Stimuli-responsive star polymers through thiol–yne core functionalization/crosslinking of block copolymer micelles†
Niels ten
Brummelhuis
and
Helmut
Schlaad
*
Max Planck Institute of Colloids and Interfaces, Department of Colloid Chemistry, Research Campus Golm, 14424, Potsdam, Germany. E-mail: Helmut.Schlaad@mpikg.mpg.de; Fax: +49 331 567 9502; Tel: +49 331 567 9514
First published on 15th February 2011
Abstract
Thiol–yne photochemistry was applied for simultaneous functionalization and crosslinking of the core of poly(2-(3-butinyl)-2-oxazoline)-block-poly(2-ethyl-2-oxazoline) micelles in water to yield functional star polymers. Samples with core-confined ionic groups exhibited stimuli-responsive properties and were sensitive to changes in temperature, pH, ionic strength, and nature of the salt. Salt-induced shifts of the cloud point temperatures were found to correlate with the Hofmeister series.
Introduction
The crosslinking of self-assembled polymer structures, e.g. micelles and vesicles in solution, gels, and films, becomes an important research topic because it produces structures with enhanced stability or robustness against environmental stimuli such as dilution. For drug delivery applications, for instance, where normally only very small amounts of the drug are administered, crosslinking prevents the drug-delivery vehicle from disintegration in the blood-stream.1,2 Also, core-crosslinked micelles, or star polymers, with core-confined catalysts have successfully been applied in homogeneous catalysis to perform reaction cascades in one pot.3Thiol–yne chemistry, which is the radical addition of usually two equivalents of a thiol to a carbon–carbon triple bond (Scheme 1a),4 has become a powerful tool for creating highly functional hyperbranched polymers, dendrimers, networks, and so on.5,6 The thiol–alkyne double addition has the characteristics of a “click” reaction as it is modular, wide in scope, proceeds rapidly to completion, and is selective for a single product.7
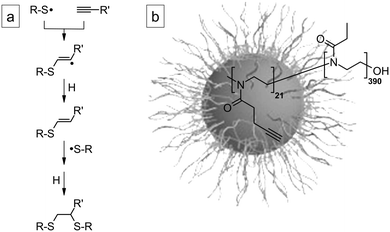 | ||
| Scheme 1 (a) Reaction pathway of radical thiol–yne addition. (b) Structure of PBOX-b-PEOX and corresponding spherical micelles in water. | ||
Here, we apply thiol–yne photochemistry in an efficient but non-“click” fashion8 for a simultaneous functionalization and crosslinking of poly(2-(3-butinyl)-2-oxazoline)-block-poly(2-ethyl-2-oxazoline) (PBOX-b-PEOX) copolymer micelles in water (see structure in Scheme 1b). The star polymers were analyzed by Raman spectroscopy and light scattering for the determination of functionality and confirmation of morphology. Stimuli-responsive (pH, salt, and temperature) star polymers were obtained by the addition of thiols carrying ionic groups and were characterized by phototurbidimetry.
Experimental part
Materials
Methyl triflate (≥98%) and acetonitrile (Chromasolv®) were received from Sigma-Aldrich, dried over CaH2, and distilled. Tetrahydrofuran (THF, 99.5%), dioxane (99.5%), methanol (99.5%), methyl 3-mercaptopropionate (≥98%), 3-mercapto-propionic acid (≥99%), sodium sulfate (≥99.0%), and sodium thiocyanate (≥99.99%) were used as received from Sigma-Aldrich. 2-Mercaptoethylamine hydrochloride (98%) was received from Acros Organics. Sodium nitrate (99.99%) was received from Merck.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 3.43 (t, 2H, CH2N), 3.91 (t, 2H, CH2O). 13C NMR (100.4 MHz, CDCl3): δ/ppm = 15.4, 27.3, 54.4, 67.5, 69.0, 82.7, 166.7.
