DOI:
10.1039/C1PY00067E
(Paper)
Polym. Chem., 2011,
2, 1190-1194
Received
8th February 2011
, Accepted 9th February 2011
First published on 24th February 2011
Abstract
A degradable polymer was prepared from α-angelica lactone, a five-membered unsaturated lactone, by ring-opening polymerization (ROP). The polymerizability of α-angelica lactone was explained by a DFT calculation. The degradability of the resultant polymer and the reaction kinetics of α-angelica lactone ROP were also considered. Owing to the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in α-angelica lactone, the ROP of five-membered cyclic lactone becomes feasible under moderate conditions and the resultant polyester exhibits good degradability under light or acidic/basic circumstances. Since α-angelica lactone can be easily obtained from the commercially available green bio-platform chemical levulinic acid, its ROP may provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
C bond in α-angelica lactone, the ROP of five-membered cyclic lactone becomes feasible under moderate conditions and the resultant polyester exhibits good degradability under light or acidic/basic circumstances. Since α-angelica lactone can be easily obtained from the commercially available green bio-platform chemical levulinic acid, its ROP may provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
1. Introduction
There has been great interest in the medical application of aliphatic polyesters in recent decades owing to their biodegradability and biocompatibility.1,2Ring-opening polymerization (ROP) of cyclic lactones or functionally related compounds is a well known approach for obtaining such degradable polymers.3,4 Among the usual lactones (Fig. 1), four-, six-, and seven-membered ones can be easily polymerized via ring-opening and the ROP of β-butyrolactone5,6 (1), δ-valerolactone7,8 (5) and ε-caprolactone9,10 (6) has been widely researched. However, it is generally considered that the five-membered cyclic lactones like γ-butyrolactone (2) and γ-valerolactone (3) are thermodynamically stable and are impracticable for ROP except under rather strict conditions.11–13 The oligomer of 2 with a molecular weight (Mn) up to 3.5 × 103 was prepared at high temperature and pressure (e.g. 160 °C and 2 × 103 MPa).14
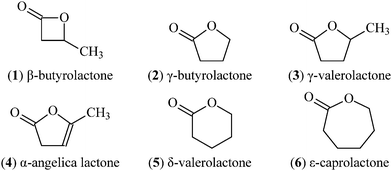 |
| | Fig. 1 Structures of lactones with 4–7 members. | |
In practice, however, it is generally accepted that the five-membered lactones cannot form high molecular weight homopolymersviaROP, because they have very small ring strain energy (SE) and the Gibbs free energy of polymerization (ΔGp) are positive.11–13,15 It was assumed that the ester in the five-membered lactone ring was not likely to break down and form a polymer chain under normal conditions; and therefore, little attention was paid to the ROP of five-membered lactones in the past. Obviously, this view neglected the feasibility of ROP of five-membered unsaturated lactones.
In this work, we describe an encouraging result for the ROP of α-angelica lactone (4), a bio-derived monomer, which can be easily obtained from the commercially available green bio-platform chemical levulinic acid16,17 (7), as shown in Scheme 1. As a five-membered unsaturated lactone, 4 can undergo ROP to produce functionalized poly(α-angelica lactone) (8). The polymer8 has carbon–carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. As a result, ROP of 4 will provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. As a result, ROP of 4 will provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
On account of this, the ROP of 4 in the presence of stannous octoateinitiator was investigated in this work. The polymerizability of 4 was explained by a DFT calculation. The degradability of the resultant polymer8 and the reaction kinetics of α-angelica lactone ROP were also considered.
2. Experimental section
General procedure for the synthesis of poly(α-angelica lactone)
5 ml of 4 (AL, 5.44 g, 0.056 mol) was first put in a flask under a flow of nitrogen. 5 ml of dry toluene and 0.186 mmol (0.075 g) of stannous octoate (Sn(OCt)2, Sn) were added with stirring under a nitrogen atmosphere. The mixture was intensively stirred and rapidly heated to 130 °C, and then kept at this temperature for 30 h to perform the ring opening polymerization (ROP). The polymerization reaction was quenched by exposing the reaction mixture to air outside the flask. After that, the solvent was evaporated. The remaining polymer8 (poly(α-angelica lactone), 4.08 g) was washed with cold hexane and then vacuum dried at 50 °C.
