)-menthol, was synthesized that displays a specific rotation of [α]20D (neat) = −90. It was further copolymerized with methyl methacrylate (MMA) either in bulk or in solution viafree radical polymerization. PMMA-co-P(−)-MnMA copolymers synthesized in bulk yielded turbid polymers, whereas copolymer synthesized in solution with 50 mol% (−)-MnMA feed was transparent with some optical rotation, and compatible for drawing into fibres. Hence, PMMA-co-P(−)-MnMA in the current study is a potential chiral material for use in POF.
1 Introduction
In the field of optics, optical rotation designates the property of certain classes of materials that can rotate the polarization plane of light. Optical rotation is a manifestation of circular birefringence i.e. the property of an optically active substance exhibiting different refractive indices for right-handed circularly polarized (RHCP) light and left-handed circularly polarized (LHCP) light. This difference in indices induces a difference in the speed of propagating light (and accumulated phase), resulting in a rotation of the polarization plane.1Circular birefringence in optical waveguides can be introduced through a break in the longitudinal symmetry of the waveguides, for example by spinning the optical fibres to produce a helical structure. This is, however, a difficult and complex technique; therefore, circular birefringence in optical fibres has not been well studied to the present.2 Another technique to introduce circular birefringence in optical fibres is via the use of chiral materials.3 The majority of chiral materials are organic and incompatible with the processing temperatures of glass optical fibres (i.e. 1800 °C). In contrast, POF, which is generally processed at 180 °C, offers unique opportunity and potential to study the effects of circular birefringence, using chiral materials in optical fibres.
To the best of our knowledge, besides theoretical studies,4–8 there is only one experimental demonstration of chiral materials incorporated into optical fibres, where a hollow core fibre was filled with a solution of chiral molecules, liquid (−)-fructose.9 Nevertheless, besides evidence of optical rotation in this fibre, the use of (−)-fructose solution is expected to be impractical as liquids in fibres are not robust and can result in leakages. Therefore, we considered using chiral polymers to generate circular birefringence in POF.10 Herein, we develop a solid and robust chiral polymer for the fabrication of optically active POF, which offers an alternative approach to the investigation of circular birefringence in optical fibres.
There are three avenues for introducing chirality into polymers. The first approach is doping PMMA, the most common POF polymeric material, with chiral compounds by swelling the polymer with solvent to allow infusion of chiral compounds (dopants)11 into the polymer. In this case, only a low loading of dopants would be expected resulting in only small optical rotation being imparted to the PMMA. The presence of residual solvent, which evaporates at elevated temperatures, may also be problematic during fibre fabrication.
The second approach is to identify a chiral monomer and homo-polymerize to produce unadulterated chiral homopolymers for use in POF (i.e. as above but without PMMA). This approach is likely to produce chiral polymers with the highest optical activities, but their suitabilities for fibre fabrication and use in POF are of concern and challenging.
The third approach is to covalently bind chiral compounds to MMA monomer viafree radical polymerization to produce PMMA-co-chiral polymers. In contrast to doping, it is expected to produce copolymers containing higher concentrations of chiral compounds with elevated optical activities. This approach also retains some of the PMMA properties that are favorable for POF fabrication.
In this study, the second and third approaches were investigated. Menthol was selected from various chiral compounds, as menthol is readily available as both left-handed (−)-menthol and right-handed (+)-menthol enantiomers, which display optical rotations of c = [α]20D = −50 in 10% ethanol, and c = [α]23D = +48 in 10% ethanol, respectively. Choosing a material for which both enantiomers are readily available means both (−) and (+) forms of the resulting chiral polymer can be realised. Compared to chiral materials with one enantiomer, they can potentially maximise optical properties (optical rotation) when incorporated into optical fibres.8,9 Our study here focuses on the use of (−)-menthol, only as a testimony of concept and potential. Our approach is shown in Scheme 1, the (−)-menthol is firstly functionalized with a methacrylate group to produce a chiral monomer, (−)-menthyl methacrylate ((−)-MnMA), and subsequently co-polymerized with MMA to produce PMMA-co-P(−)-MnMA polymers, and also homo-polymerized to produce P(−)-MnMAviafree radical polymerization. The properties of the resulting polymers and their suitability for use in optical fibres were assessed.