C), 3.43 (t, 2H, CH2N), 3.91 (t, 2H, CH2O). 13C NMR (100.4 MHz, CDCl3): δ/ppm = 15.4, 27.3, 54.4, 67.5, 69.0, 82.7, 166.7.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1 M aqueous NaOH and methanol. The polymer was isolated by dialysis (MWCO 1 kDa) against THF and freeze-drying from dioxane. 1H NMR (400.1 MHz, CDCl3): number-average molecular weight, Mn = 41.2 kDa; molar ratio BOX/EOX = 0.054. SEC: apparent polydispersity index, PDI = 1.3 (ESI†).
1 mixture of 1 M aqueous NaOH and methanol. The polymer was isolated by dialysis (MWCO 1 kDa) against THF and freeze-drying from dioxane. 1H NMR (400.1 MHz, CDCl3): number-average molecular weight, Mn = 41.2 kDa; molar ratio BOX/EOX = 0.054. SEC: apparent polydispersity index, PDI = 1.3 (ESI†).
Analytical instrumentation and methods
1H NMR measurements were carried out at room temperature using a Bruker DPX-400 spectrometer operating at 400.1 MHz (13C NMR: 100.4 MHz). CDCl3 and DMSO-d6 were used as solvents (Sigma-Aldrich); signals were referenced to the signal of the solvent at δ = 7.26 (77.0) and 2.50 ppm, respectively.Size-exclusion chromatography (SEC) with simultaneous UV/RI detection was performed with N-methyl-2-pyrrolidone (NMP + 5 wt% LiBr) as the eluent at +70 °C using a set of two 300 × 8 mm PSS-GRAM columns with average particle sizes of 7 μm and porosities of 102 and 103 Å, respectively. Calibration was done with polystyrene standards (PSS, Mainz, Germany).
Raman spectroscopy was conducted on a confocal Raman microscopeWITeC UHTS 300 (WITec, Ulm, Germany) equipped with a 532 nm Nd:YAG laser (WITec, Ulm, Germany) and a piezo-scanner (P-500, Physik Instrumente, Karlsruhe, Germany). The laser beam was focused down to a micrometre size spot on the sample, and the spectra were acquired using a CCD detector (DU401-BV, Andor, Belfast, UK) behind a grating (600 g mm−1) spectrograph (UHTS 300, WITec, Ulm, Germany). ScanCtrlSpectroscopyPlus (version 1.38, WITec, Ulm, Germany) was applied in measurement and for spectra processing.
Dynamic and static light scattering (DLS/SLS) experiments were done with dilute polymer solutions (purified from dust by centrifugation) using an ALV-7004 multiple tau digital correlator equipped with a CGS-3 compact goniometer system, 22 mW He–Ne laser (λ = 632.8 nm), and a pair of avalanche photodiodes operated in a pseudo-cross-correlation mode (ALV, Langen, Germany). The DLS autocorrelation functions were measured at a scattering angle of 90° and evaluated with the CONTIN method.10 From the obtained diffusion coefficients, apparent hydrodynamic radii (Rhapp) were calculated using the Stokes–Einstein equation. SLS was measured at angles ranging from 30–150° in steps of 10°. Radii of gyration (Rg) and apparent weight-average molar masses (Mwapp) were determined applying partial Zimm and Guinier analyses, considering only the angular variation of dilute solutions.11
ζ-Potential measurements were performed on a Zetasizer Nano-Z (Malvern Instruments LtD, Worcestershire, UK). Aqueous polymer solutions (1 wt%) were adjusted to pH 3 or 11 and analyzed at room temperature.
Phototurbimetric measurements were conducted with a photometer TP1 (Elmar Tepper, Germany) operating at λ = 670 nm; heating/cooling rate was 1 K min−1. The temperature at which transmission dropped to 80% was taken as the cloud point temperature.
