DOI:
10.1039/C0PY00401D
(Paper)
Polym. Chem., 2011,
2, 1156-1162
Low bandgap isoindigo-based copolymers: design, synthesis and photovoltaic applications
Received
11th December 2010
, Accepted 8th March 2011
First published on 16th March 2011
Abstract
Three new low bandgap isoindigo-based conjugated polymers were synthesized, namely poly {(N-octyl)-carbazole-2,7-diyl-alt-[N,N′-(2-ethylhexyl)-isoindigo]-6′,6′′-diyl} (PCzID), poly{(9, 9-dioctylfluorene)-2,7-diyl-alt-[N,N′-(2-ethylhexyl)-isoindigo]-6′,6-diyl} (PFID), and poly{4, 8-bis(2-ethylhexoxy)-benzo[1, 2-b,3,4-b]-dithiophene-2,6-diyl-alt-[N,N′-(2-ethylhexyl)-isoindigo]-6′,6′′-diyl} (PBDTID), by Stille or Suzuki coupling polymerization reaction. All of the polymers were soluble in common organic solvents, such as chloroform, tetrahydrofuran, and chlorobenzene with good film forming properties. The polymer films exhibit broad absorption bands in the wavelength region from 300 nm to 810 nm. Especially, PBDTID possesses the smallest bandgap of 1.54 eV calculated from its absorption cut-off at 806 nm. Preliminary photovoltaic cells based on the device structure of ITO/PEDOT:PSS/PBDTID:PC60BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w)/Ca/Al showed an open-circuit voltage of 0.56 V, a power conversion efficiency of 0.9% and a short circuit current of 3.81 mA cm−2.
1 w/w)/Ca/Al showed an open-circuit voltage of 0.56 V, a power conversion efficiency of 0.9% and a short circuit current of 3.81 mA cm−2.
Introduction
Polymer solar cells (PSC) have stimulated broad interest because of the prospect of low cost, solution processing, and the capability to fabricate flexible devices.1,2 In recent years, polymer photovoltaic (PV) materials have witnessed great progress.3–6 Low-bandgap polymer materials played an important role for the development of PSC, due to the improved light harvesting.7,8 In addition, high-efficiency photovoltaic materials should also show a low bandgap between 1.2 eV and 1.9 eV with a proper HOMO and LUMO energy level to optimize the open circuit voltage (Voc) and charge separation.9
Following these principles, much effort has been devoted to developing low bandgap polymers with a deep HOMO level. Among them, a diketopyrrolopyrrole (DPP) unit is appearing as an excellent electron accepting unit to constructing donor–acceptor (D–A) alternating copolymers. DPP-based polymers blended with PCBM derivatives as the active layer in PSC exhibited excellent PV properties.10,11 In fact, the isoindigo (ID) unit has a similar structure to the DPP unit (see Scheme 1), the two alkyl chains can improve the solubility and the two strong electron-withdrawing carbonyl groups can lower the HOMO level in order to increase the Voc. Moreover, the good conjugation length of ID unit should extend the absorption spectra for more light-harvesting. Very recently, Reynolds and co-workers used small molecule ID based derivatives as the electron donor blended with PC60BM as the electron acceptor which showed a high PCE up to 1.76% in molecular BHJ solar cells after modification,12a when we were preparing this manuscript, they reported a series of ID-based polymers with broad absorption spectrum, very low LUMO level and deep HOMO level, which indicated that ID based copolymers may be a kind of good acceptor in all-polymer solar cells, however, to date, the photovoltaic properties of ID-based copolymers have not been reported.12b
 |
| | Scheme 1 Chemical structures of PCzDPP and PCzID. | |
In this work, we present the synthesis and detailed characterization of three new low-bandgap ID-based copolymers which were derived from ID electron accepting units and three different common electron-donating copolymerizing groups (carbazole, fluorene and benzodithiophene). The results indicate that these copolymers possess good thermal, optical, and electrochemical properties. Photovoltaic results show that PBDTID is a promising material for efficient solar cells.
Experimental
Materials
Pd(PPh3)4, thiophene-2-boronic acid and 9,9-dioctylfluorene-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) (3) were obtained from Alfa Asia Chemical Co., and they were used without further purification. Tetrahydrofuran (THF) was dried over Na/benzophenone ketyl and freshly distilled prior to use. Other reagents and solvents were purchased commercially as analytical-grade quality and used without further purification. 2,6-Bis(trimethyltin)-4,8-bis(2-ethylhexoxy)-benzo[1,2-b,3,4-b]-dithiophene (4) and 9-octyl-2,7-bis (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole (5) were synthesized according to the reported literature.13,14
Characterization
1H NMR and 13C NMR spectra were recorded using a Bruker AV-400 spectrometer, with tetramethylsilane (TMS) as the internal reference, chemical shifts were recorded in ppm. Elemental analysis was measured on a Flash EA 1112 elemental analyzer. Molecular weight and polydispersity of the polymers were determined by gel permeation chromatography (GPC) analysis with polystyrene as standard (Waters 515 HPLCpump, a Waters 2414 differential refractometer, and three Waters Styragel columns (HT2, HT3, and HT4)) using THF (HPLC grade) as eluent at a flow rate of 1.0 mL min−1 at 35 °C. Thermogravimetric analysis (TGA) was conducted on a Shimadzu DTG-60 thermogravimetric analyzer with a heating rate of 10 K min−1 under a nitrogen atmosphere. Differential scanning calorimetry (DSC) was recorded with a Thermal Analysis (TA) DSC-2010 in nitrogen. The UV-Visabsorption spectra were recorded on the SHIMADZU UV-2450 spectrophotometer. For the solid state measurements, polymer solution in chloroform was drop-cast on quartz plates. Optical bandgap was calculated from the onset of the absorption band. The cyclic voltammograms were recorded with a computer controlled Zahner IM6e electrochemical workstation using polymer film on a platinum disk as the working electrode, platinum wire as the counter electrode and Ag/Ag+ (0.1 M) as the reference electrode in an anhydrous and argon-saturated solution of 0.1 M of tetrabutylammonium hexafluorophosphate (Bu4NPF6) in acetonitrile. Electrochemical onsets were determined at the position where the current starts to differ from the baseline.