 | ||
| Scheme 1 Functionalization of (−)-menthol to produce (−)-MnMA, co-polymerization of MMA and (−)-MnMA, and homo-polymerization of (−)-MnMA. | ||
2 Experimental methods
2.1 Materials
(1R,2S,5R)-(−)-Menthol (Aldrich, 99%) was used as received. Methyl methacrylate (MMA, 99%) was bought from Aldrich and was de-inhibited by passing through an alumina column before use. Benzoyl peroxide (BPO) from Aldrich was crystallized twice in methanol before use. Molecular sieves (Ajax Finechem, 4 Å) were first activated at a temperature of 200 °C before use. Methacryloyl chloride (Fluka, 97%), triethylamine (TEA, Sigma-Aldrich, 99%), anhydrous dichloromethane (DCM, Sigma-Aldrich, 99.8%), hydrochloric acid (HCl, Ajax Finechem, 32%), sodium hydrogen carbonate (NaHCO3, Ajax Finechem, 99.7%), sodium chloride (NaCl, Ajax Finechem, 99.9%), sodium sulfate anhydrous powder (Na2SO4, Ajax Finechem, 99%), ethyl acetate (Ajax Finechem, 99.5%), and n-hexane (Ajax Finechem, 95%) were used as received.2.2 Synthesis of (−)-MnMA
(−)-MnMA was synthesized as reported in the literature12 with some alterations. In a 250 mL flask, equipped with a magnetic stirrer, (−)-menthol (10 g, 1.06 mol) and methacryloyl chloride (7 g, 1.1 mol) were added to a solution of TEA (13.3 mL) in anhydrous DCM (60 mL) at 0 °C. Molecular sieves (4 Å) were added to the reaction mixture to absorb water produced during the reaction. The clear solution turned turbid after a few minutes (exothermic reaction). After 5 h, the suspension was extracted with a mixture of 50 mL of 0.5 N HCl and 50 mL of DCM. The extraction was washed with 50 mL of distilled water, 50 mL of saturated aqueous NaHCO3, and 50 mL of brine. The extraction was dried over Na2SO4 to give a clear orange solution. Concentrating at reduced pressure gave light brown oil. Purification by column chromatography (eluents: ethyl acetate/n-hexane = 1/8) gave 8.0 g (yield 56%) of slightly viscous (oil-like) transparent liquid. 1H NMR (CDCl3, 300 MHz) δ (ppm): 6.05 (dq, 1H, H-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 5.5 (t, 1H, H-C
C), 5.5 (t, 1H, H-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 4.74 (ddd, 1H, -CO-CH-C), 2.06 (m, 1H, CH-CH-CH), 1.93 (dd, 3H, CH3-C), 1.85 (dqq, 1H, CH3-CH-CH3), 1.75–1.60 (dd, 1H, CH2-CH-CH2), 1.74–1.64 (m, 2H, CH-CH2-CH), 1.62–1.37 (m, 2H, CH-CH2-CH), 1.18–0.93 (m, 2H, CH-CH2-CH2, CH-CH2-CH2), 0.90 (d, 3H, CH2-CH-CH3), 0.89 (d, 3H, CH-CH3), 0.77 (d, 3H, CH-CH3).
C), 4.74 (ddd, 1H, -CO-CH-C), 2.06 (m, 1H, CH-CH-CH), 1.93 (dd, 3H, CH3-C), 1.85 (dqq, 1H, CH3-CH-CH3), 1.75–1.60 (dd, 1H, CH2-CH-CH2), 1.74–1.64 (m, 2H, CH-CH2-CH), 1.62–1.37 (m, 2H, CH-CH2-CH), 1.18–0.93 (m, 2H, CH-CH2-CH2, CH-CH2-CH2), 0.90 (d, 3H, CH2-CH-CH3), 0.89 (d, 3H, CH-CH3), 0.77 (d, 3H, CH-CH3).
