DOI:
10.1039/C1PY00083G
(Review Article)
Polym. Chem., 2011,
2, 1942-1949
Polymeric chiral organocatalysts
Received
23rd February 2011
, Accepted 13th April 2011
First published on 12th May 2011
Abstract
Incorporation of a chiral quaternary ammonium salt into a polymer allows for the preparation of a polymeric chiral organocatalyst that can be utilized in various asymmetric transformations in organic synthesis. Chiral quaternary ammonium salts are among the most important and frequently used organocatalysts. There is an increased focus on new strategies for the incorporation of chiral quaternary ammonium salts into polymers. We have found that ionic-bond formation between a quaternary nitrogen and a sulfonate anion can be successfully used to link an organocatalyst to a polymer support. Stable ionic bonds formed by quaternary ammonium sulfonate can also be used in main-chain chiral polymer synthesis. The potential applications of the ionic-bond formation method in the construction of new chiral macromolecular architectures are discussed herein.
 Shinichi Itsuno | Shinichi Itsuno obtained M Eng in Polymer Chemistry from Tokyo Institute of Technology, in 1981. He then accepted a position of research associate at Toyohashi University of Technology in 1982, and obtained his PhD from Tokyo Institute of Technology in 1985. After he started as an Assistant Professor at Toyohashi University of Technology in 1986, he spent two post-doctoral years with Jean Fréchet at University of Ottawa and Cornell University, working on Reactive Polymer Synthesis and Polymer-Immobilized Chiral Catalysts. He was promoted to Associate Professor in 1993, and to Full Professor in 2003. Since 2010, he is Professor and vice Dean in the Department of Environmental and Life Sciences at Toyohashi University of Technology. |

Md. Masud Parvez | Md. Masud Parvez was born in Dinajpur, Bangladesh in 1984. He studied Bachelor of Science in chemistry (2001–2005) at University of Dhaka, Bangladesh. He completed his Master in Engineering (2008–2010) in the Department of Materials Science at Toyohashi University of Technology, Japan. He started his PhD in December 2010 in the Department of Functional Materials Engineering at Toyohashi University of Technology under the supervision of Professor Shinichi Itsuno. The focus of his research is development of chiral main-chain polymeric organocatalyst and their application to asymmetric reactions. |
 Naoki Haraguchi | Naoki Haraguchi was born in Saitama, Japan, in 1974. He studied polymer chemistry at the Tokyo Institute of Technology, where he received his PhD on the precise synthesis of functionalized polymers and branched polymers under the supervision of Prof. Akira Hirao in 2003. He joined the group of Prof. Thomas R. Hoye as a post-doctoral researcher at University of Minnesota in 2003. In 2004, he became assistant professor at Toyohashi University of Technology. His current research interest is the design and development of polymer-supported chiral organocatalysts and organometallic catalysts for asymmetric reaction. |
1. Introduction
Chiral organocatalysts have been widely developed for use in the synthesis of optically active compounds. These organocatalysts are valuable from the green chemistry perspective because they do not contain any metal species. There is no need for metal-based catalysis thus making a contribution to green chemistry. In addition, various types of chiral organocatalysts have been developed for use in asymmetric synthesis,1 and attempts have been made to produce polymer-immobilized chiral organocatalysts. Since the 1980s, many researchers have been showing interest in the development and use of metal-free polymer catalysts.2 One of the key advantages of polymer-based catalysts is that they can be easily detached from the solid (polymer) support. Excellent recyclability and feasibility of use in continuous-flow systems are the attractive features of polymer-immobilized organocatalysts. These advantages play a critical role in the development of chiral polymeric catalysts. Recently, the importance of the microenvironment of a polymer catalyst for the selectivity of the catalyst has been recognized, and extensive studies have been carried out on various kinds of polymer supports. In this review, we focus on polymer organocatalysts based on chiral quaternary ammonium salts and other related salts. Polymeric chiral catalysts can be broadly classified into two groups: polymers with side-chain chirality and polymers with main-chain chirality. We have developed some novel methods for synthesizing chiral polymers containing an enantiopure quaternary ammonium salt or an iminium salt. We have also performed studies on polymers with side-chain and main-chain chirality and reviewed some recently developed interesting synthetic routes to these polymers.
