Synthesis and characterization of birch wood xylan succinoylated in 1-n-butyl-3-methylimidazolium chloride
Natanya M. L.
Hansen
* and
David
Plackett
Risø National Laboratory for Sustainable Energy, Technical University of Denmark, P.O. 49, DK-4000, Roskilde, Denmark. E-mail: nath@risoe.dtu.dk
First published on 28th June 2011
Abstract
The chemical modification of birch wood xylan was undertaken in the ionic liquid 1-n-butyl-3-methylimidazolium chloride (C4mimCl) using three different long-chain succinic anhydrides: n-octenyl succinic anhydride (n-OSA), n-dodecenyl succinic anhydride (n-DDSA) and n-octadecenyl succinic anhydride (n-ODSA). Reactions were performed at temperatures of 80–100 °C and reaction times of 0.5–22 hours resulting in a degree of substitution (DS) of 0.08–0.27. The formation of the desired products was confirmed through the use of 1H NMR and FT-IR, while DS was determined by titration. Thermogravimetric analysis of the modified and native xylans showed a slight lowering of thermal stability with functionalization. Contact angle measurements on spin-coated surfaces of modified xylan films showed a significant increase in hydrophobicity with the introduction of the alkenyl-functionalized succinic anhydride moieties.
Introduction
In response to the increasingly limited nature of fossil fuels, lignocellulosic biomass from trees, grasses, cereals and other plants has become the focus of the developing biorefining industry.1 Plant materials are primarily made up of three biopolymers: cellulose, lignin and hemicellulose and, of these, cellulose and lignin have received by far the most attention in terms of material applications. Traditionally, hemicellulose is defined as the alkali-soluble material after the removal of pectic substances from plant cell walls2 and xyloglycans (xylans) are a sub-group within hemicelluloses consisting of β(1→4)-D-xylopyranose backbone with side groups on the 2- or 3-position.The use of renewable resources for the production of food packaging in particular has recently received increased interest.3–5 Depending upon the application, low oxygen permeability as well as mechanical strength and flexibility can be important target properties for packaging films. Although there has been development of hemicellulose coatings for paperboard,6 research has shown that free-standing films produced from unmodified hemicelluloses are hygroscopic, resulting in poor properties in high humidity.7 Consequently, chemical modification and/or the addition of plasticizers has been explored as a route to obtaining coherent films with better properties. For example, surface fluorination of arabinoxylan films8 with trifluoroacetic anhydride has been used as a method to produce hydrophobic films, in which dramatic changes in water contact angles were seen with the introduction of even small amounts of fluorine. In another example, O-acetylgalactoglucomannan was treated with benzyl chloride and cast as films, resulting in products with low oxygen permeability (8 cm3 μm m−2 d−1kPa−1) and increased resistance to solubility in water.9
A major complication during chemical modification of hemicellulose in traditional organic solvents is poor solubility resulting in heterogeneous reaction conditions. Since the complete solution of xylan in ionic liquids has been reported,10,11 this problem may potentially be overcome by utilizing ionic liquids as reaction media. The use of ionic liquids for homogeneous reactions in carbohydrate chemistry has gained interest and the modification of polysaccharides, in particular cellulose, in these solvents, has been studied extensively over the last 20 years. Ionic liquids are thermally and chemically stable salts with a melting point below the boiling point of water and are often referred to as “green” chemicals on account of their high stability and low vapour pressure, making indefinite recycling possible in principle. There are, however, also concerns regarding the “greenness” of ionic liquids12–14 and toxicity studies of the most commonly used (i.e., imidazolium-type) ionic liquids have shown that the effect on algae,15,16 bacteria17,18 and mammalian cells19,20 is in some cases comparable to traditional solvents such as methanol, chloroform and acetonitrile in EC50 tests. Furthermore, the generation of unwanted and toxic side-products may take place under certain reaction conditions.21 Nevertheless, as recently documented, research on ionic liquids as solvents and reaction media for polysaccharides and other polymers continues to attract interest.22–26 The latest research on cellulose in ionic liquids includes modification with succinic anhydride in 1-butyl-3-methylimidazolium chloride27–30 and 1-allyl-3-methylimidazolium chloride,31 while research on dissolution or ionic liquids as reaction media for carbohydrates and other macromolecules has been reviewed.22,24 Functionalization of carbohydrates with succinic anhydride and related anhydrides has been performed by a number of groups (e.g. the utilization of long-chain alkyl succinic anhydrides to increase the hydrophobicity of starch32–35). Maleic anhydride was recently used for functionalization of xylan-rich hemicelluloses from bamboo in 1-butyl-3-methylimidazolium chloride.11 Modification of hemicellulose with succinic anhydride has previously been performed by Sun et al.36,37 Similarly, xylan has been acetylated10 in ionic liquids and a number of studies have been completed on dissolving wood, including native hemicelluloses, in ionic liquids (e.g., Fort et al.,38 Kilpelainen et al.,39 and Zavrel et al.40); however, the formation of hydrophobic hemicelluloses through reaction with widely available long-chain alkenyl succinic anhydrides (ASAs) in ionic liquids has, to our knowledge, not been attempted. The ASAs described here are widely used in the paper industry and therefore available in large quantities.