Fabrication and characterization of polymer solar cells
The PSCs were fabricated in the configuration of the traditional sandwich structure with an indiumtin oxide (ITO) glass positive electrode and a metal negative electrode. Patterned ITO glass with a sheet resistance of 10 Ω/□ was purchased from CSG HOLDING Co. LTD. (China). The ITO glass was cleaned by sequential ultrasonic treatment in detergent, deionized water, acetone, and isopropanol, and then treated in an ultraviolet-ozone chamber (Ultraviolet Ozone Cleaner, Jelight Company, USA) for 20 min. Then PEDOT:PSS (poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate)) (Baytron P 4083, Germany) was filtered through a 0.45 μm filter and spin coated at 2000 rpm for 60 s on the ITO electrode. Subsequently, the PEDOT:PSS film was baked at 150 °C for 15 min in the air to give a thin film with a thickness of 40 nm. A blend of polymer and PC60BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w, 10 mg mL−1 for polymers) was dissolved in ortho-dichlorobenzene (ODCB), and spin-cast at 3000 rpm for 45 s onto the PEDOT:PSS layer. The substrates were then dried at 70 °C for 15 min. The thickness of the photoactive layer is in the range of 130 nm measured by Ambios Technology XP-2 profilometer. A bilayer cathode consisted of Ca (∼20 nm) capped with Al (∼60 nm) was thermal evaporated under a shadow mask in a base pressure of ca. 10−5 Pa. The device active area of the PSCs is 4 mm2. Device characterization was carried out under AM 1.5G irradiation with the intensity of 100 mW cm−2 (Oriel 67005, 500 W) calibrated by a standard siliconcell. J–V curves were recorded with a Keithley 236 digital source meter. AFM images were obtained using a Veeco's Dimension V atomic force microscopic (AFM) in the tapping mode.
1 w/w, 10 mg mL−1 for polymers) was dissolved in ortho-dichlorobenzene (ODCB), and spin-cast at 3000 rpm for 45 s onto the PEDOT:PSS layer. The substrates were then dried at 70 °C for 15 min. The thickness of the photoactive layer is in the range of 130 nm measured by Ambios Technology XP-2 profilometer. A bilayer cathode consisted of Ca (∼20 nm) capped with Al (∼60 nm) was thermal evaporated under a shadow mask in a base pressure of ca. 10−5 Pa. The device active area of the PSCs is 4 mm2. Device characterization was carried out under AM 1.5G irradiation with the intensity of 100 mW cm−2 (Oriel 67005, 500 W) calibrated by a standard siliconcell. J–V curves were recorded with a Keithley 236 digital source meter. AFM images were obtained using a Veeco's Dimension V atomic force microscopic (AFM) in the tapping mode.
Synthesis of the monomers and polymers
The synthetic routes of the monomers and polymers are shown in Scheme 2. The detailed synthetic processes are as follows.
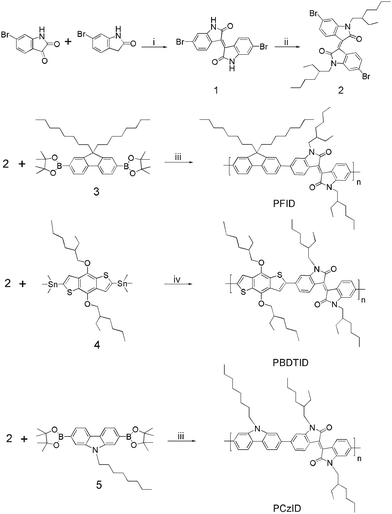 |
| | Scheme 2 Synthetic routes of the monomers and copolymers: (i) AcOH, conc. HC1 solution, reflux for 24 h; (ii) DMF, K2CO3, 1-bromo-2-ethylhexane, 100 °C for 15 h; (iii) Pd(PPh3)4, toluene, K2CO3, 95 °C for 96 h; (iv) Pd(PPh3)4, toluene, 110 °C for 24 h. | |
6, 6′-Dibromoisoindigo (1)
6-Bromooxindole (5 g, 23.6 mmol) and 6-bromoisatin (5.33 g, 23.6 mmol) were dissolved in AcOH (150 mL), followed by the addition of conc. HC1 solution (1 mL), with the reaction mixture vigorously stirring under reflux for 24 h. After cooling, the reaction solution was filtered. The solid was washed with water, ethyl alcohol and ethyl acetate. After drying under vacuum, deep-red powder was obtained (8.93 g, 85%). MS: m/z =300. 1H NMR (400 MHz, CDCl3): 7.82 (2H, s), 7.69 (2H, d), 7.40 (2H, s), 7.17 (2H, s). 13C NMR (CDCl3) δ: 170.3, 147.2, 134.0, 132.3, 127.0, 125.3, 122.6, 113.9.