Characterization of the polymer
The molecular weight (Mn) and polydispersity index (PDI) of the resultant polymer were determined by gel permeation chromatography (GPC) using polystyrene standards. The GPC measurements were made at 30 °C on Waters 150-C gel permeation chromatograph equipped with a differential refractometer. The chromatograph was fitted with a set of three columns. Tetrahydrofuran was used as the mobile phase with a flow rate of 1 ml/min. The FT-IR spectra of the polymers were collected in the range of 400–4000 cm−1 on a Nicolet-380 FT-IR spectrometer by using KBr pellet technique. 1H-NMR and 13C-NMR spectra of the polymers were acquired at 25 °C with a Bruker DRX 300 NMR spectrometer.
3. Results and discussion
For a typical polymerization, α-angelica lactone4 was first dissolved in toluene (5.0 mol L−1), Sn(OCt)2 (Sn) was added as initiator ([4]0/[Sn]0 = 300), and then the reaction was conducted at 130 °C under nitrogen atmosphere. After stirring for 30 h, the mixture is exposed to air and nearly 79.8% of 4 is converted to the polymer8 with a molecular weight (Mn) of 29357 g mol−1 and a polydispersity index (PDI) of 1.25 by gel permeation chromatography (GPC) using polystyrene standards (Table 1). After reaction for 50 h, the conversion of 4 reaches 85.6% and remains essentially unchanged with further prolonging the reaction time and the polymerization may achieve an equilibrium state, as observed for other lactone ROP. By varying the loading of the initiator, it is possible to control the Mn and PDI of the resultant polymer.18 As listed in Table 1, Mn and PDI values of 8 decrease with the increase of monomer/initiator ratio ([4]0/[Sn]0). The polymerization rate at 130 °C is far faster than that at 110 °C and does not take place below 80 °C.
|
T/°C |
[4]0/[Sn]0 |
Reaction time (h) |
Conversion (%) |
Molecular weight (g mol−1) |
PDI |
| 130 |
300 |
30 |
79.8 |
29357 |
1.25 |
| 130 |
500 |
30 |
67.3 |
24390 |
1.22 |
| 130 |
1000 |
30 |
56.6 |
17175 |
1.15 |
| 130 |
300 |
50 |
85.6 |
21076 |
1.57 |
| 120 |
300 |
30 |
51.4 |
15903 |
1.16 |
| 110 |
300 |
30 |
18.2 |
8869 |
1.12 |
| 80 |
300 |
70 |
0 |
— |
— |
The molecular weights and PDI values of resultant polymer8 are dependent on the reaction time, as shown in Fig. 2. The molecular weight increases at first with the reaction time, reaches a maximum after reaction for 30 h and then decreases with further prolonging the polymerization; meanwhile, the PDI value increases consistently from 1.09 to 1.57 with the reaction time from 10 h to 50 h. The molecular weight reduction and PDI broadening with the extension of reaction time can be ascribed to the chain transfer in the polymer by transesterification;9,10 the intramolecular chain transfer results in cyclic oligomers while the intermolecular transfer results in scrambling of chain segments.
![Dependence of molecular weight (Mn) and polydispersity index (PDI) of poly(α-angelica lactone) on reaction time. Polymerization conditions: 4 in toluene (5.0 mol L−1), 130 °C, [4]0/[Sn]0 = 300.](/image/article/2011/PY/c1py00067e/c1py00067e-f2.gif) |
| | Fig. 2 Dependence of molecular weight (Mn) and polydispersity index (PDI) of poly(α-angelica lactone) on reaction time. Polymerization conditions: 4 in toluene (5.0 mol L−1), 130 °C, [4]0/[Sn]0 = 300. | |
Characterization of poly(α-angelica lactone)
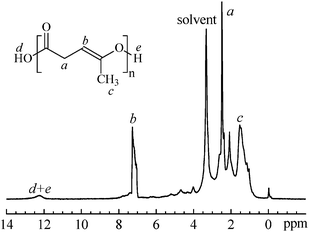
As shown in Fig. 3, 1H NMR spectrum of 8 is consistent with the iterative units resulting from the expected ROP of 4. The signal at 12.3 ppm can be assigned to the terminal carboxyl and hydroxylgroups. The peak at 7.2 ppm indicates that the carbon–carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) survive the polymerization reaction and are present in the polymer8, which are prone to generating HO˙ and CH
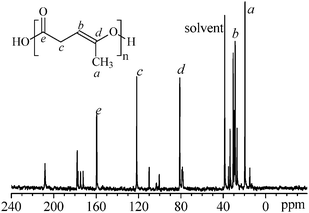
C) survive the polymerization reaction and are present in the polymer8, which are prone to generating HO˙ and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH˙ free radicals under light or heat and to trigger a series of reactions. The iterative units in the polymer8 from ROP of 4 are further confirmed by the 13C-NMR spectrum, as shown in Fig. 4.