In the research described here, birch wood xylan was modified with alkenyl chain-functionalised succinic anhydrides (ASAs) (Fig. 1) to yield novel hydrophobic polymers as a step towards new bio-derived packaging film materials. 1-Butyl-3-methylimidazolium chloride (C4mimCl) was used as the reaction medium.
 | ||
| Fig. 1 Modification of xylan with long chain alkenyl succinic anhydrides. Substitution may take place at either hydroxyl group (or both) and is shown here at C2 as an example. The structure of xylan is simplified and here represented by a D-xylopyranose ring. | ||
Experimental
Materials
Birch wood xylan and 1-n-butyl-3-methylimidazolium chloride (C4mimCl) were purchased from Sigma Aldrich (Steinheim, Germany) and used without further purification. The bottle containing C4mimCl was stored in a desiccator over silica gel after opening. n-Octenyl succinic anhydride (n-OSA), n-dodecenyl succinic anhydride (n-DDSA) and n-octadecenyl succinic anhydride (n-ODSA) were kindly supplied by Pentagon Chemicals (Workington, UK). Laboratory-grade dimethylsulfoxide (DMSO) was purchased from Sigma Aldrich (Steinheim, Germany) and anhydrous ethanol (99.9%) was obtained from Kemetyl (Køge, Denmark).Succinoylation of hemicelluloses
In a typical succinoylation, 1.00 g (7.6 mmol) of xylan and 10 g of C4mimCl (57 mmol) were placed in a round-bottomed flask equipped with a magnetic stirrer and heated at 80 °C until the ionic liquid had melted. In the next step 1.35 g (6.4 mmol) of n-OSA was added and reaction proceeded for the chosen reaction time. The reaction was stopped by quenching in ethanol. The formed precipitate was isolated by filtration, rinsed in ethanol and then dried overnight in a vacuum oven at ambient temperature. A series of such experiments was carried out in which reactant ratios (nxylan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nASA), reaction temperatures and times were varied in the ranges of 1
nASA), reaction temperatures and times were varied in the ranges of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 to 1
0.8 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 80–100 °C and 0.5–22 hours respectively. Molar amounts of xylan were calculated on the basis of xylose sub-units (M = 132 g mol−1) with two free OH-groups.
2.5, 80–100 °C and 0.5–22 hours respectively. Molar amounts of xylan were calculated on the basis of xylose sub-units (M = 132 g mol−1) with two free OH-groups.
The modified xylan yield was calculated from the following equation:
 | (1) |
In eqn (1)mproduct is the weight of the end-product, nHC and mHC are the initial amounts of hemicellulose in terms of mol and g, respectively, MASA is the molecular weight of the ASA and DS is the degree of substitution determined by titration. The theoretical maximum value of DS was estimated as two based on the number of hydroxyl groups on the predominant xylose unit.
Characterization
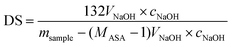 | (2) |
In eqn (2), msample is the weight of the titrated sample, VNaOH and cNaOH are the volume and concentration of NaOH used for titration, MASA is the molecular weight of the ASA and 132 g mol−1 is the molecular weight of the D-xylopyranose backbone unit. Blind tests were performed with the native xylan to determine the amount of acid present. These experiments showed that the contribution from acid groups in the unmodified xylan was negligible (less than 0.8% substitution). Four samples of each modified xylan were titrated and DS was calculated as an average of the obtained values.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.
000 g mol−1.
Results and discussion
Succinoylation of xylan
Xylan modification reaction conditions were varied for the three ASAs with respect to the ratio of reactants, reaction temperature and reaction time. Most experiments were carried out using n-OSA but selected experiments to determine the effect of chain length were also conducted with n-DDSA and n-ODSA. An overview of the experiments is shown in Table 1, in which DS determined by titration is reported.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m(C4mimCl) = 1
m(C4mimCl) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10
| Experiment | Succinic anhydride | Temperature/°C | Time/h | Yield (%) | n xylan : nASAa | DS |
|---|---|---|---|---|---|---|
| a Molar ratio of xylose units (M = 132 g mol−1) to ASA. | ||||||
| 1 | n-OSA | 80 | 0.5 | 98 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.08 |
| 2 | n-OSA | 85 | 0.5 | 96 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.15 |
| 3 | n-OSA | 90 | 0.5 | 98 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.18 |
| 4 | n-OSA | 95 | 0.5 | 98 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.24 |
| 5 | n-OSA | 100 | 0.5 | 96 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.27 |
| 6 | n-OSA | 80 | 1 | 94 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.12 |
| 7 | n-OSA | 80 | 2 | 94 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.17 |
| 8 | n-OSA | 80 | 6 | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.23 |
| 9 | n-OSA | 80 | 12 | 70 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.27 |
| 10 | n-OSA | 80 | 18 | 82 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.27 |
| 11 | n-OSA | 80 | 22 | 81 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.24 |
| 12 | n-OSA | 80 | 2 | 50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
0.16 |
| 13 | n-OSA | 80 | 2 | 62 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
0.10 |
| 14 | n-DDSA | 80 | 2 | 79 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.16 |
| 15 | n-DDSA | 80 | 2 | 86 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
0.17 |
| 16 | n-DDSA | 80 | 2 | 70 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
0.19 |
| 17 | n-ODSA | 80 | 2 | 86 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
0.16 |
The strategy of using long-chain alkenyl anhydrides instead of acetic anhydride or other similar low molecular weight anhydrides was chosen so as to avoid carboxylic acid by-products while at the same time introducing hydrophobic character. However, it must also be noted that the selected method results in the presence of a carboxylic acid group on the introduced side chain (see Fig. 1). As indicated in Table 1, the DS values were moderate with reaction efficiencies ranging from 0.2 to 0.8. This could be due to unwanted side reactions including hydrolysis of the succinic anhydride function of the ASAs, degradation of the xylan, and crosslinking (e.g., by reaction between the new side chains and the backbone functionalities).