Results and discussion
Formation of micelles
The synthesized PBOX21-b-PEOX390 (the subscripts denote the average numbers of repeat units) is an amphiphilic block copolymer (PBOX = hydrophobic, PEOX = hydrophilic) with a hydrophilic weight fraction whydro ≈ 0.93. Accordingly, the polymer was expected to form spherical micelles with a PBOX core and a PEOX shell in aqueous solution (Scheme 1b).12 At a polymer concentration of 1 wt% (at which the cross-linking of the PBOX core will have been performed, vide infra), DLS indicated the presence of aggregates with Rhapp ≈ 50 nm. SLS revealed Rg ≈ 44 nm or 38 nm, depending on whether the data were evaluated applying partial Zimm or Guinier analysis, respectively. The characteristic ratio Rg/Rhapp ≈ 0.8 is close to the value expected for a hard sphere with uniform density (0.775),13 indicating that the aggregates are indeed spherical micelles. From the apparent value of Mwapp, the number of polymer chains per micelle was estimated to be 22 (data not shown).In THF, polymer chains were dissolved on a molecular level, and no aggregates could be detected by DLS.
Thiol–yne photoaddition: model reaction
PBOX21-b-PEOX390 micelles at a concentration of 1 wt% in water were reacted with methyl 3-mercaptopropionate as a model thiol; initial molar ratio [SH]0/[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0 = 2, 5, and 10. Mixtures were irradiated with a mercury medium pressure UV lamp (λ > 300 nm) for 24 h at room temperature. Exhaustive dialysis against THF was applied to remove all unreacted thiol (as confirmed by Raman spectroscopy, ESI†), and the products were isolated by freeze-drying from dioxane.
C]0 = 2, 5, and 10. Mixtures were irradiated with a mercury medium pressure UV lamp (λ > 300 nm) for 24 h at room temperature. Exhaustive dialysis against THF was applied to remove all unreacted thiol (as confirmed by Raman spectroscopy, ESI†), and the products were isolated by freeze-drying from dioxane.
Numbers of estergroups added to the triple bonds in the core of micelles were determined by Raman spectroscopy (ESI†)—results are summarized in Table 1. For [SH]0/[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0 = 2, on average 0.6 estergroups were added to the BOX units, corresponding to a conversion of 30%, within 24 h (Table 1, entry 2); the product contained unreacted BOX units (ESI†). The low reaction rate is attributed to a low concentration of radicals (thiyl radicals were generated under UV light without photoinitiator). The number of estergroups was larger the larger was the excess of thiol, reaching a value of 1.97, i.e. close to the maximum value of 2, at [SH]0/[C
C]0 = 2, on average 0.6 estergroups were added to the BOX units, corresponding to a conversion of 30%, within 24 h (Table 1, entry 2); the product contained unreacted BOX units (ESI†). The low reaction rate is attributed to a low concentration of radicals (thiyl radicals were generated under UV light without photoinitiator). The number of estergroups was larger the larger was the excess of thiol, reaching a value of 1.97, i.e. close to the maximum value of 2, at [SH]0/[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0 = 10 (entry 4). Interestingly, for [SH]0/[C
C]0 = 10 (entry 4). Interestingly, for [SH]0/[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0 = 5 (entry 3), every BOX unit carried one estergroup at quantitative conversion of triple bonds (ESI†). The thiyl radical addition to the alkyne appears to be considerably faster than that to the vinyl thioether (Scheme 1a), which is the opposite of what was reported by Bowman et al.14 It is assumed that the second addition is sterically hindered and thus slowed down within the confinement of a micelle.
C]0 = 5 (entry 3), every BOX unit carried one estergroup at quantitative conversion of triple bonds (ESI†). The thiyl radical addition to the alkyne appears to be considerably faster than that to the vinyl thioether (Scheme 1a), which is the opposite of what was reported by Bowman et al.14 It is assumed that the second addition is sterically hindered and thus slowed down within the confinement of a micelle.