2.3 Co-polymerization of MMA and (−)-MnMA in bulk
MMA and (−)-MnMA were mixed in a glass tube, and stirred for about 30 minutes with a magnetic stirrer. BPO (0.5 mmol) as an initiator was added to the monomer solution and homogeneously mixed using an ultrasonicator for several seconds. (−)-MnMA was added into the mixture according to the different respective molar ratios (5 mol% to 50 mol%) with MMA. The reaction mixture was polymerized in a temperature controlled convection oven, where the reaction temperature was increased 5 °C per hour from room temperature until it reached 60 °C. The reaction mixture was further polymerized at 60 °C for another 24 h. The solid copolymer was recovered without purification, and was further dried in a drying oven at 70 °C over 3 days.2.4 Homo-polymerization of MMA and (−)-MnMA in bulk
MMA (0.03 mol) and (−)-MnMA (0.33 mol) were polymerized using BPO (0.5 mmol), respectively, into PMMA and P(−)-MnMA using similar reaction steps as described above.2.5 Co-polymerization of MMA and (−)-MnMA in solution
MMA and (−)-MnMA were copolymerized by the same procedure as the section on bulk polymerization except the addition of 0.5 mL anhydrous DMF as a solvent. For determination of reactivity ratio, the copolymer conversions were restricted to less than 10% in order to follow the copolymer equation, Finemann–Ross (F–R) method.2.6 Homo-polymerization of MMA and (−)-MnMA in solution
MMA and (−)-MnMA were homo-polymerized in DMF into PMMA and P(−)-MnMA, respectively, using the same procedure as the above section on copolymerization of MMA and (−)-MnMA in solution.2.7 Size Exclusion Chromatography (SEC)
Molecular weight distributions were determined by SEC with a Shimadzu modular system with N,N-dimethylacetamide (DMAc) (0.03% w/v LiBr, 0.05% BHT stabilizer) at 40 °C with a flow rate of 1.0 mL min−1. The system incorporated a DGU-12A solvent degasser, a LC-10AT pump and a CTO-10A column oven and was equipped with a RID-10A refractive index detector. A Polymer Laboratories (PL) 5.0 μm bead-size guard column (50 × 7.5 mm) followed by four 300 × 7.8 mm linear PL columns (105, 104, 103, and 500 Å) were used to separate the samples. The system was calibrated using narrow PL polystyrene standards ranging from 500 to 106 g mol−1. Filtered polymer samples using 0.45 μm RC filters were injected at concentrations of 2 mg mL−1. Chromatograms were processed using Cirrus 2.0 software from PL.2.8 Nuclear Magnetic Resonance (NMR)
All NMR spectra were recorded using a Bruker DPX-300 spectrometer with a resonance frequency of 300.2 MHz for 1H nuclei at 25 °C. All polymers were analyzed using deuterated chloroform (CDCl3) as solvent.2.9 Differential Scanning Calorimetry (DSC)
A Perkin-Elmer differential scanning calorimeter (DSC 7) and a thermal analysis controller (TAC71DX) were used in conjunction to measure glass transition temperatures. The DSC instrument was calibrated with indium and zinc standards of known mass, melting point temperature and known associated enthalpy change. Samples were analyzed in hermetic aluminium pans and lids with pin holes, sample sizes varied between 15 and 20 mg, over the temperature range of −20 to 200 °C with a scanning rate of 10 to 20 °C min−1 under N2 atmosphere.2.10 Optical rotation
The optical rotation was measured using a supercontinuum light source consisting of a silica photonic crystal fibre pumped by a pulsed Nd:YAG laser operating at 1064 nm, with a pulse width of 560 ps and a repetition rate of 7.2 kHz. The output spectrum ranges in wavelength λ from 400 nm to >1750 nm. It was used in these measurements as it is a bright source that can be easily collimated into a narrow beam of ∼1 mm diameter. The optical rotation of (−)-MnMA and copolymers synthesized was measured by polarizing the supercontinuum output using a polarizer and then passing it through the sample. A second polarizer was used to determine the polarization of the light after it had passed through the sample. An optical spectrum analyzer (OSA) was used to measure the output power at different wavelengths, and the second polarizer was rotated until the power at a particular wavelength was minimized. This would occur when the second polarizer was perpendicular to the first in the absence of a sample. Any additional rotation above/beyond 90° represents the rotation of polarization by the sample, and hence allows the optical rotation to be measured once the sample's length was taken into account.3 Results and discussions