A low degree of hemicellulose substitution has been noted in previous studies of similar reactions. Succinoylation of wheat straw hemicelluloses in alkaline solution (pH = 8.5–9.0) at temperatures of 28–45 °C yielded products with DS of 0.017–0.21 at reaction times of 0.5–16 hours.36 The authors stated that hydrolysis of succinic anhydride and the formed hemicellulose derivatives could explain the low degree of substitution. Peng et al.11 functionalized hemicellulose with maleic anhydride in C4mimCl, obtaining products with higher substitution degrees (DS of 0.095–0.75) than reported here; however, reactions were in all cases catalyzed with LiOH, while varying reaction times (40–100 min), reaction temperatures (60–100 °C) and maleic anhydride to anhydroxylose unit reactant ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 8
1 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Xie et al.35 compared acetylation and succinoylation of starch in C4mim and found that the degree of substitution was significantly lower for the latter reaction. While DS of 0.01–0.18 could be obtained for both esters without using a catalyst, the introduction of pyridine increased DS to 0.7–2.35 for acetylated starch and 0.46–0.91 for succinylated starch.
1). Xie et al.35 compared acetylation and succinoylation of starch in C4mim and found that the degree of substitution was significantly lower for the latter reaction. While DS of 0.01–0.18 could be obtained for both esters without using a catalyst, the introduction of pyridine increased DS to 0.7–2.35 for acetylated starch and 0.46–0.91 for succinylated starch.
Cellulose and starch are similar in nature to hemicelluloses in terms of being high molecular weight polysaccharides and the reactions of these biopolymers give an indication of what may be expected for xylan. Two studies on succinoylation of cellulose with succinic anhydride in ionic liquids demonstrated that the expected degree of substitution is modest. Cellulose was succinoylated in C4mimCl/DMSO by Liu et al.27 who varied reaction temperatures (85–100 °C), reaction times (5–120 min) and reactant ratios (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 14
1 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), resulting in DS of 0.038–0.53 (100% substitution of cellulose being DS = 3). Similar results were obtained in a study of succinoylation of cellulose in 1-allyl-3-methylimidazolium chloride,31 where temperatures of 60–110 °C, reaction times of 30–120 min and reactant ratios of 1
1), resulting in DS of 0.038–0.53 (100% substitution of cellulose being DS = 3). Similar results were obtained in a study of succinoylation of cellulose in 1-allyl-3-methylimidazolium chloride,31 where temperatures of 60–110 °C, reaction times of 30–120 min and reactant ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 12
1 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 yielded a DS of 0.07–0.22. Liu et al.30 improved their results by catalysing the succinoylation reaction of cellulose in C4mimCl/DMSO with iodine and increased the DS from 0.24 to 0.56–1.54 by varying reaction temperatures (85–110 °C), reaction times (30–120 min) and the reactant ratios of I2
1 yielded a DS of 0.07–0.22. Liu et al.30 improved their results by catalysing the succinoylation reaction of cellulose in C4mimCl/DMSO with iodine and increased the DS from 0.24 to 0.56–1.54 by varying reaction temperatures (85–110 °C), reaction times (30–120 min) and the reactant ratios of I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) succinic anhydride (2
succinic anhydride (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 15
1 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Succinoylation of cellulose in C4mimCl/DMSO catalyzed with 4-dimethylaminopyridine (DMAP)28 increased the DS from 0.24 to 0.94–2.34 by varying reaction temperatures (60–110 °C), reaction times (30–120 min) and the ratios of DMAP
1). Succinoylation of cellulose in C4mimCl/DMSO catalyzed with 4-dimethylaminopyridine (DMAP)28 increased the DS from 0.24 to 0.94–2.34 by varying reaction temperatures (60–110 °C), reaction times (30–120 min) and the ratios of DMAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) succinic anhydride (1–15%). A parallel study utilizing N-bromosuccininide (NBS) as a catalyst for the formation of cellulose succinylates in C4mimCl/DMSO29 yielded similar results with DS improving from 0.24 to 0.92–2.31 by varying reaction temperatures (90–120 °C), reaction times (30–240 min) and the ratios of NBS
succinic anhydride (1–15%). A parallel study utilizing N-bromosuccininide (NBS) as a catalyst for the formation of cellulose succinylates in C4mimCl/DMSO29 yielded similar results with DS improving from 0.24 to 0.92–2.31 by varying reaction temperatures (90–120 °C), reaction times (30–240 min) and the ratios of NBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) succinic anhydride (1–20%).
succinic anhydride (1–20%).
In the following section the effect of varying the reaction conditions in the present study is discussed.
![Yield and DS of n-OSA-modified xylan in C4mimCl. T = 80 °C, [xylan] : [n-OSA] = 1 : 0.8, m(xylan) : m(C4mimCl) = 1 : 10.](/image/article/2011/PY/c1py00086a/c1py00086a-f2.gif) | ||
Fig. 2 Yield and DS of n-OSA-modified xylan in C4mimCl. T = 80 °C, [xylan]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [n-OSA] = 1 [n-OSA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, m(xylan) 0.8, m(xylan)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m(C4mimCl) = 1 m(C4mimCl) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. 10. | ||
Considering other reports, an increase in DS with reaction time, with a maximum at two hours within the time range of 0.5–16 hours, was found in the modification of wheat straw hemicelluloses with succinic anhydride in an aqueous alkaline system.36 For the succinoylation of hemicelluloses in an N,N′-dimethylformamide/lithium chloride system catalyzed with pyridine, an increase in DS was observed up to 12 hours, after which a decrease was seen.37 The same tendency was seen in the succinoylation of cellulose in C4mimCl/DMSO catalyzed by DMAP28 and NBS29 respectively. Reactions of maleic anhydride and hemicellulose in C4mimCl showed an increase in DS when proceeding for up to 80 minutes, while the DS decreased for longer reaction times.11 In contrast to the mentioned studies the DS of sugar cane bagasse cellulose reacted with succinic anhydride in 1-allyl-3-methylimidazolium chloride for 30–160 min was observed to increase with increasing reaction time and temperature.31
![Yield and DS of n-OSA-modified xylan in C4mimCl. Reaction time = 0.5 hours, [xylan] : [n-OSA] = 1 : 0.8, m(xylan) : m(C4mimCl) = 1 : 10.](/image/article/2011/PY/c1py00086a/c1py00086a-f3.gif) | ||