CH˙ free radicals under light or heat and to trigger a series of reactions. The iterative units in the polymer8 from ROP of 4 are further confirmed by the 13C-NMR spectrum, as shown in Fig. 4.
The structure of the polymer8 was further characterized by the FT-IR spectrum, as shown in Fig. 5. The functional groups –OH, CH3–, –CH2–, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–O are identified in the FT-IR spectrum. The results further indicate that the iterative units resulting from the expected ROP of 4 and the carbon–carbon double bonds (C
C, and C–O are identified in the FT-IR spectrum. The results further indicate that the iterative units resulting from the expected ROP of 4 and the carbon–carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) are present in polymer8.
C) are present in polymer8.
As shown in Scheme 1, as a five-membered unsaturated lactone, 4 can undergo ROP to produce the functionalized polymer8. Similar to other lactones, the ROP of 4 may also follow a coordination-insertion mechanism with acyl-oxygen bond cleavage in which the chain grows via attaching the metal with an alkoxide bond.19,20 The polymer8 has carbon–carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. As a result, ROP of 4 will provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. As a result, ROP of 4 will provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
Degradability of poly(α-angelica lactone)
The degradability of the resultant polymer8 was then examined. Because the carbon–carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) present in the polymer8 are prone to generate HO˙ and CH
C) present in the polymer8 are prone to generate HO˙ and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH˙ free radicals under light or heat and to trigger a series of reactions, the polymer8 becomes degradable. As shown in Fig. 6, the polymer8 is stable under dark circumstances; however, the weight loss reaches 34.8% when it is exposed to daylight for 49 days. Moreover, the polymer is easily degraded under acidic or basic circumstances. As shown in Fig. 7, when it is placed in solutions with the pH values of 2.5 and 10.0, the weight losses in 49 days reach 63.3% and 52.0%, respectively, much higher than that of 13.5% in a neutral solution.
CH˙ free radicals under light or heat and to trigger a series of reactions, the polymer8 becomes degradable. As shown in Fig. 6, the polymer8 is stable under dark circumstances; however, the weight loss reaches 34.8% when it is exposed to daylight for 49 days. Moreover, the polymer is easily degraded under acidic or basic circumstances. As shown in Fig. 7, when it is placed in solutions with the pH values of 2.5 and 10.0, the weight losses in 49 days reach 63.3% and 52.0%, respectively, much higher than that of 13.5% in a neutral solution.