3.1 Requirements of chiral polymers for POF
There are several requirements which must be satisfied by the chiral polymers before they can be used to introduce circular birefringence in POF, and these are (i) transparency (i.e. over several metres of sample thickness), (ii) drawability into fibres, (iii) optical rotation, (iv) low solvent absorption, and (v) robustness for easy handling in order to explore the novel optical effects of interest. Polymers for POF must be intrinsically transparent and homogeneous to reduce absorption of light, and scattering, respectively. The latter demands the polymer to be amorphous, with no phase separations and/or clustering of substances. For a polymer to be drawn into a fibre it must be free (as much as possible) of any volatile impurities, and solvents to prevent bubbling, and be stable at temperatures required for drawing. The polymer must not be cross-linked and must be of suitable molecular weight and polydispersity index for fibre fabrication. Lastly, a high optical rotation will require sufficient loading of chiral materials into the polymer.3.2 Co-polymerization of MMA and (−)-MnMA in bulk
This co-polymerization was done in bulk (without solvent) to yield solid and solvent free copolymers. The reaction mixture of MMA and (−)-MnMA (95/5 to 50/50, mol%/mol%) was initially clear, however, turned turbid after 3 to 4 h during polymerization at 60 °C. The resultant copolymers were cloudy especially with higher content of (−)-MnMA. The cloudiness is believed to be due to the crystallization of P(−)-MnMA homo-polymer that can occur during the polymerization. In fact, P(−)-MnM having a highly ordered structure was very cloudy and mechanically brittle compared to PMMA or PMMA-co-P(−)-MnMA (see ESI†). Another reason for the cloudy copolymers could be attributed to a different monomer reactivity ratio. For this reason, the reactivity ratio between the monomers was studied using the F–R method as discussed below.3.3 Co-polymerization of MMA and (−)-MnMA in solution
This copolymerization was carried out in minimum amount of solvent (i.e.DMF), in the hope to delay crystallization of P(−)-MnMA, which is believed to cause cloudiness of the copolymers synthesized in bulk (as above). The resulting copolymers were also subsequently dried under reduced pressure to remove residual solvent. Improved transparency of the PMMA-co-P(−)-MnMA (MMA/(−)-MnMA, 95/5 to 50/50, mol%/mol%) copolymers was evident compared to copolymers polymerized in bulk (see ESI†).However, this co-polymerization in solution approach produced undesirable air bubbles in the resulting copolymers unlike the ones copolymerized in bulk. The presence of air bubbles is unacceptable in terms of optical fibre fabrication. Several approaches were employed to remove the air bubbles from the polymers synthesized. Firstly, co-polymerization was conducted under vacuum to aid the escape of air bubbles from the reaction mixture solutions. Fewer air bubbles were observed in the resulting copolymers however, complete elimination of bubbles was not possible. Another approach was to increase the reaction temperature very slowly (i.e. 5 °C per hour), from room temperature to the desired co-polymerization temperature of 60 °C. The initiator in the reaction solution will decompose gradually, leading to a more steady propagation of monomers with minimum gases given off (air bubbles) compared to a sudden thermal shock co-polymerization. At the same time, inevitable air bubbles generated will have enough time to escape from the reaction mixture as the viscosity increases slowly during the process of co-polymerization. As a result, gradually increasing the reaction temperature was the most efficient way to reduce air bubbles in the current study.
In fact, when high vacuum was applied simultaneously with the slow increase in reaction temperature approach during co-polymerization of MMA and (−)-MnMA in solution, transparent and bubble free PMMA-co-P(−)-MnMA copolymers were obtained even up to (MMA/(−)-MnMA, 50/50, mol%/mol%).