DLS showed the presence of micelles (Rhapp ≥ 35 nm) in THF at a polymer concentration of 0.1 wt% (Table 1), though PBOX-b-PEOX is soluble in this solvent (vide supra), thus confirming crosslinking of the core of micelles and formation of star polymers. Interestingly, the size of the crosslinked micelles was larger (Rhapp = 35 nm–56 nm) the higher was the molar ratio of [SH]0/[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0. It is assumed that, under the given reaction conditions, the hydrophobic methyl 3-mercaptopropionate was incorporated in the micelles, leading to a swelling of the PBOX core and a change of the effective packing parameter.15
C]0. It is assumed that, under the given reaction conditions, the hydrophobic methyl 3-mercaptopropionate was incorporated in the micelles, leading to a swelling of the PBOX core and a change of the effective packing parameter.15
At this point it is important to note that crosslinking did not occur when a sample of PBOX21-b-PEOX390 in water was exposed to UV light in the absence of the thiol (Table 1, entry 1). Crosslinking was also suppressed when the thiol–yne reaction was performed in THF solution, i.e. when the block copolymer chains were dissolved molecularly and not assembled in micelles. Interestingly, the crosslinking of the core of poly(2-(3-butenyl)-2-oxazoline)-block-poly(2-ethyl-2-oxazoline)9 micelles failed with methyl 3-mercaptopropionate, though it could be achieved with 1,3-propanedithiol (data not shown).
Hence, one prerequisite for crosslinking to occur appears to be the spatial confinement of several chains within the core of a micelle. Furthermore, the reactivity of the intermediate radical species needs to be high, like that of a vinylsulfide radical,16 and the rate of hydrogen transfer be relatively low. It is believed that the main reaction leading to crosslinking is the addition of the vinylsulfide radical to a carbon–carbon triple or double bond (Scheme 2). A recombination of radicals cannot be excluded but appears to be rather unlikely due to the low concentration of radicals (vide supra).
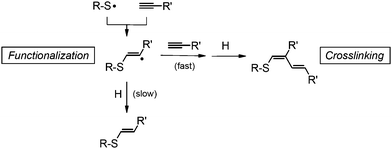 | ||
| Scheme 2 Tentative mechanism of the thiol–yne functionalization and crosslinking of PBOX-b-PEOX micelles (one possible pathway). | ||
Stimuli-responsive star polymers
The thiol–yne functionalization/crosslinking reaction was used to prepare stimuli-responsive star polymers being sensitive to changes of pH, ionic strength, and temperature. The thiols used were 3-mercaptopropionic acid (anionic) and 2-mercaptoethylamine hydrochloride (cationic). Due to the solubility of these thiols in water, contrary to methyl 3-mercaptopropionate, the experimental procedure was slightly changed: mixtures of PBOX21-b-PEOX390 (1 wt% in water) and thiol ([SH]0/[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0 = 1) were exposed to UV light for ∼14 h. Then, another 5 equivalents of thiol were added, and the mixture was irradiated for further 6 h. The product was purified by dialysis against water and isolated by freeze-drying.
C]0 = 1) were exposed to UV light for ∼14 h. Then, another 5 equivalents of thiol were added, and the mixture was irradiated for further 6 h. The product was purified by dialysis against water and isolated by freeze-drying.
According to DLS (0.1 wt% in THF), the hydrodynamic radii of cross-linked micelles were in the range of Rhapp ≈ 25–30 nm. The number of ionic groups added to the core of micelles could neither be determined by 1H NMR nor Raman spectroscopy nor by acid–base titration. Measurements of the ζ-potential confirmed, at least qualitatively, that the star polymers carried carboxylate (ξ = −3.43 mV at pH 11) and ammoniumgroups (ξ = +7.59 mV at pH 3), respectively (ESI†).