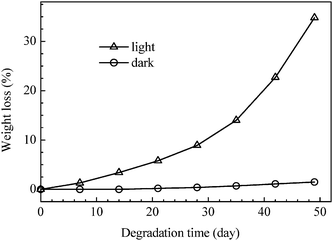 |
| | Fig. 6 Comparison of the weight losses of poly(α-angelica lactone) by degradation under daylight illumination and dark conditions. | |
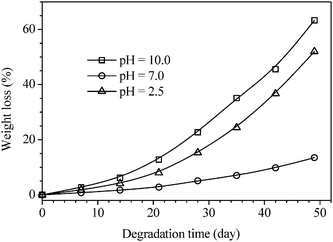 |
| | Fig. 7 Weight losses of poly(α-angelica lactone) by degradation in solution of different pH values. | |
![Semilogarithmic plot of the relative α-angelica lactone concentration against the reaction time under different temperatures (Reaction conditions: [AL]0 = 5.0 mol L−1 in toluene, [AL]0/[Sn]0 = 500): k = 3.07 × 10−6 s−1 (R = 0.9988) at 110 °C; k = 8.78 × 10−6 s−1 (R = 0.9975) at 120 °C; k = 1.70 × 10−5 s−1 (R = 0.9964) at 130 °C.](/image/article/2011/PY/c1py00067e/c1py00067e-f8.gif) |
| | Fig. 8 Semilogarithmic plot of the relative α-angelica lactone concentration against the reaction time under different temperatures (Reaction conditions: [AL]0 = 5.0 mol L−1 in toluene, [AL]0/[Sn]0 = 500): k = 3.07 × 10−6 s−1 (R = 0.9988) at 110 °C; k = 8.78 × 10−6 s−1 (R = 0.9975) at 120 °C; k = 1.70 × 10−5 s−1 (R = 0.9964) at 130 °C. | |
![Plot of ln(k) versus 1/T. Ea = 110.1 kJ mol−1 (linear fit, R = −0.99912). Reaction conditions: [AL]0 = 5.0 mol L−1 in toluene, [AL]0/[Sn]0 = 500. (△, ○ and ▽ represent 3 sets of independent experiments performed under the same conditions).](/image/article/2011/PY/c1py00067e/c1py00067e-f9.gif) |
| | Fig. 9 Plot of ln(k) versus 1/T. Ea = 110.1 kJ mol−1 (linear fit, R = −0.99912). Reaction conditions: [AL]0 = 5.0 mol L−1 in toluene, [AL]0/[Sn]0 = 500. (△, ○ and ▽ represent 3 sets of independent experiments performed under the same conditions). | |
Molecular modeling of lactones
Why does α-angelica lactone4 polymerize under moderate conditions but γ-butyrolactone2 and γ-valerolactone3 do not? Thermodynamically, the polymerizability of cyclic esters is determined by the Gibbs' free energy change of polymerization (ΔGp).11–13,21,22 Because the entropy of polymerization (ΔSp) is almost independent of the ring size, ΔGp at given temperature depends mainly on the enthalpy of polymerization (ΔHp) which is related to the ring strain.11–13,15,21–23 The release of ring strain through the polymerization process provides the principal driving force for the polymerization of cyclic monomers.24,25 Molecular modeling with the program suite of Gaussian 03 was used to evaluate the ring strain and the enthalpy change for lactonepolymerization. Geometry optimizations and frequency calculations of lactones were performed by using the B3LYP density functional theory (DFT) in conjunction with the 6-31G(d) basis set.26,27 The thermodynamic parameters were evaluated by the CBS-Q method. Strain energy (SE, CBS-Q) for the carbonyl derivatives is based upon the insertion/extrusion of –CH2–/–O– energy equivalents,28 as shown in Fig. 10.
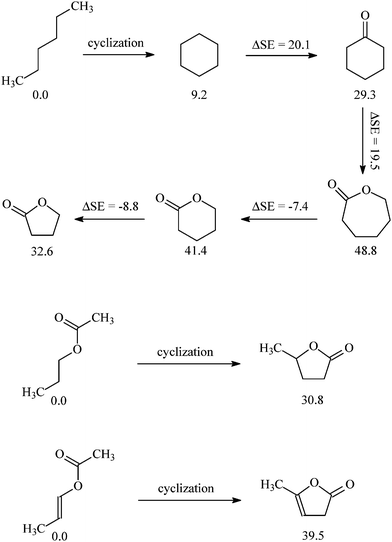 |
| | Fig. 10 Strain energies (CBS-Q, kJ mol−1) calculated for different lactones. | |
Table 2 lists the calculated results of a series of cyclic esters.15,29 Various lactones exhibit little difference in the bond lengths of O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] and O–C, whereas they are significantly different in the bond angles of ∠C–O–[C
O] and O–C, whereas they are significantly different in the bond angles of ∠C–O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] and ∠O–[C
O] and ∠O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]–C, which show the distortion around the breaking bond for ROP. This suggests that the major contribution to the ring strain is bond angle distortion. The bond angles for ∠C–O–[C
O]–C, which show the distortion around the breaking bond for ROP. This suggests that the major contribution to the ring strain is bond angle distortion. The bond angles for ∠C–O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] and ∠O–[C
O] and ∠O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]–C in 4 are 105.2° and 106.9°, which are 4.3° and 3.1° deviation from the forcefield equilibrium values, respectively. In contrast, the corresponding bond angles in 3 are only 0.3° and 0.9° deviation from the forcefield equilibrium values, respectively.