3.4 Physical properties and optical rotation of (−)-MnMA
(−)-MnMA after purifying by column chromatography is a slightly viscous (oil-like) transparent liquid as seen in the ESI†. Fig. 1 illustrates the measurement of optical rotation of (−)-MnMA as a function of wavelength. The specific rotation value of (−)-MnMA synthesized ([α]20D (neat) = −90 to −91) is in good agreement with literature values;13 ‘D’ and ‘neat’ refer to the D line of a sodium lamp (589 nm) and an undiluted liquid, respectively. The optical rotation of (−)-menthol was also characterized. The sample was used undiluted, and heated to 60 °C, so it was in its liquid state (mp ≈ 40 °C). The results are also shown in Fig. 1, and a value of ([α]60D (neat) = −51) was measured. | ||
| Fig. 1 Optical rotation of (−)-menthol, and (−)-MnMA as (a) a function of wavelength and (b) a function of (wavelength)−2. | ||
The rotation of the plane of linear polarization is most commonly described through the optical rotation, α (in degrees per decimetre), however other methods of expressing it become more intuitive in the context of optics and optical fibres.
From Fig. 1, it can be seen that α depends on the wavelength λ, with an α ∝ λ−2 dependence. The effect of chirality on polarization can also be expressed in terms of the chirality parameter γ, which has units of metres and is independent of wavelength. This chirality parameter can be extracted from the slope of the graph in Fig. 1(b), and is related to α through,
 | (1) |
 | (2) |
A third quantity of interest is the length of propagation required to achieve a significant change in the polarization of light. This ‘characteristic length’, which describes the strength of the optical rotation, is the beat length LB, which is defined as the length of propagation required to bring the light back to its original polarization, i.e. a rotation of 180°. This depends on the other parameters as
 | (3) |
Hence, the measurement of α and γ in Fig. 2 allows these parameters to be determined. The value of these parameters for the resulting polymers can be used to assess the feasibility of their use in optical fibres.
 | ||
| Fig. 2 Optical rotation measurement of (−)-MnMA and the chiral co-polymers at a wavelength of 589 nm (D line) at room temperature. (A) (−)-MnMA (neat); (B) the copolymer (10 mol% (−)-MnMA in feed); (C) the copolymer (30 mol% (−)-MnMA in feed); (D) the copolymer (50 mol% (−)-MnMA in feed). | ||
3.5 Optical rotation of copolymers synthesized in solution
The results of the measurement of optical rotation of the various samples at a wavelength of 589 nm (D line) at room temperature are shown in Fig. 2. Optical rotation of the copolymers gradually increases as the amount of (−)-MnMA in the feed increases.Measurements of the optical rotation of different samples containing different (−)-MnMA feeds indicated that the optical rotation simply scaled linearly with the (−)-MnMA content. Extrapolating this to 100% feed (i.e.P(−)-MnMA only) reproduced the optical rotation of the (−)-MnMA monomer. Thus it was concluded that the optical rotation is not appreciably changed by the polymerization. It should be noted that the optical rotation of these copolymers was measured using solid samples of polymer, therefore it is not possible to compare directly with values of optical rotation in the literature, which were generally derived from measurements using solutions. Furthermore, optical rotation depends on the temperature, wavelength, concentration, and solvent used.