2.
Polymers with side-chain chirality
Polymer-immobilized chiral organocatalysts have received considerable attention in the last few decades. A wide variety of chiral organocatalysts have been attached to the side-chains of different functionalized polymer-support.3 While various types of chiral organocatalysts have been reported, we make a special mention of chiral quaternary ammonium salt organocatalysts and iminium salt organocatalysts, which have been recognized as important catalysts for asymmetric synthesis. The ionic structure of these catalysts facilitates polymer immobilization through ionic-bond formation.
2.1 Synthesis of polymer-immobilized chiral quaternary ammonium salts through covalent-bond formation
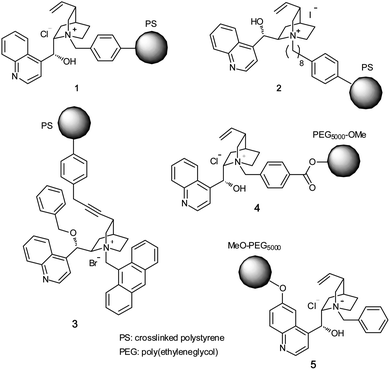
Chiral-quaternary-ammonium-salt-containing polymers are the most extensively studied catalysts because they can be easily synthesized from readily available precursors.4 Various types of polymer-immobilized chiral quaternary ammonium salts derived from cinchona alkaloids have been synthesized and used as catalysts in asymmetric reactions (Fig. 1). Initial approach of polymer-immobilized cinchona alkaloid quaternary ammonium salt involved the quaternization of cinchonidine with benzyl chloride moiety in Merrifield resin (1).5 A catalyst 3 in which a quaternary salt is immobilized on polystyrene by covalent-bond formation with the C-3 vinyl group has also been reported.6Water-soluble PEG-supported cinchona ammonium salts 4 and 5, which can be used for reactions in aqueous media, have also been synthesized.7
2.2 Synthesis of polymer-immobilized chiral quaternary ammonium salts through ionic-bond formation
In all the catalysts shown in Fig. 1, the quaternized cinchona alkaloid molecules are covalently bonded to the polymer support, because of which the catalytic performance degrades. We have developed a novel method by which a quaternized chiral ammonium salt can be noncovalently linked to a polymer support. We chose a quaternary ammonium sulfonate salt since it has a stable structure. The quaternary ammonium salt of cinchonidine 6 was treated with sodium styrene sulfonate 7 to give the quaternary ammonium sulfonate monomer 8 in quantitative yield (Scheme 1). Polymerization of 8 with styrene using divinylbenzene as the crosslinking agent afforded the corresponding polymer 9, which had a chiral quaternary ammonium salt. In the case of 9, the chiral catalyst was attached to the polymer support via an ionic bond. An alternative method to obtain the polymer-immobilized quaternary ammonium salt involves an ion-exchange reaction between the chiral quaternary ammonium salt and the polymeric sulfonate (Scheme 2). This method is useful for quaternary ammonium salts having a radical-sensitive structure. The styrene sulfonate monomer 12 containing an anthryl moiety did not undergo radical polymerization because of the formation of the stabilized radical. Ion-exchange reaction between 10 and 11 proceeded smoothly to give the corresponding chiral sulfonated polymer 13. By using the abovementioned two methods, any quaternary ammonium salt can be linked to the polymer support through ionic-bond formation. One of the main advantages of ionically immobilized polymeric chiral quaternary ammonium salts is the simplicity and ease of synthesis. More importantly, the chiral quaternary ammonium salt can be linked to the polymer support without