6, 6′-Dibromo-N,N′-(2-ethylhexyl)-isoindigo (2)
Under nitrogen, compound 1 (4.2 g, 10 mmol), potassium carbonate (8.29 g, 50 mmol) and dimethylformaldehyde (DMF) (20 mL) were placed in a 150 mL three-necked flask, the mixture was stirred for half an hour, 1-bromo-2-ethylhexane (4.25 g, 22 mmol) was added by a syringe, and then this mixture was stirred for 15 h at 100 °C. The reaction mixture was poured into water, extracted with methylene chloride, washed by water and then dried with MgSO4. The resulting solid was purified by column chromatography using CH2Cl2: PE (petroleum ether) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent, red powder was obtained (5.5 g, 85%). GC-Ms: m/z = 281. 1H NMR (400 MHz, CDCl3): 7.49–6.91 (10H, m), 3.68 (2H, t), 1.77–1.36(12H, m), 0.91 (3H, t). 13C NMR (CDCl3) δ: 170.3, 147.2, 134.0, 132.3, 127.0, 125.3, 122.6, 113.9.
1 as eluent, red powder was obtained (5.5 g, 85%). GC-Ms: m/z = 281. 1H NMR (400 MHz, CDCl3): 7.49–6.91 (10H, m), 3.68 (2H, t), 1.77–1.36(12H, m), 0.91 (3H, t). 13C NMR (CDCl3) δ: 170.3, 147.2, 134.0, 132.3, 127.0, 125.3, 122.6, 113.9.
Synthesis of polymer (PFID)
9,9-Dioctylfluorene-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) (3) (231.3 mg, 0.36 mmol), 6,6′-dibromo-N,N′-(2-ethylhexyl)-isoindigo (2) (231.8 mg, 0.36 mmol), dry toluene (15 mL) and aqueous potassium carbonate (2.0 M, 4.5 mL) were added to a 50 mL two-necked round bottom flask. The reaction was purged with N2 for 20 min to remove O2, then Pd(PPh3)4 (20 mg) was added. The reaction mixture was stirred at 95 °C for 96 h. Then the dark red sticky solution was cooled down to room temperature and poured into CH3OH (50 mL), the precipitates were collected by filtration, and extracted using Soxhlet apparatus with methanol, hexanes, chloroform. The chloroform fraction was condensed under reduced pressure, precipitated in methanol/H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 50 mL), then filtered. After that, the final polymer was obtained as a dark red powder after vacuum-drying at 50 °C overnight. (143 mg, yield: 46%). 1H NMR (400 MHz, CDCl3): 8.07–6.97 (12H, br), 3.65 (4H, br), 1.25–2.12 (46H, m), 0.78–0.90 (18H, br). Anal. Calcd for (C61H80N2O2)n (%): C, 83.90; H, 9.23; N, 3.21;O, 3.66. Found (%): C, 82.48; H, 9.34; N, 3.24.
1, 50 mL), then filtered. After that, the final polymer was obtained as a dark red powder after vacuum-drying at 50 °C overnight. (143 mg, yield: 46%). 1H NMR (400 MHz, CDCl3): 8.07–6.97 (12H, br), 3.65 (4H, br), 1.25–2.12 (46H, m), 0.78–0.90 (18H, br). Anal. Calcd for (C61H80N2O2)n (%): C, 83.90; H, 9.23; N, 3.21;O, 3.66. Found (%): C, 82.48; H, 9.34; N, 3.24.
Synthesis of polymer (PBDTID)
2, 6-Bis(trimethyltin)-4,8-bis(2-ethylhexoxy)benzo[1,2-b;3,4-b]dithiophene (4) (231.6 mg, 0.3 mmol), 6,6′-dibromo-N,N′-(2-ethylhexyl)-isoindigo (2) (193.2 mg, 0.3 mmol), and 10 mL of dry toluene were put into a two-necked flask. The solution was flushed with N2 for 20 min, then Pd(PPh3)4 (17 mg) was added into the flask. The solution was flushed with N2 again for 10 min. The oil bath was heated to 100 °C carefully, and the reactant was stirred for 24 h at this temperature under N2 atmosphere. Then the reaction mixture was cooled to room temperature, and the polymer was precipitated by the addition of 50 mL of methanol and filtered through a Soxhlet thimble, which was then subjected to Soxhlet extraction with methanol, hexanes and chloroform. The polymer was recovered as a solid from the chloroform fraction by rotary evaporation. The bluish-green solid was dried under vacuum at 50 °C overnight to get the final product. (234 mg, yield: 86%). 1H NMR (400 MHz, CDCl3): 8.07–6.97 (8H, br), 4.03–3.62(8H, br), 1.25–2.21(36H, br), 0.81–0.90(24H, br). Anal. Calcd for (C58H76N2O4S2)n (%): C, 74.96; H, 8.24; N, 3.02; O, 6.89;.S, 6.90. Found (%): C, 73.48; H, 8.28; N, 3.08.
Synthesis of polymer (PCzID)
PCzID was obtained by a similar procedure to the synthesis of PFID starting from 9-octyl-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole (5) (106.3 mg, 0.2 mmol), 6,6′-dibromo-N,N′-(2-ethylhexyl)-isoindigo (128.8 mg, 0.2 mmol). Finally, the dark red solid was obtained. (112 mg, yield: 74%). 1H NMR (400 MHz, CDCl3): 8.17–6.97 (12H, m), 4.13(4H, br), 1.25–2.18 (30H, br), 0.70–0.90 (15H, br). Anal. Calcd for (C52H63N3O2)n (%): C, 81.96; H, 8.33; N, 5.51; O, 4.20. Found (%): C, 81.48; H, 8.33; N, 5.58.