3.6 Monomer reactivity ratios
The determination of reactivity ratios can be a way to understand the copolymerization behavior of the co-monomers. Monomer feed (M1) versusco-polymer (m1) composition was plotted in Fig. 3.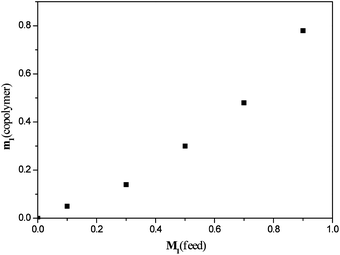 | ||
| Fig. 3 Copolymer vs. feed composition of MMA and (−)-MnMA. M1, mole fraction of (−)-MnMA in feed; m1, mole fraction of (−)-MnMA in copolymer. | ||
The co-polymerization reactivity ratios were calculated using the F–R method, which is governed by the fundamental equation:
 | (4) |
| Entry | x = M1/M2 | y = m1/m2 | G = x(y − 1)/y | F = x2/y | M 1((−)-MnMA) | M 2(MMA) | m 1((−)-MnMA) | m 2(MMA) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.11 | 0.05 | −1.98 | 0.23 | 0.1 | 0.9 | 0.05 | 0.94 |
| 2 | 0.43 | 0.16 | −2.20 | 1.13 | 0.3 | 0.7 | 0.14 | 0.86 |
| 3 | 1.00 | 0.43 | −1.33 | 2.33 | 0.5 | 0.5 | 0.3 | 0.7 |
| 4 | 2.33 | 0.92 | −0.19 | 5.90 | 0.7 | 0.3 | 0.48 | 0.52 |
| 5 | 9.00 | 3.55 | 6.46 | 22.85 | 0.9 | 0.1 | 0.78 | 0.22 |
 | ||
| Fig. 4 F–R plot for co-polymerization of (−)-MnMA and MMA in solution. | ||
P(−)-MnMA is a thoroughly white brittle polymer as seen in the ESI†. Therefore, the cloudiness of the copolymer is attributed to the P(−)-MnMA block, which is influenced by the feed of (−)-MnMA.
3.7 Molecular weight of PMMA-co-P(−)-MnMA
Another important factor to consider in drawing POF is the polymer molecular weight and its distributions (polydispersity index, PDI). The typical number average molecular weights (Mn) of PMMA suitable for POF drawing are between 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 50
000 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 with broad PDI (Mw/Mn), generally beyond 2. For example, samples with molecular weights that are too large or low PDI become rubbery upon heating and tear under tension, rather than drawing to produce fibres.
000 g mol−1 with broad PDI (Mw/Mn), generally beyond 2. For example, samples with molecular weights that are too large or low PDI become rubbery upon heating and tear under tension, rather than drawing to produce fibres.
Table 2 lists molecular weights and PDI of P(−)-MnMA, and PMMA-co-P(−)-MnMA regarding initial monomer feed in solution.
| Entry | (−)-MnMA in feed (mol%) | MMA in feed (mol%) | M n/g mol−1 | M w/g mol−1 | M n/Mw (PDI) |
|---|---|---|---|---|---|
| M1 | 0 | 100 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.1 |
| M2 | 5 | 95 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.2 |
| M3 | 10 | 90 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.1 |
| M4 | 30 | 70 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
218![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
5.3 |
| M5 | 50 | 50 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
404![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
4.6 |
| M6 | 100 | 0 | 124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
337![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
3.1 |
As shown in Table 2, the homo-polymerizations of MMA (M1) and (−)-MnMA (M6) have a PDI of approximately 2, while an upsurge of PDI was noticed with increasing concentrations of (−)-MnMA in the copolymers. Upon further investigation, the copolymer GPC spectra in Fig. 5 exhibited multi-modal distributions, beginning with the copolymer with 10 mol% of (−)-MnMA. The magnitude of the peaks also increased and intensified as the percentage of (−)-MnMA increased in the copolymers.
 | ||
| Fig. 5 GPC spectra of PMMA-co-P(−)-MnMA. M1, PMMA; M2, PMMA-co-P(−)-MnMA (5 mol% (−)-MnMA); M3, PMMA-co-P(−)-MnMA (10 mol% (−)-MnMA); M4, PMMA-co-P(−)-MnMA (30 mol% (−)-MnMA); M5, PMMA-co-P(−)-MnMA (50 mol% (−)-MnMA); M6, P(−)-MnMA. | ||
A multi-modal distributed GPC spectrum of polymers is indicative either of several populations of PMMA-co-P(−)-MnMA copolymers or an assortment of PMMA, P(−)-MnMA homo-polymers, and PMMA-co-P(−)-MnMA copolymers in the samples or even due to no purification step after polymerization. It is believed that the latter is prevalent in this study because bulk homo-polymerization of (−)-MnMA resulted in opaque polymers and bulk copolymerization of MMA and (−)-MnMA with 30 or more mol% of (−)-MnMA, resulted in less opaque polymers. It is suggested that the systems in this study are preferential to homo-polymerization of similar monomers instead of the desired co-polymerization of various monomers. This can be assigned to a significant difference in reactivity ratios of the various types of monomers in the systems as discussed above.