Results of phototurbimetric analyses of aqueous solutions of PBOX21-b-PEOX390 micelles (1 wt%) and anionic/cationic star polymers (star-COOH/COO− and star-NH2/NH3+) (0.1 wt%) at pH 3, 7, and 11 are shown in Fig. 1. PEOX in water exhibits lower critical solution behavior and turns insoluble upon an increase of temperature.17,18 The cloud point temperatures (TCP) of PBOX21-b-PEOX390 micelles at pH 3–11 were found to be in the narrow range of 56.3 to 57.4 °C.‡
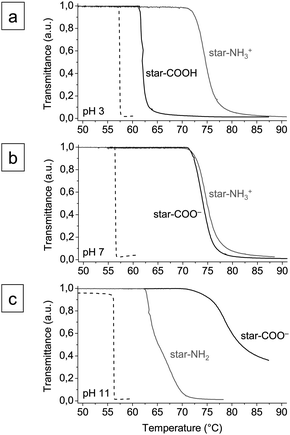 | ||
| Fig. 1 Turbidity curves of aqueous solutions of PBOX-b-PEOX micelles (1 wt%; dashed lines), anionic star polymer (0.1 wt%; black solid lines), and cationic star polymer (0.1 wt%; grey solid lines) at (a) pH 3, (b) pH 7, and (c) pH 11. | ||
The dependency of the cloud point temperatures of anionic star polymer solutions on pH vanished upon the addition of salt, 0.1 M NaNO3 or NaSCN and 0.01 M Na2SO4 (Fig. 2a and ESI†), which can be attributed to a screening of the charges. The cloud point temperatures were found to depend on the nature of the salt (Fig. 2a) and concentration (Fig. 2b and ESI†).18 At an ionic strength of 0.1 M at pH 7, for instance, the cloud point temperatures were 47.8 °C (Na2SO4), 62.7 °C (NaNO3), and 81.3 °C (NaSCN). The phase transition could thus be tuned within a temperature range of about 35 degrees, just by variation of salt and concentration. The shifts of TCP could be correlated with the Hofmeister series of anions:20
| kosmotropic SO42− > NO3− > SCN−chaotropic |
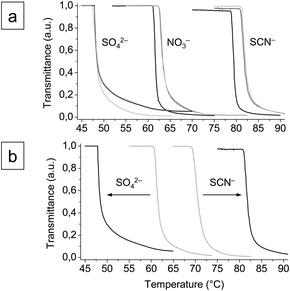 | ||
| Fig. 2 Turbidity curves of the anionic star polymer (0.1 wt%) (a) in 0.1 M aqueous salt solutions (Na2SO4, NaNO3, and NaSCN) at pH 3 (black lines), 7 (dark grey lines), and 11 (grey lines) and (b) in 0.01 M (grey lines) and 0.1 M (black lines) aqueous salt solutions (Na2SO4 and NaSCN) at pH 7. | ||
The sulfate anion is expected to decrease the solubility of the solute (“salting out”), thus causing a decrease of TCP, and the thiocyanate anion should have the opposite effect (“salting in”). The nitrate anion is considered not to have a pronounced effect on solubility.21 Though the observed changes in the phase behavior appear to be in agreement with the Hofmeister series, however, the true origin of this effect is not clear yet.
Conclusions
The micelles of poly(2-(3-butinyl)-2-oxazoline)-block-poly(2-ethyl-2-oxazoline) were simultaneously functionalized and crosslinked by thiol–yne photochemistry in water to yield well-defined star polymers. Crosslinking requires some spatial confinement (chains with triple bonds in the core of micelles) and is thought to occur via the addition of an intermediate vinylsulfide radical to a carbon–carbon triple or double bond. Star polymer samples with core-confined carboxylate or aminegroups exhibited stimuli-responsive properties and were sensitive to changes in temperature, pH, ionic strength, and nature of the salt. The shifting of the cloud point temperature, TCP ≈ 48–82 °C, with concentration and nature of the salt could be well correlated with the Hofmeister series.Current investigations deal with a systematic study of the Hofmeister effect and application of the star polymers as “smart” drug-carrier systems and as nanoreactors for catalysis. Also, the concept of thiol–yne functionalization/crosslinking shall be applied to other amphiphilic block copolymers and morphologies such as vesicles, lyotropic phases, films, etc.