Results and discussion
Synthesis and characterization of polymers
The synthesis of the monomers and the corresponding polymers are sketched in Scheme 2. 6-Bromooxindole and 6-bromoisatin were used as the starting materials for the preparation of compound 1, which was in turn converted to 2 by using 1-bromo-2-ethylhexane. The polymers were synthesized with the ditin derivative (4) or di-oxaborolane derivatives (3 and 5) in the presence of dibromide2 using Stille or Suzuki coupling reaction. The synthesized polymers were purified by continuous extraction with methanol, hexane and chloroform, and the chloroform fractions were recovered. The chemical structures of the polymers were verified by 1H NMR, the representative spectrum of PCzID is shown in Fig. 1, the broad peaks between 8.5 ppm–7.0 ppm belong to the aromatic ring protons, the broad peak from 3.6 ppm–4.0 ppm is attributed to the protons of the methylene group connected to the nitrogen atom, the peaks from 0.6 ppm–2.0 ppm correspond to the protons of the long alkyl chain. The absence of the characteristic singlet peak at 0.4 ppm in the 1H NMR spectrum reveals that no trimethyltin end groups are present in PBDTID. These results, combined with elemental analysis, indicate that the polymerization reaction is successful and complete. Gel permeation chromatography (GPC) results (using polystyrene as the standard and CHCl3 as eluent) have shown that the polymers have relatively high average molecular weight (Mw) values ranging from 13687 to 71359 with polydispersities of around 1.5–3.0. The obtained copolymers from chloroform fractions are readily soluble in common organic solvents such as chlorobenzene, dichlorobenzene and tetrahydrofuran, etc.Table 1 lists the polymerization results including molecular weight, PDI, yield, and thermal data for the polymers.
|
Polymers
|
M
n (g mol−1)a |
M
w (g mol−1)a |
PDI |
T
d (°C)b |
Yield (%) |
|
Determined by GPC in CHCl3 based on polystyrene standards.
Temperature at 5% weight loss under nitrogen.
|
|
PBDTID
|
11229 |
25125 |
2.2 |
366 |
86 |
|
PFID
|
23627 |
71359 |
3.0 |
326 |
46 |
|
PCzID
|
8956 |
13687 |
1.5 |
345 |
74 |
Thermal stability
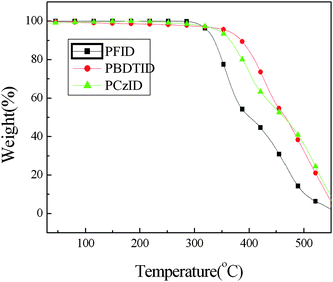
The thermal stability of the polymers is important for device fabrication. Fig. 2 shows the TGA thermograms of PBDTID, PFID and PCzID. The TGA analysis reveals that, under the protection of the inert atmosphere, the onset points of the weight loss (5%) of PBDTID, PFID and PCzID are ca. 366 °C, 326 °C and 345 °C, respectively, mainly due to the leaving of an alkoxy or alkyl group. Good thermal stability of the resulting copolymers prevents the deformation of the copolymer morphology and the degradation of the polymeric active layer under applied electric field. The thermal data of the copolymers are also summarized in Table 1. The polymers show no glass transition in differential scanning calorimetry (DSC) analysis.
Optical properties
The electronic structure and optical properties of the conjugated polymers can be obtained from UV-Visabsorption spectra. The absorption spectra of the synthesized copolymers in solution and thin-film states are shown in Fig. 3, and the related absorption information is summarized in Table 2. As shown in Fig. 3a, three polymers exhibit broad absorption plateau in the CHCl3 solution. The polymers show the two absorption bands, the peaks in the long wavelength region locate at 682 nm, 563 nm and 576 nm, the peaks in the short wavelength region lie at 455 nm, 462 nm and 458 nm, respectively for PBDTID, PFID and PCzID. The long wavelength absorption is due to D–A charge transfer state, which led to extended absorption. Compared to the solution state, three polymers have more red-shifted and broader absorption in the film states, as shown in Fig. 3b. The broader and better absorption is originated from better planarity of the polymer and stronger electronic interaction between the individual polymer chains in the film states.15 From the onset absorption, we can get optical bandgaps of 1.54 eV, 1.79 eV and 1.67 eV, respectively for PBDTID, PFID and PCzID. UV-Visabsorption spectra show that PBDTID, PFID and PCzID are low bandgap polymers with broad absorption band, which is very beneficial for obtaining high efficiency polymer solar cells.
|
Polymers
|
Absorption spectra
|
Cyclic voltammetry (vsAg/Ag+) |
| Solutiona |
Filmb |
p-doping |
n-doping |
— |
|
λ
max (nm) |
λ
max (nm) |
λ
onset (nm) |
E
optg
(eV) |
E
oxon/HOMOd (V)/(eV) |
E
redon/LUMOd (V)/(eV) |
E
ECg (eV) |
|
Measured in chloroform solution.
Cast from chloroform solution.
Bandgap estimated from the onset wavelength of the optical absorption.