the need for chemical modification of the chiral catalyst. Ionically immobilized catalysts were successfully used in the asymmetric alkylation of 14 to obtain 16 with high enantioselectivity (Scheme 3). When immobilizing a successfully designed catalyst on a polymer support, it is imperative that the original structure of the catalyst is unaltered, so that the catalytic activity is retained even after immobilization. For example, the Maruoka catalyst, which shows very strong activity in the asymmetric alkylation of glycine derivatives, can be easily immobilized on a polymer support by the ionically immobilized method. The original structure of the Maruoka catalyst is unaltered after immobilization on the polymer, and hence, high catalytic activity and high enantioselectivity are observed.8
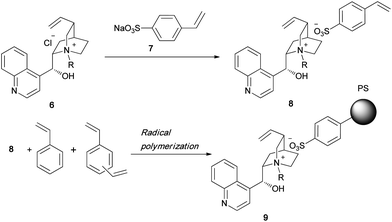 |
| | Scheme 1 | |
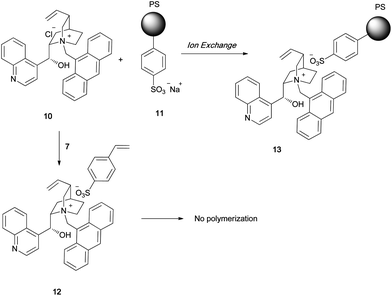 |
| | Scheme 2 | |
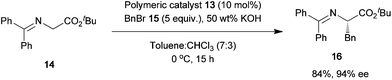 |
| | Scheme 3 | |
When a polystyrene-based crosslinked polymer is used as the support, the reaction proceeds smoothly in a triphase system to afford the chiral product in quantitative yield. The enantioselectivity obtained when using the polymeric catalyst is much higher than that obtained when using the unsupported catalyst. Although the actual reaction mechanism when using the chiral quaternary ammonium sulfonate has not been clarified, we believe that aggregated ion pairs (18) are involved in the reaction (Scheme 4). The results obtained for the aforementioned polymer-immobilized catalysts lead us to propose that the polymeric sulfonate plays a major role in the formation of the transition state. Owing to the strong affinity between the sulfonate anions and ammonium cations, the polymeric organocatalyst 17 can be easily recovered. Our ionic-bond formation strategy for polymeric chiral catalyst synthesis can be extended to other catalyst systems as well. One such example is the immobilization of a chiral amine catalystvia the formation of ammonium sulfonate linkages between a polymer and an amine catalyst.9 The resulting catalyst effectively catalyzes the asymmetric aldol reaction.
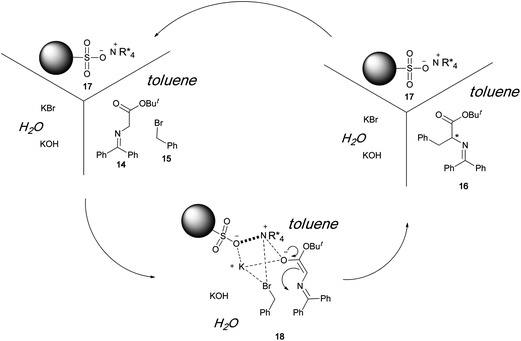 |
| | Scheme 4 | |
2.3 Synthesis of polymer-immobilized MacMillan catalysts by ionic-bond formation
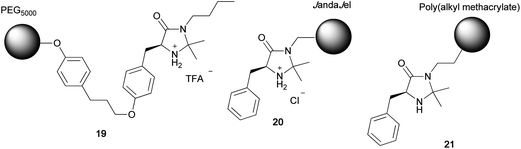
Among the numerous chiral organocatalysts reported, the imidazolidin-4-one derivatives designed and synthesized by D. W. C. MacMillan10–14 have been widely utilized as efficient chiral catalysts for various asymmetric transformations.15–22 There are some reports on the immobilization of MacMillan catalysts on polymer supports. Benaglia et al. were the first to immobilize a MacMillan catalyst on a PEG5000 support.23 The PEG-immobilized MacMillan catalyst 19 was used for the asymmetric Diels–Alder reaction of acrolein with 1,3-cyclohexadiene to obtain the corresponding Diels–Alder adduct in moderate yield (67%) and with high enantioselectivity (92% ee) (Fig. 2).23 The same catalyst effectively catalyzed asymmetric 1,3-dipolar cycloadditions.24 JandaJel-immobilized MacMillan catalyst 20 was also prepared and used as a catalyst for Diels–Alder reactions.25 Another polymer-immobilized MacMillan catalyst 21 was obtained by polymerization of a methacrylated MacMillan monomer.26 In all these polymer catalysts, the MacMillan catalyst was covalently bonded to the polymer support.