Fig. 3 Yield and DS of n-OSA-modified xylan in C4mimCl. Reaction time = 0.5 hours, [xylan]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [n-OSA] = 1 [n-OSA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, m(xylan) 0.8, m(xylan)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m(C4mimCl) = 1 m(C4mimCl) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. 10. | ||
 | ||
| Fig. 4 Appearance of final products from reaction of birch wood xylan with n-OSA at various temperatures. | ||
As with the findings reported here, reaction temperature increased the DS in a seemingly linear fashion in the n-DDSA modification of corn starch performed in an aqueous slurry under alkaline conditions.33 The same tendency was seen for both NBS-catalyzed29 and non-catalyzed27 reactions of cellulose with succinic anhydride in C4mimCl/DMSO. Increased functionalization at elevated temperatures is mainly due to increased diffusion of the ASA species11,27 and may also stem from increased solubility of this compound in the solvent.28,29,33 For maleic anhydride functionalization of hemicellulose an increase in DS was seen in the temperature range of 60–80 °C, while a decrease in DS was observed for elevated temperatures (80–120 °C), most likely due to enhanced solubility of all species leading to more unwanted side reactions.11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ASA. The chosen ratios were 1.0.8, 1
ASA. The chosen ratios were 1.0.8, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 and 1
1.7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5. For n-OSA the DS decreased with increased amount of n-OSA from 0.17 to 0.10, while a slight increase in DS from 0.16 to 0.19 was seen for n-DDSA. Furthermore, an experiment using xylan
2.5. For n-OSA the DS decreased with increased amount of n-OSA from 0.17 to 0.10, while a slight increase in DS from 0.16 to 0.19 was seen for n-DDSA. Furthermore, an experiment using xylan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-ODSA of 1
n-ODSA of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 was carried out, which yielded a product with DS of 0.16. Contrary to earlier observations on other systems there was no clear dependency on the ratio of ASA to xylan in the studied range of reactant ratios, indicating that DS ∼0.3 was probably the limit of the reaction under the given conditions. A more pronounced variation in the DS might have been observed in the current study with a larger excess of the anhydride.
0.8 was carried out, which yielded a product with DS of 0.16. Contrary to earlier observations on other systems there was no clear dependency on the ratio of ASA to xylan in the studied range of reactant ratios, indicating that DS ∼0.3 was probably the limit of the reaction under the given conditions. A more pronounced variation in the DS might have been observed in the current study with a larger excess of the anhydride.
In the literature, the efficiency of the reaction of xylan and maleic anhydride increased with addition of the anhydride until a maximum in DS was achieved with a four-fold excess of anhydride (relative to anhydroxylose units).11 In the functionalization of carboxymethyl starch with octenyl succinic anhydride in a solvent system of dimethylsulfoxide and p-toluenesulfonic acid, the DS increased with increasing ratio of anhydride to starch.34 A similar tendency was seen for acylation of both starch44 and cellulose45 in C4mimCl using acetic anhydride, in which the DS increased with increasing anhydride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) polysaccharide ratio. Succinoylation reactions of cellulose27,30,31 and starch35 under similar conditions as those used in the current study showed the same tendency. In these latter studies, the ratio of anhydride to polymer was varied to a greater extent than in the present study with a ratio of anhydride to hydroxyl functionality of up to 14
polysaccharide ratio. Succinoylation reactions of cellulose27,30,31 and starch35 under similar conditions as those used in the current study showed the same tendency. In these latter studies, the ratio of anhydride to polymer was varied to a greater extent than in the present study with a ratio of anhydride to hydroxyl functionality of up to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ASA ratio did not show the same tendency for n-OSA and n-DDSA. The DS values obtained for these two ASAs are, however, in the same range (0.10–0.19) under the chosen reaction conditions at 80 °C. The DS appears to be independent of chain length, as functionalization with n-DDSA and n-ODSA reached the same levels as with n-OSA. This may indicate that the rate-determining step is the opening of the cyclic anhydride rather than diffusion of this species to the reactive sites (i.e., the OH-groups on xylan). Otherwise, the larger and more sterically hindered n-DDSA and in particular n-ODSA would exhibit lower modification of xylan under the same conditions as used for n-OSA.
ASA ratio did not show the same tendency for n-OSA and n-DDSA. The DS values obtained for these two ASAs are, however, in the same range (0.10–0.19) under the chosen reaction conditions at 80 °C. The DS appears to be independent of chain length, as functionalization with n-DDSA and n-ODSA reached the same levels as with n-OSA. This may indicate that the rate-determining step is the opening of the cyclic anhydride rather than diffusion of this species to the reactive sites (i.e., the OH-groups on xylan). Otherwise, the larger and more sterically hindered n-DDSA and in particular n-ODSA would exhibit lower modification of xylan under the same conditions as used for n-OSA.
Considering relevant literature reports, in the esterification of starch with alkenyl succinates using an aqueous system, the length of the alkenyl chain has been shown to have an influence on the ease of substitution due to differences in hydrophobicity and therefore differences in diffusion in the chosen media.32 The behavior of the ASAs in an ionic liquid is expected to differ from that in an aqueous system and therefore steric factors may outweigh the diffusion of the reactive species in the chosen system. The effect of steric hindrance was seen by Xie et al.35 in their study, which compared the efficiency of starch esterification with acetic anhydride and succinic anhydride respectively. DS was significantly higher when using acetic anhydride, as opposed to succinic anhydride, which requires a more sterically hindered ring-opening of the anhydride for reaction with the hydroxyl groups on the carbohydrate to take place.