HOMO = −e(Eoxon + 4.71) (eV); LUMO = −e(Eredon + 4.71) (eV) using Ag/Ag+ as the reference electrode.
|
|
PBDTID
|
682 |
686 |
806 |
1.54 |
0.40/−5.11 |
−1.05/−3.66 |
1.45 |
|
PFID
|
563 |
566 |
693 |
1.79 |
0.43/−5.14 |
−1.2/−3.51 |
1.63 |
|
PCzID
|
576 |
578 |
743 |
1.67 |
0.52/−5.23 |
−1.11/−3.6 |
1.63 |
Electrochemical properties
Cyclic voltammetry was usually performed to estimate the HOMO and LUMO energy levels of conjugated polymers.16 The onset oxidation and reduction potentials obtained from the cyclic voltammograms correspond to the HOMO and LUMO energy levels, respectively.17 We studied the electrochemical properties of PBDTID, PFID and PCzID by cyclic voltammetry. Fig. 4 shows the cyclic voltammograms of PBDTID, PFID and PCzID films on Ptelectrode with 0.1 mol L−1tetrabutylammonium tetrafluoroborate (Bu4NPF6)/CH3CN as the electrolyte at a scan rate of 50 mV s−1 using Ag/Ag+ as the reference electrode. It can be seen that PBDTID, PFID and PCzID exhibit quasi-reversible or reversible p-doping/dedoping (oxidation/re-reduction) processes over a positive potential range and irreversible n-doping/dedoping (reduction/re-oxidation) processes over a negative potential range. We calculated the HOMO and LUMO energy levels of the polymers according to the equations:18 HOMO = −e(Eoxon + 4.71) (eV); LUMO = −e(Eredon + 4.71) (eV). The onset reduction potentials of PBDTID, PFID and PCzID are −1.05 V, −1.2 V, and −1.11 V vs.Ag/Ag+, respectively. The LUMO energy levels of PBDTID, PFID and PCzID are −3.66 eV, −3.51 eV and −3.60 eV, which are similar to those of the DPP based polymers.10 The onset oxidation potentials of PBDTID, PFID and PCzID are 0.4 V, 0.43 V and 0.52 V vs.Ag/Ag+, respectively, which indicates that these polymers are air-stable.19 The HOMO energy levels of PBDTID, PFID and PCzID are −5.11 eV, −5.14 eV and −5.23 eV, respectively. The deep HOMO energy level of the polymers is desirable for a higher open circuit voltage of the PSCs. The electrochemical energy gaps of PBDTID, PFID and PCzID are 1.45 eV, 1.63 eV and 1.63 eV from the electrochemical measurement. The most electron-rich effect of the benzodithiophenegroup should be the main reason of the greatest bathochromic shift of PBDTID. The electrochemical bandgaps are generally in good agreement with the optical bandgaps of the polymers. The related CV data (Eoxon/Eredon, HOMO and LUMO energy levels, ECEg) are summarized in Table 2.
Photovoltaic properties
In order to investigate the potential applications of the three copolymers in solar cells, the bulk heterojunction PSCs were fabricated with a device structure of ITO/PEDOT:PSS/polymers:PCBM (w/w)/Ca/Al. We have measured the devices using the PBDTID/PCBM with different ratios (such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) under simulated 100 mW cm−2, AM 1.5G illumination. The active area was 4 mm2 for the device in this work. When the weight ratio of PBDTID/PCBM was 1
3) under simulated 100 mW cm−2, AM 1.5G illumination. The active area was 4 mm2 for the device in this work. When the weight ratio of PBDTID/PCBM was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the highest PCE was achieved. Fig. 5 shows the typical current density versus voltage (J–V) curve of polymer/PCBM (1
1, the highest PCE was achieved. Fig. 5 shows the typical current density versus voltage (J–V) curve of polymer/PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) based device and the open-circuit voltage (Voc), short circuit current (Jsc), fill factor (FF), and power conversion efficiency (PCE) of the polymers are summarized in Table 3. PBDTID demonstrated better photovoltaic performance over the other two copolymers with Jsc of 3.81 mA cm−2, Voc of 0.56 V, FF of 0.41, leading to a PCE of 0.9%, because the best optical properties among the three polymers from the progressively improved absorption spectra brought by the enhanced ICT interaction, and better charge separation from relatively coarse morphology (RMS: 8.51 nm, shown in Fig. 6) should account for the increase in Jsc in PBDTID based solar cells.20 However, PFID had the poorest photovoltaic performance, maybe due to poor charge mobility and morphology. The device performance can be believed to be improved by the modification of the chemical structure and device engineering such as optimized thickness, using PC71BM as the acceptor, solvent, additive, buffer layer etc. The fill factors of three polymers are relatively low, it's possibly due to the poor film quality, the unbalance charge transport and poor molecular packing of the blend layer.21