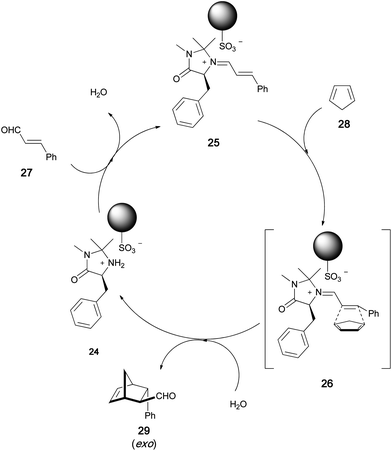
Recently, some examples of noncovalently immobilized MacMillan catalysts have been reported. Montmorillonite clay was found to readily entrap MacMillan catalysts, and the resulting heterogeneous chiral organocatalyst was utilized in Diels–Alder reactions.27 In another case, silica gel soaked in an ionic liquid such as a medium containing 1-butyl-3-methylimidazolium salt was used for the immobilization of MacMillan catalysts.28 We have developed a new polymer immobilization method based on ionic-bond formation between a MacMillan catalyst and a polymer support. Since the chiral quaternary ammonium sulfonate was sufficiently stable for immobilization on a polymer, as mentioned earlier in the document, we applied this strategy for the ionic immobilization of a MacMillan catalyst on a polymer (Scheme 5).29 The polymer-immobilized MacMillan catalyst 24 was easily obtained by polymerization of the MacMillan monomer 23 or by ion exchange between McMillan chloride 22 and the sulfonated polymer 11. The MacMillan imidazolidinone was successfully linked to the polymer through the formation of an ionic bond, which was stable under the asymmetric Diels–Alder reaction conditions. The reaction between cinnamaldehyde 27 and cyclopentadiene 28 proceeded smoothly in the presence of 24 to afford the Diels–Alder adduct in high yield (Table 1). On the basis of the plausible reaction mechanism shown in Scheme 6, we state that polymer sulfonate anion always stays near the nitrogen cation so that the catalyst remains linked to the polymer. We used several polymers as ionic supports for the MacMillan catalyst (Fig. 3). In the presence of the sulfonated-polystyrene-immobilized MacMillan catalyst 30, asymmetric Diels–Alder reaction readily occurred to give the chiral adduct in quantitative yield with high enantioselectivity (Table 1). The hydrophilic–hydrophobic balance of the polymer structure was found to be important for the catalyst performance in an asymmetric reaction. The poly(N-isopropylacrylamide)-immobilized catalyst 32 showed the best performance in the Diels–Alder reaction between cinnamaldehyde and cyclopentadiene. This catalyst could be reused without significant loss of its activity.
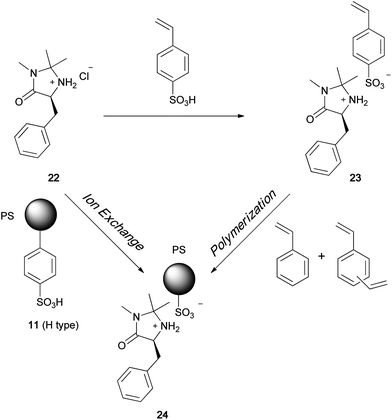 |
| | Scheme 5 | |
|
Catalyst
|
Conv. (%) |
Exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo endo |
Ee (exo) (%) |
Ee (endo) (%) |
|
30
|
>99 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
84 |
83 |
|
31
|
68 |
56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
91 |
88 |
|
32
|
>99 |
56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
88 |
91 |
 |
| | Scheme 6 | |
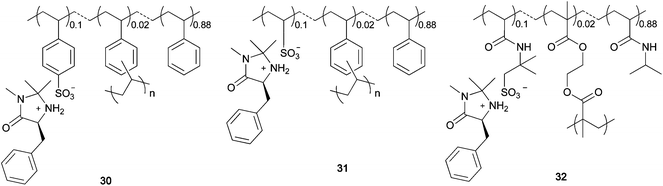 |
| | Fig. 3 Ionically immobilized polymeric MacMillan catalysts. | |
3.