O]–C in 4 are 105.2° and 106.9°, which are 4.3° and 3.1° deviation from the forcefield equilibrium values, respectively. In contrast, the corresponding bond angles in 3 are only 0.3° and 0.9° deviation from the forcefield equilibrium values, respectively.
Table 2 Calculated structure and thermodynamic parameters of several lactones
|
Lactone
|
Bond angle (°) |
Bond length (Å) |
SE (kJ mol−1) |
ΔHp
a (kJ mol−1) |
ΔHp
b (kJ mol−1) |
C–O–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
O–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C O)–C |
O–C |
O–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|
From reference of 29.
This work.
Forcefield equilibrium value.15
|
|
2
|
109.1 |
110.9 |
1.442 |
1.397 |
32.6 |
−15.4 |
−13.2 |
|
3
|
109.2 |
110.9 |
1.442 |
1.367 |
30.8 |
−7.1 |
−7.4 |
|
4
|
105.2 |
106.9 |
1.440 |
1.392 |
39.5 |
|
−26.3 |
|
5
|
121.5 |
108.7 |
1.444 |
1.360 |
41.4 |
−26.8 |
−27.6 |
|
6
|
122.5 |
117.8 |
1.439 |
1.361 |
48.8 |
−35.9 |
−35.9 |
|
7
|
109.5 |
110.0 |
1.425 |
1.370 |
|
|
|
The minimized energy of both the lactone monomer (M) and the 3- and 2-mers of the polymer (P3 and P2) were obtained, as listed in Table 3. The internal energy of a single polymer unit was estimated as the difference in energy between the 3- and 2-mers, and so the change in internal energy upon polymerization could be calculated from: ΔHp = (E(P3) − E(P2)) − E(M).
Table 3 Calculated energy (CBS-Q) of the monomer (M) and 2- (P2) and 3-mers (P3) of the polymer from several lactones and the polymerization enthalpy (ΔHp)a
|
Lactone entry |
Energy (a.u.) |
ΔHp (a.u.) |
| M |
P2 |
P3 |
|
1 a.u. = 2625.5 kJ mol−1; ΔHp = (E(P3) − E(P2)) − E(M).
|
|
2
|
−306.47181 |
−612.94985 |
−919.42669 |
−0.00503 |
|
3
|
−305.85986 |
−611.72398 |
−917.586657 |
−0.00282 |
|
4
|
−344.07452 |
−688.16901 |
−1032.25354 |
−0.01001 |
|
5
|
−345.26643 |
−690.73132 |
−1036.00827 |
−0.01052 |
|
6
|
−366.85785 |
−733.79357 |
−1100.66510 |
−0.01368 |
The bond angles for ∠C–O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] and ∠O–[C
O] and ∠O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]–C in 4 are far distorted due to the presence of C
O]–C in 4 are far distorted due to the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the cyclic ring, suggesting a higher strain than that in 3. As shown in Table 2, the strain energy (SE) in 4 is comparable to that in a six-membered lactone (5), much higher than that in 3. Accordingly, the enthalpy change (–ΔHp) for ROP of 4 is close to that of 5, much higher than that of 3; actually, 4 also exhibits similar ROP ability to 5. All these indicate that the presence of a C
C in the cyclic ring, suggesting a higher strain than that in 3. As shown in Table 2, the strain energy (SE) in 4 is comparable to that in a six-membered lactone (5), much higher than that in 3. Accordingly, the enthalpy change (–ΔHp) for ROP of 4 is close to that of 5, much higher than that of 3; actually, 4 also exhibits similar ROP ability to 5. All these indicate that the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the unsaturated lactone enhance the strain energy and –ΔHp for ROP, which makes the ROP of five-membered α-angelica lactone feasible under moderated conditions.
C bond in the unsaturated lactone enhance the strain energy and –ΔHp for ROP, which makes the ROP of five-membered α-angelica lactone feasible under moderated conditions.
4. Conclusions
A degradable polymer was prepared from α-angelica lactone, a five-membered unsaturated lactone, by ring-opening polymerization. Owing to the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in α-angelica lactone that significantly enhances the strain energy of pentagon ring, the ROP of five-membered cyclic lactone becomes feasible under moderate conditions and the resultant polyester exhibits good degradability under light or acidic/basic circumstances.