1, w/w) based device and the open-circuit voltage (Voc), short circuit current (Jsc), fill factor (FF), and power conversion efficiency (PCE) of the polymers are summarized in Table 3. PBDTID demonstrated better photovoltaic performance over the other two copolymers with Jsc of 3.81 mA cm−2, Voc of 0.56 V, FF of 0.41, leading to a PCE of 0.9%, because the best optical properties among the three polymers from the progressively improved absorption spectra brought by the enhanced ICT interaction, and better charge separation from relatively coarse morphology (RMS: 8.51 nm, shown in Fig. 6) should account for the increase in Jsc in PBDTID based solar cells.20 However, PFID had the poorest photovoltaic performance, maybe due to poor charge mobility and morphology. The device performance can be believed to be improved by the modification of the chemical structure and device engineering such as optimized thickness, using PC71BM as the acceptor, solvent, additive, buffer layer etc. The fill factors of three polymers are relatively low, it's possibly due to the poor film quality, the unbalance charge transport and poor molecular packing of the blend layer.21
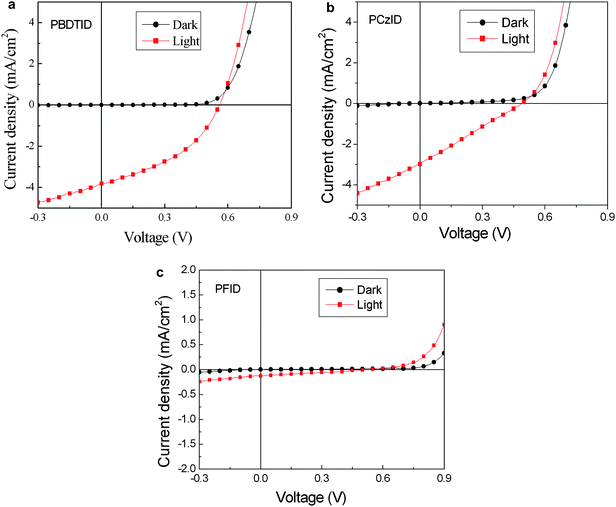 |
| | Fig. 5
J–V curves of the polymer solar cell based on PBDTID:PCBM blend (a), PczID:PCBM blend (b) and PFID:PCBM blend (c) at the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), under the illumination of AM 1.5 G, 100 mW cm−12. 1 (w/w), under the illumination of AM 1.5 G, 100 mW cm−12. | |
Table 3 Photovoltaic properties of the PSCs based on ID-based copolymers with PCBM
| Active layer |
J
sc (mA cm−2) |
V
oc (V) |
FF |
PCE (%) |
PBDTID:PCBM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.81 |
0.56 |
0.41 |
0.9 |
PBDTID:PCBM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2.05 |
0.58 |
33.0 |
0.40 |
PFID:PCBM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.13 |
0.53 |
0.26 |
0.02 |
PczID:PCBM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.98 |
0.49 |
0.25 |
0.4 |
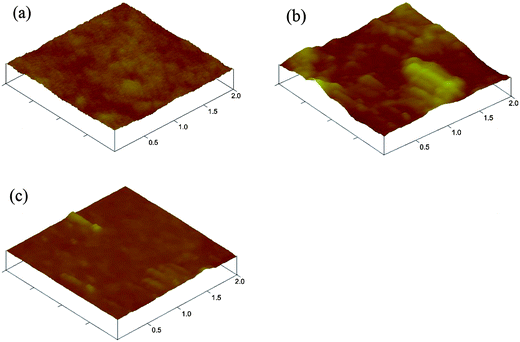 |
| | Fig. 6
AFM topography images of ID-based polymer/PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend films. (a) PFID; (b) PBDTID; (c) PCzID. RMS surface roughness values are (a) 1.04 nm; (b) 8.51 nm; (c) 2.54 nm. 1) blend films. (a) PFID; (b) PBDTID; (c) PCzID. RMS surface roughness values are (a) 1.04 nm; (b) 8.51 nm; (c) 2.54 nm. | |
Conclusions
In summary, we have designed and synthesized three new low bandgap isoindigo containing copolymers. They have been well characterized by 1H NMR, TGA, UV absorption, cyclic voltammetry. The copolymers exhibit good solubility in the common solvents and have broad absorption plateau in the visible-near infrared region, which ranges from 300 nm to 810 nm. Electrochemical measurements show that the polymers have low LUMO level, which is due to the introduction of the electron-withdrawing group. PBDTID exhibited better photovoltaic properties with PCE up to 0.9%.
Acknowledgements
This work was supported by National Natural Science Foundation of China (NO.50825102), Lieying Project, the Fundamental Research Funds for the Central Universities (No.2010QZZD0112), Doctoral Fund of Ministry of Education of China (No.20100162120033), Key Laboratory of Photochemical Conversion and Optoelectronic Materials, TIPC, Chinese Academy of Sciences (PCOM201025), the Opening Fund of State Key Laboratory of Powder Metallurgy.
References
- Y. Cheng, S. Yang and C. Hsu, Chem. Rev., 2009, 11, 5868 CrossRef.
- Y. Li and Y. Zou, Adv. Mater., 2008, 20, 2952 CrossRef CAS.
- J. Chen and Y. Cao, Acc. Chem. Res., 2009, 11, 1709 CrossRef.
- E. Ahmed, F. Kim, H. Xin and S. Jenekhe, Macromolecules, 2009, 42, 8615 CrossRef CAS.
- S. Park, A. Roy, S. Beaupré, S. Cho, N. Coates, J. Moon, D. Moses, M. Leclerc, K. Lee and A. Heeger, Nat. Photonics, 2009, 3, 297 CrossRef CAS.
- J. Wu, Y. Cheng, M. Dubosc, C. Hsieh, C. Chang and C. Hsu, Chem. Commun., 2009, 3259 Search PubMed.
- X. Zhan, Z. Tan, B. Domercq, Z. An, X. Zhang, S. Barlow, Y. Li, D. Zhu, B. Kippelen and S. Marder, J. Am. Chem. Soc., 2007, 129, 7246 CrossRef CAS.
-
(a) Y. Zou, A. Najari, P. Berrouard, S. Beaupré, R. Aich, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2010, 132, 5330 CrossRef CAS;
(b) A. Najari, S. Beaupré, P. Berrouard, Y. Zou, J. Pouliot, C. Pérusse and M. Leclerc, Adv. Funct. Mater., 2011, 21, 718 CrossRef CAS.
- R. Mondal, S. Ko, J. Norton, N. Miyaki, H. Becerril, E. Verploegen, M. Toney, J. Bredas, M. McGehee and Z. Bao, J. Mater. Chem., 2009, 19, 7195 RSC.