3.8 Glass transition temperature (Tg)
It is fundamental to determine the optimum draw temperatures of the materials of POF. These optimum temperatures are typically about 60 °C above the glass transition temperature (Tg) of the POF materials. Experience has shown that drawing POF at non-optimum temperatures can lead to undesirable effects including fibre breakages during drawing, air pockets or bubbles along the fibre, and stresses introduced into the fibre.Table 3 is a summary of Tg values of the synthesized PMMA-co-PMnMA copolymers in this study, determined using DSC. (−)-Menthol does not have a Tg but three melting points were prominent from its compositions, isomer (∼33 °C), racemic (∼38 °C) and form- (∼44 °C). After functionalizing (−)-MnMA with a methacrylate group into (−)-MnMA and polymerization into P(−)-MnMA (M6), the (−)-menthol-based polymer exhibited an average Tg of approximately 83 °C. This temperature is lower than that of PMMA (M1), commonly used for POF. The Tg values of PMMA-co-P(−)-MnMA copolymers synthesized were determined to be among these temperatures as anticipated. In fact, reductions in Tg values were evident in copolymers with elevating concentrations of P(−)-MnMA such as copolymers M5 (∼95 °C), M4 (∼96 °C), M3 (∼100 °C) and M2 (∼104 °C). The copolymer with 5 mol% of (−)-MnMA has Tg values adjacent to that of PMMA (M1, ∼112.5 °C). These results suggest that current drawing temperatures (about 170–190 °C) for drawing PMMA POF can be applied with minimum alterations to draw chiral-based fibres from copolymers.
| Entry | (−)-MnMA in feed (mol%) | MMA in feed (mol%) | T g/°C |
|---|---|---|---|
| M1 | 0 | 100 | 110–115 |
| M2 | 5 | 95 | 102–106 |
| M3 | 10 | 90 | 99–101 |
| M4 | 30 | 70 | 95–97 |
| M5 | 50 | 50 | 93–97 |
| M6 | 100 | 0 | 81–85 |
3.9 Potential for fabrication of chiral POF
The copolymers discussed above are suitable in principle for use in POF as per the initial aim of this work. Such polymers incorporated into the core of a POF will provide a circular birefringence arising from the chiral nature of the material.Taking into account the value of the chiral parameter measured above, the amount of circular birefringence will be given by
 | (5) |
 | (6) |
An estimate of beat length for a fibre with a large 50 mol% (−)-MnMA copolymer core ranges from 18 to 64 cm for wavelengths across the visible. Hence, the strength of the optical rotation is sufficient for the polarization of light to be manipulated over lengths of 10s of cm, meaning the use of this polymer in optical fibres is practical and feasible in this sense.
To produce optical fibres however, there are additional criteria that the polymer must satisfy as discussed above. It must be able to be drawn into the fibre upon heating, and must not contain any volatile impurities (e.g.solvents) that will evaporate and cause bubbling, etc. To test these criteria, a 4 mm diameter × 2 cm length sample of PMMA-co-P(−)-MnMA with a 50 mol% (−)-MnMA feed was heated at 90 °C, under vacuum for several days, to ensure that any remaining solvent had been evaporated. This was placed inside a PMMA tube with an inner diameter of 6 mm and an outer diameter of 12 mm. Vacuum was applied to the PMMA tube to remove air, and the tube was heated and drawn to a fibre of diameter <1 mm. The fibre was cleaved and inspected to show no evidence of bubbling and good adhesion between the two polymers, as expected given the PMMA content of the copolymer (Fig. 6).
 | ||
| Fig. 6 Optical microscope image of a preliminary chiral optical fibre consisting of PMMA-co-P(−)-MnMA core and pure PMMA cladding. | ||
Although this produced a fibre with a PMMA-co-P(−)-MnMA core and PMMA cladding, the difference in reflective index between the two polymers (i.e. core and cladding) was insufficient to produce strong guidance of light. Nevertheless, this result proves that the chiral polymer developed is suitable for use in POF and compatible with established PMMA–POF approaches.