C bond in α-angelica lactone that significantly enhances the strain energy of pentagon ring, the ROP of five-membered cyclic lactone becomes feasible under moderate conditions and the resultant polyester exhibits good degradability under light or acidic/basic circumstances.
α-Angelica lactone as a bio-derived monomer can be easily obtained from the commercially available green bio-platform chemical levulinic acid. The ring-opening polymerization of it produces functionalized poly(α-angelica lactone) with carbon-carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. This may provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. This may provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
Acknowledgements
The authors are grateful for the financial support of the National Basic Research Program of China (2006CB202504) and the Knowledge Innovation Program of the Chinese Academy of Sciences (KJCX2.YW.H16, YZ200933).
References
- A. C. Albertsson and I. K. Varma, Biomacromolecules, 2003, 4, 1466–1486 CrossRef CAS
 .
.
- L. S. Nair and C. T. Laurencin, Prog. Polym. Sci., 2007, 32, 762–798 CrossRef CAS
 .
.
- J. Wu, T.-L. Yu, C.-T. Chen and C.-C. Lin, Coord. Chem. Rev., 2006, 250, 602–626 CrossRef CAS
 .
.
- O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147–6176 CrossRef
 .
.
- C. Guillaume, J.-F. Carpentier and S. M. Guillaume, Polymer, 2009, 50, 5909–5917 CrossRef CAS
 .
.
- P. Kurcok, P. Dubois and R. Jérôme, Polym. Int., 1996, 41, 479–485 CrossRef CAS
 .
.
- X. Lou, C. Detrembleur and R. Jérôme, Macromolecules, 2002, 35, 1190–1195 CrossRef CAS
 .
.
- R. R. Gowda and D. Chakraborty, J. Mol. Catal. A: Chem., 2009, 301, 84–92 CrossRef CAS
 .
.
- J. H. Khan, F. Schue and G. A. George, Polym. Int., 2009, 58, 296–301 CrossRef CAS
 .
.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC
 .
.
-
R. Seymour and C. Carraher, Structure Property Relationships in Polymers, Plenum Press, New York, 1984 Search PubMed
 .
.
-
H. R. Allcock, F. W. Lampe and J. E. Mark, Contemporary Polymer Chemistry, Pearson Education, Upper Saddle River, New Jersey, 3rd. edn, 2003 Search PubMed
 .
.
- K. N. Houk, A. Jabbari, H. K. Hall Jr and C. Alemán, J. Org. Chem., 2008, 73, 2674–2678 CrossRef CAS
 .
.
- F. Korte and H. Glet, J. Polym. Sci., Part C: Polym. Lett., 1966, 4, 685–689 Search PubMed
 .
.
- W. Saiyasombat, R. Molloy, T. M. Nicholson, A. F. Johnson, I. M. Ward and S. Poshyachinda, Polymer, 1998, 39, 5581–5585 CrossRef CAS
 .
.
-
R. H. Leonard and Pensacola, U.S. Pat., 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 809
809![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 203, 1957.
203, 1957.
- T. Chen, T. Deng and X. Hou, Fine Chemicals, 2009, 26, 885–888 Search PubMed
 .
.
- M. Tang, A. J. P. White, M. M. Stevens and C. K. Williams, Chem. Commun., 2009, 941–943 RSC
 .
.
- Y. Nakayama, K. Sasaki, N. Watanabe, Z. Cai and T. Shiono, Polymer, 2009, 50, 4788–4793 CrossRef CAS
 .
.
- N. Ropson, Ph. Dubois, R. Jérôme and Ph. Teyssié, Macromolecules, 1994, 27, 5950–5956 CrossRef CAS
 .
.
- J. Casanovas and C. Alemán, J. Comput.-Aided Mol. Des., 1994, 8, 441–448 CrossRef CAS
 .
.
- C. Alemán, Biopolymers, 1994, 34, 841–847 CrossRef CAS
 .
.
- A. Duda, J. Libiszowski, J. Mosnacek and S. Penczek, Macromol. Symp., 2005, 226, 109–119 CrossRef CAS
 .
.
- H. R. Allcock, Polym. Rev., 1970, 4, 149–189 Search PubMed
 .