Characterization of modified xylans
 | ||
| Fig. 5 1H NMR spectra of birch wood xylan (A) and n-OSA-modified xylan (B) (experiment 10, Table 1). Signals from the xylan ring are found at δ 3.0–5.4 ppm (∼6H) and signals from the terminal part of the alkenyl chain of n-OSA are seen at δ 1.23 ppm (6H) and δ 0.84 ppm (3H). (The prominent peak at δ 2.5 is from the solvent, DMSO.) | ||
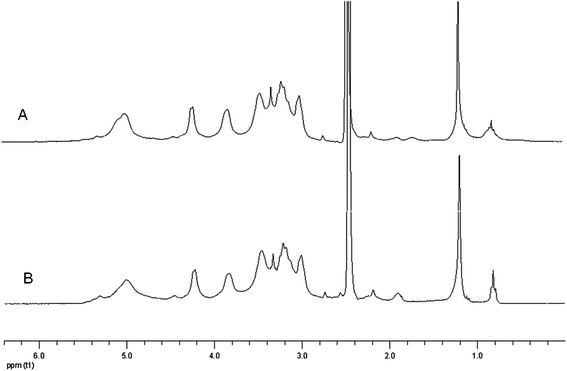 | ||
| Fig. 6 1H NMR spectra of ASA-modified xylans. A: n-ODSA-modified xylan (experiment 17, Table 1). Signals from the xylan ring are found at δ 3.0–5.4 ppm (∼6H) and the signal from the terminal part of the alkenyl chain of n-ODSA are seen at δ 1.21 ppm (26H) and δ 0.82 ppm (3H). B: n-DDSA-modified xylan (experiment 14, Table 1). Signals from the alkenyl chain of n-DDSA are seen at δ 1.22 ppm (14H) and δ 0.82 ppm (3H). (The prominent peak at δ 2.5 is from the solvent, DMSO.) | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester peak increased with DS (see Fig. 8). The signal from the new carboxylic acid may overlap with the signals from the inherent carboxylic acid groups36 as well as from absorbed water. The intensity of the signal at 2920 cm−1 was increased and also shifted slightly due to the presence of the long carbohydrate chains in the new side groups.10,36,52
O ester peak increased with DS (see Fig. 8). The signal from the new carboxylic acid may overlap with the signals from the inherent carboxylic acid groups36 as well as from absorbed water. The intensity of the signal at 2920 cm−1 was increased and also shifted slightly due to the presence of the long carbohydrate chains in the new side groups.10,36,52
 | ||
| Fig. 7 FT-IR spectra of xylan (top), n-DDSA-modified xylan (middle) with DS = 0.16 (experiment 14, Table 1), and n-DDSA (bottom). | ||
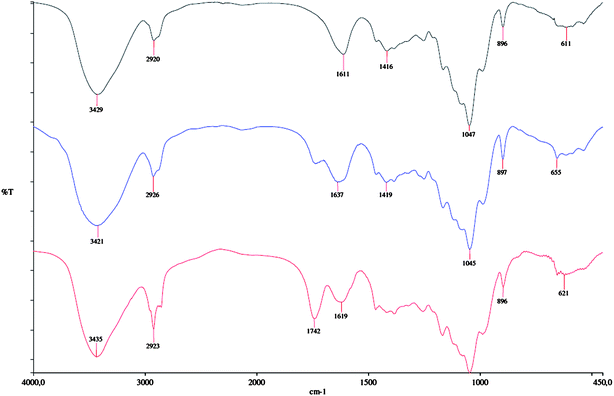 | ||
| Fig. 8 FT-IR spectra of xylan (top) and n-OSA-modified xylans with DS = 0.08 (middle) and DS = 0.27 (bottom) (experiments 1 and 9, Table 1 respectively). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 as determined by SEC giving Dp ∼265. Fig. 9 shows SEC-traces of an n-OSA-modified xylan, an n-DDSA-modified xylan and the unmodified xylan. SEC data for modified xylans are shown in Table 2. In most cases a slight increase in molecular weight was found, which was most pronounced for the n-ODSA- and n-DDSA-modified xylans. This is not unexpected, as the introduction of side-groups should only give a minor change in conformation of the xylan molecule, thereby affecting the hydrodynamic volume only to a small degree. Limited degradation could also reduce molecular weights slightly, as seen for maleic anhydride-functionalized xylans where, as reported, the modified products exhibited lower molecular weights than the initial species.11
000 g mol−1 as determined by SEC giving Dp ∼265. Fig. 9 shows SEC-traces of an n-OSA-modified xylan, an n-DDSA-modified xylan and the unmodified xylan. SEC data for modified xylans are shown in Table 2. In most cases a slight increase in molecular weight was found, which was most pronounced for the n-ODSA- and n-DDSA-modified xylans. This is not unexpected, as the introduction of side-groups should only give a minor change in conformation of the xylan molecule, thereby affecting the hydrodynamic volume only to a small degree. Limited degradation could also reduce molecular weights slightly, as seen for maleic anhydride-functionalized xylans where, as reported, the modified products exhibited lower molecular weights than the initial species.11
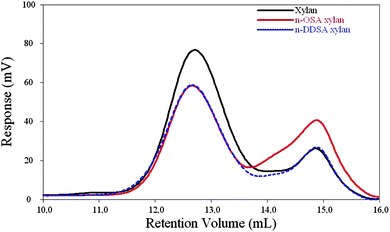 | ||
| Fig. 9 SEC-traces for xylan (black), n-OSA-modified xylan (red) and n-DDSA-modified xylan (dashed). The modified xylans are products from experiment 9 (DS = 0.27) and experiment 14 (DS= 0.16) respectively. The peak at retention volume near 15 ml is an artifact from the solvent. | ||
| Experiment No | Succinic derivative | DS |
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w/g mol−1
w/g mol−1 |
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n/g mol−1
n/g mol−1 |
M peak/g mol−1 |
|---|---|---|---|---|---|