4 Conclusions
Chiral monomer, (−)-MnMA, was synthesized from a reaction between methacryloyl chloride and (−)-menthol, and its optical rotation ([α]20D (neat) = −90) is in good agreement with values from the literature. (−)-MnMA was subsequently copolymerized with MMA according to the different respective molar ratios (5 to 50 mol%) either in bulk (without solvent) or in solution (with solvent, i.e.DMF) viafree radical polymerization. PMMA-co-P(−)-MnMA synthesized in bulk was turbid even with a small feed of (−)-MnMA due to rapid crystallization of P(−)-MnMA and a significant difference in monomer reactivity of the two monomers. Interestingly, the transparency of PMMA-co-P(−)-MnMA synthesized in solution was maintained even when the (−)-MnMA feed was up to 50 mol%. This copolymer also possessed favorable properties of molecular weight, polydispersity index, and Tg, which is amenable for fibre drawing. This PMMA-co-P(−)-MnMA was successfully co-drawn with PMMA into a fibre, showing that it is potentially usable as POF. Characterizations of the copolymers indicated that a certain useful level of optical rotation or circular birefringence is retained, and a fibre made of this material would allow the circular polarization of light to be manipulated over practical lengths of 10s of centimetres. Having shown the feasibility of menthol-based chiral polymers in this work, future work will focus on producing optical fibres with better optical rotation either from these copolymers or other chiral materials. Most importantly, this work would provide numerous opportunities to investigate further the characteristics and applications of circular birefringence in optical fibres.Acknowledgements
The authors would like to thank the Australia Research Council (ARC) for finance support (grant no: DP0880882). The authors also like to thank the Centre of Advanced Macromolecular Design (CAMD) for using GPC and DSC.References
- D. B. Amabilino, Chirality at the Nanoscale: Nanoparticles, Surfaces, Materials and More, Weinheim: Wiley-VCH, Weinheim, 2009 Search PubMed.
- A. Argyros, J. Pla, F. Ladouceur and L. Polandian, Opt. Express, 2009, 17, 15983–15990 CrossRef CAS.
- N. S. Pujari, M. R. Kulkarni, M. C. J. Large, M. Bassett and S. Ponrathnam, J. Appl. Polym. Sci., 2005, 98, 58–65 CrossRef CAS.
- F. M. Janeiro, C. R. Paiva and A. L. J. Topa, J. Opt. Soc. Am. B, 2002, 19, 2558–2566 CrossRef CAS.
- P. K. Choudhury and T. Yoshino, Optik (Jena, Germany), 2002, 113, 89–95 CAS.
- R. C. Qiu and I. T. Lu, IEE Proc.: Optoelectron., 1998, 145, 155–158 CrossRef CAS.
- A. K. Singh, K. S. Singh, P. Khastgir, S. P. Ojha and O. N. J. Singh, J. Opt. Soc. Am. B, 1994, 11, 1283–1287 CrossRef CAS.
- L. Poladian, M. Straton, A. Docherty and A. Argyros, Opt. Express, 2011, 19, 968–980 CrossRef CAS.
- F. M. Cox, A. Argyros and M. C. J. Large, Opt. Express, 2006, 14, 4135–4140 CrossRef CAS.
- A. Argyros, M. Straton, A. Docherty, E. H. Min, Z. Ge, K. H. Wong, F. Ladouceur and L. Polandian, Front. Optoelectron. China, 2010, 3, 67–70 CrossRef.
- M. C. J. Large, S. Ponrathnam, A. Argyros, N. S. Pujari and F. Cox, Opt. Express, 2004, 12, 1966–1971 CrossRef CAS.
- L. Thijs, R. Keltjens and G. J. B. Ettema, Application: US, Synthon Ip Inc., USA, 2004, p. 23, Cont-in-part of US Ser No. 261,492 Search PubMed.
- Y. Shi, B. Yu, M. Ding and F. Wang, Polym. J., 1996, 28, 465–466 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1py00056j |
| This journal is © The Royal Society of Chemistry 2011 |