Polymers with main-chain chirality
Polymers with main-chain chirality, which are widely used for chiral separation, are being increasingly used for catalytic applications as well. Typical examples of polymers with main-chain chirality used as asymmetric catalysts are chiral polythioureas,30 chiral 1,1′-binaphthyl polymers,31 chiral isotactic polystyrene,32 poly(amino acid)s,33polypeptides,34 synthetic peptides,35 helically chiral poly(phenylacetylene)s,36,37 helically chiral polyquinoxalines,38 and helically chiral poly(methacrylate)s.39,40 Some of these polymers have been used as chiral organocatalysts. An important example of such an application is the use of poly((S)-alanine) as a catalyst for the asymmetric epoxidation of chalcone by alkaline hydrogen peroxide.41 On the basis of this report, many chiral polymers have been synthesized for use as asymmetric organocatalysts.42 However, main-chain chiral quaternary ammonium salt polymers have not been reported till date. We have developed several polymers containing chiral quaternary ammonium salts in their main chain. These chiral polymers show excellent catalytic activity when used in asymmetric reactions.
3.1 Main-chain chiral organocatalyst polymers
Ammonium polyionenes are ion-containing polymers in which quaternary nitrogen atoms are present in the polymer main chain of the repeating unit, and not in the pendant position. Various types of ionene polymers have been synthesized from achiral monomers, and these polymers have many potential uses in biomedical applications. However, till date, optically active ionene polymers have not been synthesized and used as asymmetric organocatalysts. Menschutkin reaction between an optically active tertiary amine and a dihalide readily affords the corresponding dimeric quaternary ammonium salt. Cinchonidine 33 is an optically active tertiary amine having a hydroxy group. The dimeric quaternary ammonium salt 34 of 33 has two hydroxy groups (Scheme 7). Diol 34 can be polymerized with a dihalide in the presence of NaH to afford chiral polyether 35, which has a chiral quaternary ammonium moiety in its main chain.43 When equimolar amounts of dihalide and cinchonidine are allowed to react, a quaternary ammonium salt 36 is formed, as shown in Scheme 8. In the presence of NaH, 36 polymerizes by self-polycondensation to give the chiral polymer 37. An alternative method to prepare the main-chain chiral polymer involves the use of the chiral tertiary amine dimer 38, which can be easily prepared from 2 equiv. of cinchonidine and a dibromide. Repeated quaternization reaction between 38 and a dihalide gives the main-chain chiral polymer 35 (Scheme 9).44
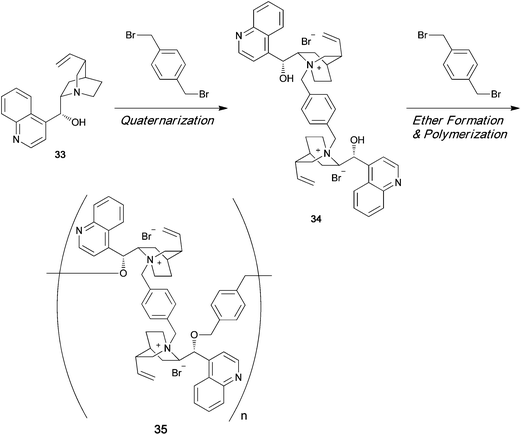 |
| | Scheme 7 | |
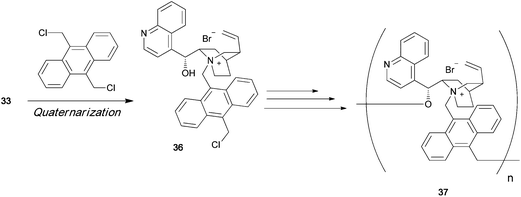 |
| | Scheme 8 | |
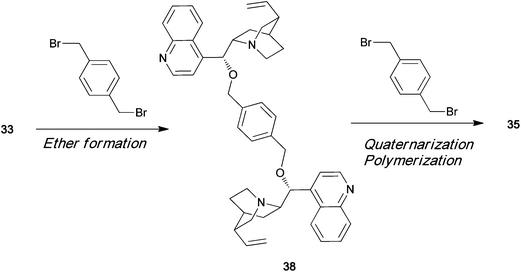 |
| | Scheme 9 | |
We used these chiral main-chain polymers as catalysts for the asymmetric benzylation of N-diphenylmethylidene glycine tert-butyl ester 14 (Scheme 10). After the asymmetric benzylation, the polymers could be easily recovered by precipitation in hexane and reused for the same reaction without any notable loss of catalytic activity.