| Xylan | — | — | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 13 | n-OSA | 0.10 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
| 2 | n-OSA | 0.15 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| 12 | n-OSA | 0.16 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 7 | n-OSA | 0.17 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| 4 | n-OSA | 0.24 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
| 11 | n-OSA | 0.24 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 9 | n-OSA | 0.27 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 10 | n-OSA | 0.27 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| 5 | n-OSA | 0.27 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| 14 | n-DDSA | 0.16 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
| 15 | n-DDSA | 0.17 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 16 | n-DDSA | 0.19 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| 17 | n-ODSA | 0.16 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
| Exp. No | Succinic derivative | DS | T initial/°C | T peak/°C |
|---|---|---|---|---|
| Pure xylan | None | — | 202 | 299 |
| 1 | n-OSA | 0.08 | 206 | 282 |
| 13 | n-OSA | 0.10 | 210 | 295 |
| 2 | n-OSA | 0.15 | 198 | 266 |
| 12 | n-OSA | 0.16 | 196 | 242 |
| 7 | n-OSA | 0.17 | 199 | 242 |
| 3 | n-OSA | 0.18 | 193 | 242 |
| 8 | n-OSA | 0.23 | 187 | 229 |
| 4 | n-OSA | 0.24 | 193 | 254 |
| 5 | n-OSA | 0.27 | 198 | 244 |
| 9 | n-OSA | 0.27 | 203 | 247 |
| 10 | n-OSA | 0.27 | 208 | 249 |
| 14 | n-DDSA | 0.16 | 199 | 254 |
| 15 | n-DDSA | 0.17 | 193 | 239 |
| 16 | n-DDSA | 0.19 | 193 | 239 |
| 17 | n-ODSA | 0.16 | 213 | 264 |
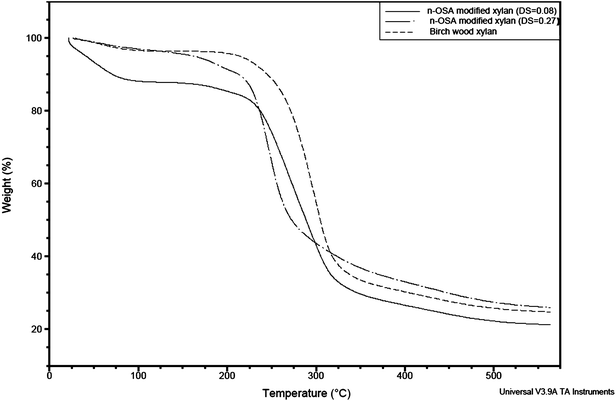 | ||
| Fig. 10 TGA plots of birch wood xylan and n-OSA-modified xylans with DS of 0.08 and 0.27, respectively (experiments 1 and 9, Table 1). | ||
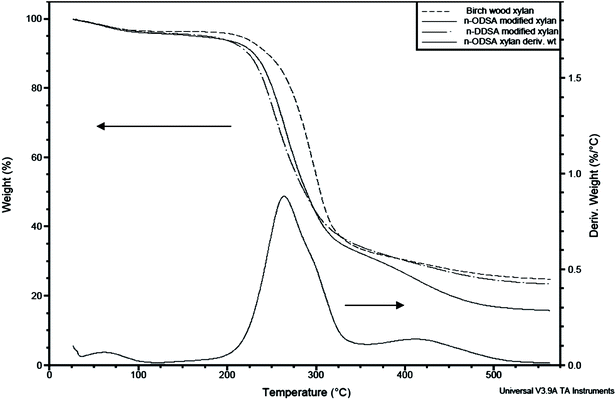 | ||
| Fig. 11 TGA plots of birch wood xylan, n-DDSA and n-ODSA-modified xylans both with DS of 0.16 (experiments 14 and 17, Table 1 respectively). The corresponding first derivative of the weight loss of n-ODSA-modified xylan is also shown. | ||
Referring to previous reports, maleic anhydride-functionalized hemicelluloses exhibited similar thermal stability behavior as the functionalization led to less thermally stable products.11 A range of wheat straw hemicellulose esters were also observed by Xu et al.50 to have lower thermal stability than the native hemicelluloses and this was also the case for wheat straw hemicellulosic succinates synthesized by Sun et al.37Xylan succinylates obtained by microwave irradiation57 were less thermally stable than the native xylan, although this in part could be due to degradation of the polymer as a result of the chosen modification process. Ren et al.51 found that lauroylated hemicelluloses with low DS (<0.78) were less thermally stable than the native biopolymer, while higher DS (>1.56) lead to compounds which were more thermally stable than the starting material. Stearoylated43 and acetylated10,48 hemicelluloses have previously been synthesized and found to be more thermally stable than the non-functionalized hemicelluloses. Succinylated celluloses modified in ionic liquids were less thermally stable than the native cellulose,27,31 while starch succinates (DS < 0.7) from various plant sources were more thermally stable than the native compound.58
| Exp. No | Succinic derivative | DS | θ/° |
|---|---|---|---|
| Pure xylan | None | — | 28 (±1.7) |
| 1 | n-OSA | 0.08 | 55 (±1.4) |
| 7 | n-OSA | 0.17 | 60 (±2.2) |
| 10 | n-OSA | 0.27 | 65 (±3.3) |
| 14 | n-DDSA | 0.16 | 81 (±3.0) |
| 17 | n-ODSA | 0.16 | 76 (±1.6) |
The longer alkenyl chains of n-DDSA and n-ODSA appear to have a considerable influence on surface properties yielding more hydrophobic xylan films even at low DS. It would be expected for n-ODSA to give the most hydrophobic surface, as it has the longest alkenyl chain, which is however not the case. This could be due to differences in surface roughness of the samples, which is in part dependent on the solubility of the polymer in the chosen solvent. However, the difference between the contact angle results obtained for the n-DDSA- and n-ODSA-modified xylans is not significant with the method employed.