-
(a) Y. Zou, D. Gendron, R. Badrou-Aïch, A. Najari, T. Ye and M. Leclerc, Macromolecules, 2009, 42, 2891 CrossRef CAS;
(b) R. Badrou-Aïch, Y. Zou, M. Leclerc and Y. Tao, Org. Electron., 2010, 6, 1053 CrossRef;
(c) J. Jo, D. Gendron, A. Najari, J. S. Moon, S. Cho, M. Leclerc and A. J. Heeger, Appl. Phys. Lett., 2010, 97, 203303 CrossRef.
- L. Huo, J. Hou, H. Chen, S. Zhang, Y. Jiang, T. Chen and Y. Yang, Macromolecules, 2009, 17, 6564 CrossRef.
-
(a) J. Mei, K. Graham, R. Stalder and J. Reynolds, Org. Lett., 2010, 4, 660 CrossRef;
(b) R. Stalder, J. Mei and J. Reynolds, Macromolecules, 2010, 43, 8348 CrossRef CAS.
- Y. Liang, Y. Wu, D. Feng, S. Tsai, H. Son, G. Li and L. Yu, J. Am. Chem. Soc., 2009, 1, 56 CrossRef.
- N. Blouin, A. Michaud and M. Leclerc, Adv. Mater., 2007, 19, 2295 CrossRef CAS.
-
(a) P. Shen, B. Zhao, X. Huang, H. Huang and S. Tan, Eur. Polym. J., 2009, 45, 2726 CrossRef CAS;
(b) Y. Li, L. Xue, H. Li, Z. Li, B. Xu, S. Wen and W. Tian, Macromolecules, 2009, 13, 4491 CrossRef.
- Y. Li, Y. Cao, J. Gao, D. Wang, G. Yu and A. Heeger, Synth. Met., 1999, 99, 243 CrossRef CAS.
-
(a) Y. Zou, G. Sang, W. Wu, Y. Liu and Y. Li, Synth. Met., 2009, 159, 182 CrossRef CAS;
(b) B. Liu, A. Najari, C. Pan, M. Leclerc, D. Xiao and Y. Zou, Macromol. Rapid Commun., 2009, 31, 391;
(c) B. Liu, W. Wu, B. Peng, Y. Liu, Y. He, C. Pan and Y. Zou, Polym. Chem., 2010, 1, 678 RSC;
(d) Z. Zhang, B. Peng, B. Liu, C. Pan, Y. Li, Y. He, K. Zhou and Y. Zou, Polym. Chem., 2010, 1, 1441 RSC.
- Q. Sun, H. Wang, C. Yang and Y. Li, J. Mater. Chem., 2003, 13, 800 RSC.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. Plesu, M. Belletête, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732 CrossRef CAS.
-
(a) X. N. Yang, J. Loos, S. C. Veenstra, W. J. H. Verhees, M. M. Wienk, J. M. Kroon, M. A. J. Michels and R. A. J. Janssen, Nano Lett., 2005, 5, 579 CrossRef CAS;
(b) J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619 CrossRef CAS;
(c) W. Z. Cai, X. Gong and Y. Cao, Sol. Energy Mater. Sol. Cells, 2010, 94, 114 CrossRef CAS;
(d) J. C. Bijleveld, R. A. M. Verstrijden, M. M. Wienk and R. A. J. Janssen, Appl. Phys. Lett., 2010, 97, 073304 CrossRef.
-
(a) M. S. Kim, B. G. Kim and J. Kim, ACS Appl. Mater. Interfaces, 2009, 1, 1264 CrossRef CAS;
(b) R. Qin, W. Li, C. Li, C. Du, C. Veit, H. F. Schleiermacher, M. Andersson, Z. Bo, Z. Liu, O. Inganas, U. Wuerfel and F. Zhang, J. Am. Chem. Soc., 2009, 131, 14612 CrossRef CAS;
(c) C. Hoven, X. Dang, R. Coffin, J. Peet, T. Nguyen and G. Bazan, Adv. Mater., 2010, 22, 1.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w)/Ca/Al showed an open-circuit voltage of 0.56 V, a power conversion efficiency of 0.9% and a short circuit current of 3.81 mA cm−2.
1 w/w)/Ca/Al showed an open-circuit voltage of 0.56 V, a power conversion efficiency of 0.9% and a short circuit current of 3.81 mA cm−2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w, 10 mg mL−1 for polymers) was dissolved in ortho-dichlorobenzene (ODCB), and spin-cast at 3000 rpm for 45 s onto the PEDOT:PSS layer. The substrates were then dried at 70 °C for 15 min. The thickness of the photoactive layer is in the range of 130 nm measured by Ambios Technology XP-2 profilometer. A bilayer cathode consisted of Ca (∼20 nm) capped with Al (∼60 nm) was thermal evaporated under a shadow mask in a base pressure of ca. 10−5 Pa. The device active area of the PSCs is 4 mm2. Device characterization was carried out under AM 1.5G irradiation with the intensity of 100 mW cm−2 (Oriel 67005, 500 W) calibrated by a standard siliconcell. J–V curves were recorded with a Keithley 236 digital source meter. AFM images were obtained using a Veeco's Dimension V atomic force microscopic (AFM) in the tapping mode.