Acknowledgements
Jessica Brandt, Katharina Ostwald, Marlies Gräwert, Olaf Niemeyer, Peter Černoch, and Admir Mašić are thanked for technical assistance and helping hands. Financial support was given by the Max Planck Society through the Network of Excellence “Synthetic Bioactive Surfaces”.References
- A. Harada and K. Kataoka, Prog. Polym. Sci., 2006, 31, 949–982 CrossRef CAS.
- R. Hoogenboom, Angew. Chem., Int. Ed., 2009, 48, 7978–7994 CrossRef CAS.
- B. Helms, S. J. Guillaudeu, Y. Xie, M. McMurdo, C. J. Hawker and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2005, 44, 6384–6387 CrossRef CAS.
- K. Griesbaum, Angew. Chem., Int. Ed. Engl., 1970, 9, 273–287 CAS.
- A. B. Lowe, C. E. Hoyle and C. N. Bowman, J. Mater. Chem., 2010, 20, 4745–4750 RSC.
- R. Hoogenboom, Angew. Chem., Int. Ed., 2010, 49, 3415–3417 CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- C. Barner-Kowollik, F. E. Du Prez, P. Espeel, C. J. Hawker, T. Junkers, H. Schlaad and W. Van Camp, Angew. Chem., Int. Ed., 2011, 50, 60–62 CrossRef CAS.
- A. Gress, A. Völkel and H. Schlaad, Macromolecules, 2007, 40, 7928–7933 CrossRef CAS.
- S. W. Provencher, Comput. Phys. Commun., 1982, 27, 229–242 CrossRef.
- P. A. Fuierer, B. Li and H. S. Jeon, J. Sol-Gel Sci. Technol., 2003, 27, 185–192 CrossRef CAS.
- S. Jain and F. S. Bates, Science, 2003, 300, 460–464 CrossRef CAS.
- W. Burchard, Adv. Polym. Sci., 1983, 48, 1–124 CAS.
- B. D. Fairbanks, T. F. Scott, C. J. Kloxin, K. S. Anseth and C. N. Bowman, Macromolecules, 2009, 42, 211–217 CrossRef CAS.
- R. Sigel, T. Krasia-Christoforou, I. Below and H. Schlaad, Macromolecules, 2009, 42, 4257–4261 CrossRef CAS.
- B. D. Fairbanks, E. A. Sims, K. S. Anseth and C. N. Bowman, Macromolecules, 2010, 43, 4113–4119 CrossRef CAS.
- D. Christova, R. Velichkova, W. Loos, E. J. Goethals and F. Du Prez, Polymer, 2003, 44, 2255–2261 CrossRef CAS.
- I. Dimitrov, B. Trzebicka, A. H. E. Müller, A. Dworak and C. B. Tsvetanov, Prog. Polym. Sci., 2007, 32, 1275–1343 CrossRef CAS.
- C. Diehl and H. Schlaad, Macromol. Biosci., 2009, 9, 157–161 CrossRef CAS.
- F. Hofmeister, Arch. Exp. Pathol. Pharmakol., 1888, 24, 247–260.
- Y. Zhang and P. S. Cremer, Curr. Opin. Chem. Biol., 2006, 10, 658–663 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Monomer synthesis (including intermediate reaction steps), polymer synthesis and characterization (1H NMR and SEC), Raman spectra, ζ-potential and turbidimetric measurements. See DOI: 10.1039/c1py00002k |
| ‡ Cloud point temperatures of PBOX21-b-PEOX390 at 0.1 wt% in water were in the range of TCP = 59.7–64.3 °C, however, considerably depending on pH of the solution (highest TCP at pH 3). Likewise, the cloud point temperatures of a PEOX (50 kDa, PDI = 3–4, Sigma-Aldrich) at 0.1 wt% in water varied between 62.5 °C (pH 11) and 64.3 °C (pH 3) (ESI†). The reason for this unexpected dependency of cloud point temperatures on pH is not known yet. |
| This journal is © The Royal Society of Chemistry 2011 |