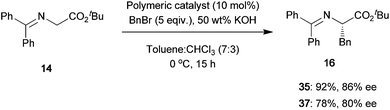 |
| | Scheme 10 | |
3.2 Main-chain ionic chiral polymers as organocatalysts
After confirming the high stability of quaternary ammonium sulfonate, as mentioned in Section 2.2, we attempted to carry out polymeric catalyst synthesis via quaternary ammonium sulfonate formation. As shown in the synthesis of polymer-immobilized quaternary ammonium organocatalysts via ionic-bond formation, the quaternary ammonium halide readily reacts with sodium sulfonate to give a stable quaternary ammonium sulfonate in quantitative yield. Repeated reaction between the dimer of the chiral quaternary ammonium salt and the disulfonate should give the corresponding chiral polymer, in which there is a main-chain ionic bond between the quaternary nitrogen cation and the sulfonate anion.45 A chiral quaternary ammonium dimer can be easily prepared from an enantiopure tertiary amine and a dihalide. For example, the dimeric quaternary ammonium salt of cinchonidine 39 is prepared from cinchonidine and a dihalide. Reaction of 39 and disodium 2,6-naphthalene sulfonate 40 proceeds smoothly when equimolar amounts of these compounds are allowed to react in methanolic water (Scheme 11). The precipitated polymer 41 can be easily isolated by filtration. To the best of our knowledge, this is the first example of an optically active polymer with a main-chain ionic bond. The catalytic activity of the ionic polymers 41 is evaluated for the asymmetric benzylation of 14 (Table 2). The asymmetric reaction proceeds smoothly in the presence of 41 to give 16 with high enantioselectivity. Various structural modifications can be carried out on the chiral quaternary ammonium dimer obtained from cinchonidine. A variety of disulfonates can also be used for the polymerization.
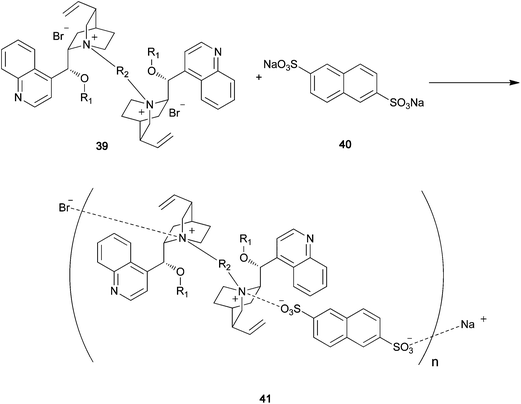 |
| | Scheme 11 | |
Table 2 Asymmetric benzylation of N-diphenylmethylene glycine tert-butyl ester 14 by using polymeric catalyst 41 at −20 °C
| Polymeric Catalyst 41 |
Time/h |
Yield (%) |
Ee (%) |
| R1 |
R2 |
|
Allyl
|
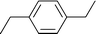
|
20 |
93 |
94 |
|
Allyl
|
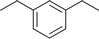
|
20 |
85 |
94 |
| H |
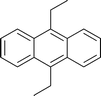
|
40 |
90 |
93 |
4. Conclusion
This paper highlights the synthesis of polymeric chiral organocatalysts and their applications in asymmetric reactions. Optically active quaternary ammonium salts are typical asymmetric organocatalysts that can be attached to the side chain of a polymer support through ammonium sulfonate linkages. The ionic bond between the polymeric sulfonate anion and the chiral quaternary nitrogen cation is sufficiently stable to ensure recyclability of the polymeric organocatalyst even under highly basic conditions. This ionic-bond formation method is also used to synthesize main-chain chiral polymers. Polymerization reaction between a chiral quaternary ammonium halide dimer and a disulfonate proceeds smoothly via ionic-bond formation to yield chiral ionic polymers. These polymers, which contain a chiral quaternary ammonium moiety, show excellent catalytic activity for asymmetric alkylation reactions.