1 w/w, 10 mg mL−1 for polymers) was dissolved in ortho-dichlorobenzene (ODCB), and spin-cast at 3000 rpm for 45 s onto the PEDOT:PSS layer. The substrates were then dried at 70 °C for 15 min. The thickness of the photoactive layer is in the range of 130 nm measured by Ambios Technology XP-2 profilometer. A bilayer cathode consisted of Ca (∼20 nm) capped with Al (∼60 nm) was thermal evaporated under a shadow mask in a base pressure of ca. 10−5 Pa. The device active area of the PSCs is 4 mm2. Device characterization was carried out under AM 1.5G irradiation with the intensity of 100 mW cm−2 (Oriel 67005, 500 W) calibrated by a standard siliconcell. J–V curves were recorded with a Keithley 236 digital source meter. AFM images were obtained using a Veeco's Dimension V atomic force microscopic (AFM) in the tapping mode.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent, red powder was obtained (5.5 g, 85%). GC-Ms: m/z = 281. 1H NMR (400 MHz, CDCl3): 7.49–6.91 (10H, m), 3.68 (2H, t), 1.77–1.36(12H, m), 0.91 (3H, t). 13C NMR (CDCl3) δ: 170.3, 147.2, 134.0, 132.3, 127.0, 125.3, 122.6, 113.9.
1 as eluent, red powder was obtained (5.5 g, 85%). GC-Ms: m/z = 281. 1H NMR (400 MHz, CDCl3): 7.49–6.91 (10H, m), 3.68 (2H, t), 1.77–1.36(12H, m), 0.91 (3H, t). 13C NMR (CDCl3) δ: 170.3, 147.2, 134.0, 132.3, 127.0, 125.3, 122.6, 113.9.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 50 mL), then filtered. After that, the final polymer was obtained as a dark red powder after vacuum-drying at 50 °C overnight. (143 mg, yield: 46%). 1H NMR (400 MHz, CDCl3): 8.07–6.97 (12H, br), 3.65 (4H, br), 1.25–2.12 (46H, m), 0.78–0.90 (18H, br). Anal. Calcd for (C61H80N2O2)n (%): C, 83.90; H, 9.23; N, 3.21;O, 3.66. Found (%): C, 82.48; H, 9.34; N, 3.24.
1, 50 mL), then filtered. After that, the final polymer was obtained as a dark red powder after vacuum-drying at 50 °C overnight. (143 mg, yield: 46%). 1H NMR (400 MHz, CDCl3): 8.07–6.97 (12H, br), 3.65 (4H, br), 1.25–2.12 (46H, m), 0.78–0.90 (18H, br). Anal. Calcd for (C61H80N2O2)n (%): C, 83.90; H, 9.23; N, 3.21;O, 3.66. Found (%): C, 82.48; H, 9.34; N, 3.24.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) under simulated 100 mW cm−2, AM 1.5G illumination. The active area was 4 mm2 for the device in this work. When the weight ratio of PBDTID/PCBM was 1
3) under simulated 100 mW cm−2, AM 1.5G illumination. The active area was 4 mm2 for the device in this work. When the weight ratio of PBDTID/PCBM was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the highest PCE was achieved. Fig. 5 shows the typical current density versus voltage (J–V) curve of polymer/PCBM (1
1, the highest PCE was achieved. Fig. 5 shows the typical current density versus voltage (J–V) curve of polymer/PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) based device and the open-circuit voltage (Voc), short circuit current (Jsc), fill factor (FF), and power conversion efficiency (PCE) of the polymers are summarized in Table 3. PBDTID demonstrated better photovoltaic performance over the other two copolymers with Jsc of 3.81 mA cm−2, Voc of 0.56 V, FF of 0.41, leading to a PCE of 0.9%, because the best optical properties among the three polymers from the progressively improved absorption spectra brought by the enhanced ICT interaction, and better charge separation from relatively coarse morphology (RMS: 8.51 nm, shown in Fig. 6) should account for the increase in Jsc in PBDTID based solar cells.20 However, PFID had the poorest photovoltaic performance, maybe due to poor charge mobility and morphology. The device performance can be believed to be improved by the modification of the chemical structure and device engineering such as optimized thickness, using PC71BM as the acceptor, solvent, additive, buffer layer etc. The fill factors of three polymers are relatively low, it's possibly due to the poor film quality, the unbalance charge transport and poor molecular packing of the blend layer.21
1, w/w) based device and the open-circuit voltage (Voc), short circuit current (Jsc), fill factor (FF), and power conversion efficiency (PCE) of the polymers are summarized in Table 3. PBDTID demonstrated better photovoltaic performance over the other two copolymers with Jsc of 3.81 mA cm−2, Voc of 0.56 V, FF of 0.41, leading to a PCE of 0.9%, because the best optical properties among the three polymers from the progressively improved absorption spectra brought by the enhanced ICT interaction, and better charge separation from relatively coarse morphology (RMS: 8.51 nm, shown in Fig. 6) should account for the increase in Jsc in PBDTID based solar cells.20 However, PFID had the poorest photovoltaic performance, maybe due to poor charge mobility and morphology. The device performance can be believed to be improved by the modification of the chemical structure and device engineering such as optimized thickness, using PC71BM as the acceptor, solvent, additive, buffer layer etc. The fill factors of three polymers are relatively low, it's possibly due to the poor film quality, the unbalance charge transport and poor molecular packing of the blend layer.21

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), under the illumination of AM 1.5 G, 100 mW cm−12.
1 (w/w), under the illumination of AM 1.5 G, 100 mW cm−12.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) blend films. (a) PFID; (b) PBDTID; (c) PCzID. RMS surface roughness values are (a) 1.04 nm; (b) 8.51 nm; (c) 2.54 nm.
1) blend films. (a) PFID; (b) PBDTID; (c) PCzID. RMS surface roughness values are (a) 1.04 nm; (b) 8.51 nm; (c) 2.54 nm.