.
-
H. Sawada, Thermodynamics of Polymerization. Marcel Dekker, New York, 1976 Search PubMed
 .
.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS
 .
.
- P. C. Hariharan and J. A. Pople, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS
 .
.
- R. D. Bach and O. Dmitrenko, J. Am. Chem. Soc., 2006, 128, 4598–4611 CrossRef CAS
 .
.
-
J. Brandrup, E. H. Immergut, E. A. Grulke, A. Abe and D. R. Bloch, Polymer Handbook, John Wiley & Sons, New York, 4th edn, 1999 Search PubMed
 .
.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in α-angelica lactone, the ROP of five-membered cyclic lactone becomes feasible under moderate conditions and the resultant polyester exhibits good degradability under light or acidic/basic circumstances. Since α-angelica lactone can be easily obtained from the commercially available green bio-platform chemical levulinic acid, its ROP may provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
C bond in α-angelica lactone, the ROP of five-membered cyclic lactone becomes feasible under moderate conditions and the resultant polyester exhibits good degradability under light or acidic/basic circumstances. Since α-angelica lactone can be easily obtained from the commercially available green bio-platform chemical levulinic acid, its ROP may provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. As a result, ROP of 4 will provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. As a result, ROP of 4 will provide a potential route to produce functionalized aliphatic polyesters from renewable resources.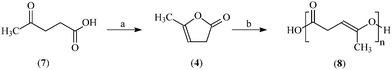
![Dependence of molecular weight (Mn) and polydispersity index (PDI) of poly(α-angelica lactone) on reaction time. Polymerization conditions: 4 in toluene (5.0 mol L−1), 130 °C, [4]0/[Sn]0 = 300.](/image/article/2011/PY/c1py00067e/c1py00067e-f2.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) survive the polymerization reaction and are present in the polymer8, which are prone to generating HO˙ and CH
C) survive the polymerization reaction and are present in the polymer8, which are prone to generating HO˙ and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH˙ free radicals under light or heat and to trigger a series of reactions. The iterative units in the polymer8 from ROP of 4 are further confirmed by the 13C-NMR spectrum, as shown in Fig. 4.
CH˙ free radicals under light or heat and to trigger a series of reactions. The iterative units in the polymer8 from ROP of 4 are further confirmed by the 13C-NMR spectrum, as shown in Fig. 4.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–O are identified in the FT-IR spectrum. The results further indicate that the iterative units resulting from the expected ROP of 4 and the carbon–carbon double bonds (C
C, and C–O are identified in the FT-IR spectrum. The results further indicate that the iterative units resulting from the expected ROP of 4 and the carbon–carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) are present in polymer8.
C) are present in polymer8.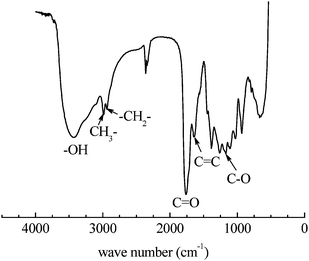
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. As a result, ROP of 4 will provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. As a result, ROP of 4 will provide a potential route to produce functionalized aliphatic polyesters from renewable resources.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) present in the polymer8 are prone to generate HO˙ and CH
C) present in the polymer8 are prone to generate HO˙ and CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH˙ free radicals under light or heat and to trigger a series of reactions, the polymer8 becomes degradable. As shown in Fig. 6, the polymer8 is stable under dark circumstances; however, the weight loss reaches 34.8% when it is exposed to daylight for 49 days. Moreover, the polymer is easily degraded under acidic or basic circumstances. As shown in Fig. 7, when it is placed in solutions with the pH values of 2.5 and 10.0, the weight losses in 49 days reach 63.3% and 52.0%, respectively, much higher than that of 13.5% in a neutral solution.
CH˙ free radicals under light or heat and to trigger a series of reactions, the polymer8 becomes degradable. As shown in Fig. 6, the polymer8 is stable under dark circumstances; however, the weight loss reaches 34.8% when it is exposed to daylight for 49 days. Moreover, the polymer is easily degraded under acidic or basic circumstances. As shown in Fig. 7, when it is placed in solutions with the pH values of 2.5 and 10.0, the weight losses in 49 days reach 63.3% and 52.0%, respectively, much higher than that of 13.5% in a neutral solution.