References
-
(a)
A. Berkessel and H. Groeger, Asymmetric Organocatalysis, Wiley-VCHWeinheim, 2005 Search PubMed;
(b) B. List, Chem. Rev., 2007, 107, 5413–5883 CrossRef CAS;
(c) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138–5175 CrossRef CAS;
(d) D. Enders, C. Grondal and M. R. M. Hüttl, Angew. Chem., Int. Ed., 2007, 46, 1570–1581 CrossRef CAS;
(e) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2001, 40, 3726–3748 CrossRef CAS.
- M. Aglietto, E. Chiellini, S. D'Antone, C. Fegeri and R. Solam, Pure Appl. Chem., 1988, 60, 415–430 CAS.
-
N. Haraguchi, in Polymeric Chiral Catalyst Design and Chiral Polymer Synthesis, ed. S. Itsuno, Wiley, Hoboken, 2011, ch. 2 Search PubMed.
-
Handbook of Asymmetric Heterogeneous Catalysis, ed. K. Ding and Y. Uozumi, Wiley-VCHWeinheim, 2008 Search PubMed.
- Z. Zhengpu, Y. Yongmer, W. Zhen and P. Hodge, React. Funct. Polym., 1999, 41, 37–43 Search PubMed.
- B. Thierry, J. C. Plaquevent and D. Cahard, Mol. Diversity, 2005, 9, 277–290 Search PubMed.
- S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471–5569 CrossRef CAS.
- Y. Arakawa, N. Haraguchi and S. Itsuno, Angew. Chem., Int. Ed., 2008, 47, 8232–8235 CrossRef CAS.
- S. Luo, J. Li, L. Zhang, H. Xu and J. P. Cheng, Chem.–Eur. J., 2008, 14, 1273–1281 CrossRef CAS.
- G. Lelais and D. W. C. MacMillan, Aldrichimica Acta, 2006, 39, 79–87 CAS.
- D. W. C. MacMillan, Nature, 2008, 455, 304–308 CrossRef CAS.
- A. Erkkila, I. Majander and P. M. Pihko, Chem. Rev., 2007, 107, 5416–5470 CrossRef.
- S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471–5569 CrossRef CAS.
-
G. Lelais and D. W. D. MacMillan, in New Frontiers in Asymmetric Catalysis ed. K. Mikami and M. Lautens, John & Wiley Sons, 2007, pp. 319–331 Search PubMed.
- K. A. Ahrendt, C. J. Borths and D. W. C. MacMillan, J. Am. Chem. Soc., 2000, 122, 4243–4244 CrossRef CAS.
- W. S. Jen, J. J. M. Wiener and D. W. C. MacMillan, J. Am. Chem. Soc., 2000, 122, 9874–9875 CrossRef CAS.
- S. Lee and D. W. C. MacMillan, J. Am. Chem. Soc., 2007, 129, 15438–15439 CrossRef CAS.
- N. A. Pares and D. W. C. MacMillan, J. Am. Chem. Soc., 2001, 123, 4370–4371 CrossRef CAS.
- J. F. Austin and D. W. C. MacMillan, J. Am. Chem. Soc., 2002, 124, 1172–1173 CrossRef CAS.