Conclusions
Chemical modification of birch wood xylan in C4mimCl using long-chain alkenyl succinic anhydrides was confirmed through use of 1H NMR and FT-IR spectroscopy, while titration was used to determine DS. Products with low DS (0.08–0.27) were obtained in good yield. The low DS values are consistent with previous reports in the literature for various hemicellulose modification methods. An increase in temperature and reaction time increased the DS; however product discoloration also occurred, which was attributed to unwanted side reactions. There was no obvious effect of chain length on the DS obtained for the three ASAs under similar reaction conditions. The thermal stability of the ASA-modified xylans was found to be lower than that of the unmodified xylan; however, this difference may not be of significance for application purposes. The introduction of the alkenyl side chains on the xylan backbone induced higher hydrophobicity as seen from increased contact angles of water on the surface of spin-coated modified xylan films.Acknowledgements
The authors wish to thank the Danish Agency for Science Technology and Innovation for funding this project (# 09-065804). We are grateful to Kim Chi Szabo (Danish Polymer Centre, Department of Chemical and Biochemical Engineering, Technical University of Denmark) for technical assistance with DSC and TGA measurements, Lotte Nielsen (Department of Micro- and Nanotechnology, Technical University of Denmark) for performing the SEC experiments, and Zsuzsa Sarossy (Biosystems Division, Risø National Laboratory for Sustainable Energy, Technical University of Denmark) for performing the acid hydrolysis and sugar analyses. The supply of ASAs by Pentagon Chemicals is also gratefully acknowledged.References
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS
.
-
R. C. Sun, X. F. Sun, and J. Tomkinson. in Hemicellulose: Science and Technology, ed. P. Gatenholm and M. Tenkanen, American Chemical Society, Washington, DC, 2004, pp. 2–22 Search PubMed
.
-
Biobased Packaging Materials for the Food Industry—Status and Perspectives, ed. C. J. Weber, KVL, Department of Dairy and Food Science, Frederiksberg C, Denmark, 2000 Search PubMed
.
- G. Davis and J. H. Song, Ind. Crops Prod., 2006, 23, 147–161 CrossRef CAS
.
- G. Kale, T. Kijchavengkul, R. Auras, M. Rubino, S. E. Selke and S. P. Singh, Macromol. Biosci., 2007, 7, 255–277 CrossRef CAS
.
- http://www.xylophane.com/ .
- N. M. L. Hansen and D. Plackett, Biomacromolecules, 2008, 9, 1493–1505 CrossRef CAS
.
- M. Grondahl, A. Gustafsson and P. Gatenholm, Macromolecules, 2006, 39, 2718–2721 CrossRef
.
- J. Hartman, A. C. Albertsson and J. Sjoberg, Biomacromolecules, 2006, 7, 1983–1989 CrossRef CAS
.
- J. L. Ren, R. C. Sun, C. F. Liu, Z. N. Cao and W. Luo, Carbohydr. Polym., 2007, 70, 406–414 CrossRef CAS
.
- X. W. Peng, J. L. Ren and R. C. Sun, Biomacromolecules, 2010, 11, 3519–3524 CrossRef CAS
.
- D. B. Zhao, Y. C. Liao and Z. D. Zhang, Clean, 2007, 35, 42–48 CAS
.
- S. Zhu, R. Chen, Y. Wu, Q. Chen, X. Zhang and Z. Yu, Chem. Biochem. Eng. Q., 2009, 23, 207–211 CAS
.
- T. Welton, Green Chem., 2008, 10, 483 RSC
.
- A. Latala, M. Nedzi and P. Stepnowski, Green Chem., 2009, 11, 1371–1376 RSC
.
- A. S. Wells and V. T. Coombe, Org. Process Res. Dev., 2006, 10, 794–798 CrossRef CAS
.
- K. M. Docherty and C. F. Kulpa, Green Chem., 2005, 7, 185–189 RSC
.
- J. Ranke, K. Molter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffmann, B. Ondruschka, J. Filser and B. Jastorff, Ecotoxicol. Environ. Saf., 2004, 58, 396–404 CrossRef CAS
.
- R. A. Kumar, N. Papaiconomou, J. M. Lee, J. Salminen, D. S. Clark and J. M. Prausnitz, Environ. Toxicol., 2009, 24, 388–395 CrossRef CAS
.
- X. F. Wang, C. A. Ohlin, Q. H. Lu, Z. F. Fei, J. Hu and P. J. Dyson, Green Chem., 2007, 9, 1191–1197 RSC
.
- J. Dupont and J. Spencer, Angew. Chem., Int. Ed., 2004, 43, 5296–5297 CrossRef CAS
.
- O. A. El Seoud, A. Koschella, L. C. Fidale, S. Dorn and T. Heinze, Biomacromolecules, 2007, 8, 2629–2647 CrossRef CAS
.
- L. Feng and Z. I. Chen, J. Mol. Liq., 2008, 142, 1–5 CrossRef
.
- T. Ueki and M. Watanabe, Macromolecules, 2008, 41, 3739–3749 CrossRef CAS
.
- P. Kubisa, Prog. Polym. Sci., 2009, 34, 1333–1347 CrossRef CAS
.
- M. E. Zakrzewska, E. Bogel-Lukasik and R. Bogel-Lukasik, Energy Fuels, 2010, 24, 737–745 CrossRef CAS
.
- C. F. Liu, R. C. Sun, A. P. Zhang, J. L. Ren and Z. C. Geng, Polym. Degrad. Stab., 2006, 91, 3040–3047 CrossRef CAS
.
- W. Y. Li, A. X. Jin, C. F. Liu, R. C. Sun, A. P. Zhang and J. F. Kennedy, Carbohydr. Polym., 2009, 78, 389–395 CrossRef CAS
.