![Semilogarithmic plot of the relative α-angelica lactone concentration against the reaction time under different temperatures (Reaction conditions: [AL]0 = 5.0 mol L−1 in toluene, [AL]0/[Sn]0 = 500): k = 3.07 × 10−6 s−1 (R = 0.9988) at 110 °C; k = 8.78 × 10−6 s−1 (R = 0.9975) at 120 °C; k = 1.70 × 10−5 s−1 (R = 0.9964) at 130 °C.](/image/article/2011/PY/c1py00067e/c1py00067e-f8.gif)
![Plot of ln(k) versus 1/T. Ea = 110.1 kJ mol−1 (linear fit, R = −0.99912). Reaction conditions: [AL]0 = 5.0 mol L−1 in toluene, [AL]0/[Sn]0 = 500. (△, ○ and ▽ represent 3 sets of independent experiments performed under the same conditions).](/image/article/2011/PY/c1py00067e/c1py00067e-f9.gif)

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] and O–C, whereas they are significantly different in the bond angles of ∠C–O–[C
O] and O–C, whereas they are significantly different in the bond angles of ∠C–O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] and ∠O–[C
O] and ∠O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]–C, which show the distortion around the breaking bond for ROP. This suggests that the major contribution to the ring strain is bond angle distortion. The bond angles for ∠C–O–[C
O]–C, which show the distortion around the breaking bond for ROP. This suggests that the major contribution to the ring strain is bond angle distortion. The bond angles for ∠C–O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] and ∠O–[C
O] and ∠O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]–C in 4 are 105.2° and 106.9°, which are 4.3° and 3.1° deviation from the forcefield equilibrium values, respectively. In contrast, the corresponding bond angles in 3 are only 0.3° and 0.9° deviation from the forcefield equilibrium values, respectively.
O]–C in 4 are 105.2° and 106.9°, which are 4.3° and 3.1° deviation from the forcefield equilibrium values, respectively. In contrast, the corresponding bond angles in 3 are only 0.3° and 0.9° deviation from the forcefield equilibrium values, respectively.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)
O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C
O)–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)
O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] and ∠O–[C
O] and ∠O–[C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]–C in 4 are far distorted due to the presence of C
O]–C in 4 are far distorted due to the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the cyclic ring, suggesting a higher strain than that in 3. As shown in Table 2, the strain energy (SE) in 4 is comparable to that in a six-membered lactone (5), much higher than that in 3. Accordingly, the enthalpy change (–ΔHp) for ROP of 4 is close to that of 5, much higher than that of 3; actually, 4 also exhibits similar ROP ability to 5. All these indicate that the presence of a C
C in the cyclic ring, suggesting a higher strain than that in 3. As shown in Table 2, the strain energy (SE) in 4 is comparable to that in a six-membered lactone (5), much higher than that in 3. Accordingly, the enthalpy change (–ΔHp) for ROP of 4 is close to that of 5, much higher than that of 3; actually, 4 also exhibits similar ROP ability to 5. All these indicate that the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the unsaturated lactone enhance the strain energy and –ΔHp for ROP, which makes the ROP of five-membered α-angelica lactone feasible under moderated conditions.
C bond in the unsaturated lactone enhance the strain energy and –ΔHp for ROP, which makes the ROP of five-membered α-angelica lactone feasible under moderated conditions.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in α-angelica lactone that significantly enhances the strain energy of pentagon ring, the ROP of five-membered cyclic lactone becomes feasible under moderate conditions and the resultant polyester exhibits good degradability under light or acidic/basic circumstances.
C bond in α-angelica lactone that significantly enhances the strain energy of pentagon ring, the ROP of five-membered cyclic lactone becomes feasible under moderate conditions and the resultant polyester exhibits good degradability under light or acidic/basic circumstances.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. This may provide a potential route to produce functionalized aliphatic polyesters from renewable resources.
C) in its iterative units, which makes it degradable and/or possible to form numerous polymers by chemical modification. This may provide a potential route to produce functionalized aliphatic polyesters from renewable resources..
.
.
.
.
.
.
.
.
.
.
.
.
.
.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 809
809![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 203, 1957.
203, 1957..
.
.
.
.
.
.
.
.
.
.
.
.