- M. P. Brochu, S. P. Brown and D. W. C. MacMillan, J. Am. Chem. Soc., 2004, 126, 4108–4109 CrossRef CAS.
- I. K. Mangion, A. B. Northrup and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2004, 43, 6722–6724 CrossRef CAS.
- S. Lee and D. W. C. MacMillan, Tetrahedron, 2006, 62, 11413–11424 CrossRef CAS.
- M. Benaglia, G. Celentano, M. Cinquini, A. Puglisi and F. Cozzi, Adv. Synth. Catal., 2002, 344, 149–152 CrossRef CAS.
- A. Puglisi, M. Benaglia, M. Cinquini, F. Cozzi and G. Celentano, Eur. J. Org. Chem., 2004, 567–573 CrossRef CAS.
- S. A. Selkälä, J. Tois, P. M. Pihko and A. M. P. Koskinen, Adv. Synth. Catal., 2002, 344, 941–945 CrossRef CAS.
- T. E. Kristensen, K. Vestli, M. G. Jakobsen, F. K. Hansen and T. J. Hansen, J. Org. Chem., 2010, 75, 1620–1629 Search PubMed.
- S. Luo, J. Li, L. Zhang, H. Xu and J. P. Cheng, Chem.–Eur. J., 2008, 14, 1273–1281 CrossRef CAS.
- H. Hagiwara, T. Kuroda, T. Hoshi and T. Suzuki, Adv. Synth. Catal., 2010, 352(8), 909–916 CAS.
- N. Haraguchi, Y. Takemura and S. Itsuno, Tetrahedron Lett., 2010, 51, 1205–1208 CrossRef CAS.
- F. Touchard, F. Fache and M. Lemaire, Eur. J. Org. Chem., 2000, 3787–3792 Search PubMed.
- L. Pu, Chem.–Eur. J., 1999, 5, 2227–2232 CrossRef CAS.
- T. Kawasaki, C. Hohberger, Y. Araki, K. Hatase, K. Beckerle, J. Okuda and K. Soai, Chem. Commun., 2009, 5621 RSC.
- S. Colonna, D. Perdicchia and E. D. Mauro, Tetrahedron: Asymmetry, 2009, 20, 1709–1714 CrossRef CAS.
- D. R. Kelly and S. M. Roberts, Biopolymers, 2006, 84, 74–89 CrossRef CAS.
- E. A. C. Davie, S. M. Mennen, Y. Xu and S. J. Miller, Chem. Rev., 2007, 107, 5759–5812 CrossRef.
- K. Maeda, K. Tanaka, K. Morino and E. Yashima, Macromolecules, 2007, 40, 6783–6785 CrossRef CAS.
- K. Terada, T. Masuda and F. Sanda, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4971–4981 CrossRef CAS.
- M. Y. Suginome, J. Am. Chem. Soc., 2010, 132, 7899–7901 Search PubMed.
- M. Reggelin, M. Schultz and M. Holbach, Angew. Chem., Int. Ed., 2002, 41, 1614–1617 Search PubMed.
- C. A. Müller, T. Hoffart, M. Holbach and M. Reggelin, Macromolecules, 2005, 38, 5375–5380 Search PubMed.
- S. Juliá, J. Masana and J. C. Vega, Angew. Chem., Int. Ed. Engl., 1980, 19, 929–931 CrossRef.
-
K. Kudo, in Polymeric Chiral Catalyst Design and Chiral Polymer Synthesis, ed. S. Itsuno, Wiley, Hoboken, 2011, ch. 4 Search PubMed.
- S. Itsuno, D. K. Paul and N. Haraguchi, Chem. Lett., 2010, 39, 86–87 Search PubMed.
- M. Ishimoto, N. Haraguchi and S. Itsuno, Polymer Preprint, 2010, 59, 204 Search PubMed.
- S. Itsuno, D. K. Paul, M. A. Salam and N. Haraguchi, J. Am. Chem. Soc., 2010, 132, 2864–2865 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo
endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44
44