- C. F. Liu, A. P. Zhang, W. Y. Li, F. X. Yue and R. C. Sun, J. Agric. Food Chem., 2009, 57, 1814–1820 CrossRef CAS
.
- C. F. Liu, A. P. Zhang, W. Y. Li, F. X. Yue and R. C. Sun, Ind. Crops Prod., 2010, 31, 363–369 CrossRef CAS
.
- C. F. Liu, R. C. Sun, A. P. Zhang, J. L. Ren, X. A. Wang, M. H. Qin, Z. N. Chao and W. Luo, Carbohydr. Res., 2007, 342, 919–926 CrossRef CAS
.
- Y. S. Jeon, A. Viswanathan and R. A. Gross, Starch, 1999, 51, 90–93 CrossRef CAS
.
- H. Chi, K. Xu, D. H. Xue, C. L. Song, W. D. Zhang and P. X. Wang, Food Res. Int., 2007, 40, 232–238 CrossRef CAS
.
- A. Cizova, A. Koschella, T. Heinze, A. Ebringerova and I. Srokova, Starch, 2007, 59, 482–492 CrossRef CAS
.
- W. L. Xie, L. Shao and Y. W. Liu, J. Appl. Polym. Sci., 2010, 116, 218–224 CrossRef CAS
.
- R. C. Sun, X. F. Sun and X. Bing, J. Appl. Polym. Sci., 2002, 83, 757–766 CrossRef CAS
.
- R. C. Sun, S. T. Min and X. F. Sun, Int. J. Polym. Anal. Charact., 2002, 7, 130–144 CrossRef CAS
.
- D. A. Fort, R. C. Remsing, R. P. Swatloski, P. Moyna, G. Moyna and R. D. Rogers, Green Chem., 2007, 9, 63–69 RSC
.
- I. Kilpelainen, H. Xie, A. King, M. Granstrom, S. Heikkinen and D. S. Argyropoulos, J. Agric. Food Chem., 2007, 55, 9142–9148 CrossRef
.
- M. Zavrel, D. Bross, M. Funke, J. Buchs and A. C. Spiess, Bioresour. Technol., 2009, 100, 2580–2587 CrossRef CAS
.
- A. S. Schmidt and A. B. Thomsen, Bioresour. Technol., 1998, 64, 139–151 CrossRef CAS
.
- Z. Stojanovic, K. Jeremic, S. Jovanovic and M. D. Lechner, Starch, 2005, 57, 79–83 CrossRef CAS
.
- R. C. Sun, J. M. Fang and J. Tomkinson, Polym. Degrad. Stab., 2000, 67, 345–353 CrossRef CAS
.
- A. Biswas, R. L. Shogren, D. G. Stevenson, J. L. Willett and P. K. Bhowmik, Carbohydr. Polym., 2006, 66, 546–550 CrossRef CAS
.
- S. Barthel and T. Heinze, Green Chem., 2006, 8, 301–306 RSC
.
- I. Gabrielii, P. Gatenholm, W. G. Glasser, R. K. Jain and L. Kenne, Carbohydr. Polym., 2000, 43, 367–374 CrossRef CAS
.
- R. C. Sun, J. Tomkinson, Y. X. Wang and B. Xiao, Polymer, 2000, 41, 2647–2656 CrossRef CAS
.
- J. M. Fang, R. C. Sun, J. Tomkinson and P. Fowler, Carbohydr. Polym., 2000, 41, 379–387 CrossRef CAS
.
- D. S. Ruzene, D. P. Silva, A. A. Vicente, A. R. Goncalves and J. A. Teixeira, Appl. Biochem. Biotechnol., 2008, 147, 85–96 CrossRef CAS
.
- F. Xu, J. X. Jiang, R. C. Sun, D. She, B. Peng, J. X. Sun and J. F. Kennedy, Carbohydr. Polym., 2008, 73, 612–620 CrossRef CAS
.
- J. L. Ren, F. Xu, R. C. Sun, B. Peng and J. X. Sun, J. Agric. Food Chem., 2008, 56, 1251–1258 CrossRef CAS
.
- F. Peng, J. L. Ren, B. Peng, F. Xu, R. C. Sun and J. X. Sun, Carbohydr. Res., 2008, 343, 2956–2962 CrossRef CAS
.
- J. Hartman, A. C. Albertsson, M. S. Lindblad and J. Sjoberg, J. Appl. Polym. Sci., 2006, 100, 2985–2991 CrossRef CAS
.
- H. M. Shaikh, K. V. Pandare, G. Nair and A. J. Varma, Carbohydr. Polym., 2009, 76, 23–29 CrossRef CAS
.
- C. M. Buchanan, N. L. Buchanan, J. S. Debenham, P. Gatenholm, M. Jacobsson, M. C. Shelton, T. L. Watterson and M. D. Wood, Carbohydr. Polym., 2003, 52, 345–357 CrossRef CAS
.
- R. K. Jain, M. Sjostedt and W. G. Glasser, Cellulose, 2000, 7, 319–336 CrossRef CAS
.
- F. Peng, J. L. Ren, X. F. Sun, F. Xu, R. C. Sun, B. Peng and J. X. Sun, e-Polym., 2008, 108 Search PubMed
.
- E. Rudnik, G. Matuschek, N. Milanov and A. Kettrup, Thermochim. Acta, 2005, 427, 163–166 CrossRef CAS
.
- K. Hettrich, S. Fischer, N. Schroder, J. Engelhardt, U. Drechsler and F. Loth, Macromol. Symp., 2006, 232, 37–48 CrossRef CAS
.
- J. G. Wang and C. K. Ober, Macromolecules, 1997, 30, 7560–7567 CrossRef CAS
.
- C. Biver, G. de Crevoisier, S. Girault, A. Mourran, R. Pirri, J. C. Razet and L. Leibler, Macromolecules, 2002, 35, 2552–2559 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2011 |
