An efficient strategy for preparation of super-hydrophobic silane fluorinated polystyrene films with monomethoxypolyethyleneglycol-graft-polyethoxydisiloxane gel†
Guodong
Jiang
*,
Xiaodong
Shen
and
Tingwei
Wang
College of Material Science and Technology, Nanjing University of Technology, Nanjing, 210009, PR China. E-mail: gdjiang@njut.edu.cn; Fax: +86 25 83587266; Tel: +86 25 83587266
First published on 6th April 2011
Abstract
The present study reports a super-hydrophobic silane fluorinated polystyrene surface conglutinated with SiO2 nano-spheres prepared via the hydrolysis and condensation of the monomethoxypolyethyleneglycol-graft-polyethoxydisiloxane (MPEG-g-PEDS) micron gel particles and then fluorinated modification of the MPEG-g-PEDS gel/polystyrene film dried under dry air at room temperature.
With a contact angle (CA) greater than 150°, the super-hydrophobic surface shows excellent water-repellent properties which exhibits various applications.1 In order to form such a surface, the super-hydrophobic surface is fabricated by both coating with low-surface energy materials and forming micro- and/or nano-structures on the surface.2 Much attention is now focused on the design of the microstructure surface with the advancement of nano-techniques,3 such as the template method, etching, chemical vapor deposition (CVD) and vapor-induced phase separation (VIPS), etc. However, some of these methods have certain limitations such as severe conditions, complex process control, special equipment and expensive raw materials, etc.
The nano-particles incorporated into polymers to tailor the surface micro-and/or nano-structures of the inorganic/organic composite surface4 have been recently studied because of relatively simple fabrication technology. Unfortunately, the nano-particles can't obviously promote the roughness of a composite surface because the nano-particles are embedded in the polymer. What's more, the mechanical strength of composite films will rapidly decline with the increasing nano-particle content. For the purpose of improving the surface roughness of composites, Hou and Wang5 placed a nano-silica/polystyrene composite at 180 °C, the thermoplastic polymer melting caused that macromolecule was submerged under nano-silica. It is not suitable for fabricating silica/polystyrene super-hydrophobic films at room temperature. Furthermore, the high fluid viscosity of nano-particle/polymer solution leads to poor fluidity and poor film-forming because of the enormous special surface area of the nano-particle. In order to overcome the above defects, the initial strategy: the micron level of silica ethanol gels were directly dispersed in the polymer film, and then the super-hydrophobic xerogels on the composite film were obtained according to the preparation of nano-silica xerogels at ambient pressure.6 Disappointingly, the evaporation of ethanol in silica ethanol gels led to the film shrinkage and silica nano-particle agglomeration during film-forming.
In this communication, we report a new approach for fabricating the super-hydrophobic silane fluorinated polystyrene surface at room temperature. Monomethoxypolyethyleneglycol (MPEG) was substituted for the common ethanol solvent. For the complete miscibility of polyethoxydisiloxane (PEDS)7 and MPEG, MPEG was grafted to PEDS due to the reaction of the hydroxyl end-group of MPEG with the ethoxy group of PEDS. Then the MPEG-g-PEDS micron gel particles were dispersed in polymer. Furthermore, the nano-structure was obtained via the hydrolysis and condensation of MPEG-g-PEDS gel and fluorinated modification on the surface of the MPEG-g-PEDS gel/polystyrene film. Compared with the previous approaches, our method is characteristic of coating technology-free, good fluidity and good film-forming of the coating solution and good mechanical strength of the super-hydrophobic film.
The experimental procedure can be illustrated as shown in Fig. 1. ① Preparation of MPEG-g-PEDS gel particles: the PEDS should first be grafted to MPEG with the weight ratio of mMPEG:mPEDS = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 at 80 °C under the pressure of 2 kPa. The MPEG-g-PEDS gel was obtained after some water (volume ratio of water/ethanol 1
0.5 at 80 °C under the pressure of 2 kPa. The MPEG-g-PEDS gel was obtained after some water (volume ratio of water/ethanol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was slowly added into the aforementioned product with the weight ratio of H2O/product, 0.05
2) was slowly added into the aforementioned product with the weight ratio of H2O/product, 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and then kept at 105 °C for 6 h under the pressure of 2 kPa. ② Preparation of MPEG-g-PEDS gel/polystyrene films: a slurry of the MPEG-g-PEDS gel and toluene with the weight ratio of 1
1 and then kept at 105 °C for 6 h under the pressure of 2 kPa. ② Preparation of MPEG-g-PEDS gel/polystyrene films: a slurry of the MPEG-g-PEDS gel and toluene with the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was mixed with 20 wt% of polystyrene/toluene solution. The resulting mixed solution was coated on the surfaces of low density polyethylene (LDPE) substrate using a wire bar coater and dried at room temperature under RH = 15% and RH = 50%. ③ Preparation of super-hydrophobic silane fluorinated polystyrene films: the obtained MPEG-g-PEDS gel/polystyrene films were soaked in a 0.01 M perfluoroalkyltriethoxysilane (FTEOS) solution with the volume ratio of water and ethanol 1
1 was mixed with 20 wt% of polystyrene/toluene solution. The resulting mixed solution was coated on the surfaces of low density polyethylene (LDPE) substrate using a wire bar coater and dried at room temperature under RH = 15% and RH = 50%. ③ Preparation of super-hydrophobic silane fluorinated polystyrene films: the obtained MPEG-g-PEDS gel/polystyrene films were soaked in a 0.01 M perfluoroalkyltriethoxysilane (FTEOS) solution with the volume ratio of water and ethanol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 for 24 h, the resulting treated films were washed in hexane for 30 min to remove the excess FTEOS.
10 for 24 h, the resulting treated films were washed in hexane for 30 min to remove the excess FTEOS.
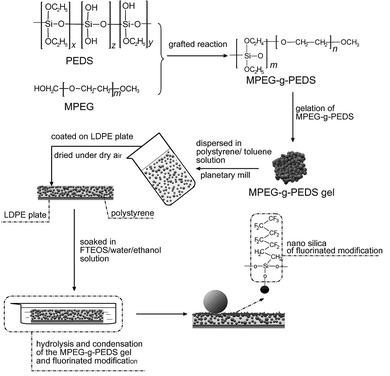 | ||
| Fig. 1 Scheme showing creation of a super-hydrophobic film via the hydrolysis and condensation of the MPEG-g-PEDS gel and fluorinated modification. | ||
The reaction of MPEG with PEDS was further studied by Fourier-transform infrared (FT-IR) spectra. The reaction solution gradually changed from opaque to transparent due to the reaction of the hydroxyl end-group of MPEG with the ethoxy group of PEDS as shown in Formula S1 (See ESI).† As shown in Fig. 2-a, the stretching vibrations of C–H bonds are observed at 2927 cm−1(a) and shoulder around 2865 cm−1(b). The asymmetric deformation vibrations of CH3 and CH2 are absorbed at 1455 cm−1(d), and the symmetric deformation vibration of CH3 is at 1352 cm−1(e). Obviously, the peaks of C–H bonds, CH2 and CH3 of MPEG-g-PEDS gel (A) are stronger than PEDS gel (B). It indicates that MPEG has been grafted to PEDS. Accessorial attenuated total reflection infrared (ATR) spectroscopy was used to determine the structure information of exterior of substance. As shown in Fig. 2-b, the blending vibrations of Si–OH near 1637 cm−1 (c) and 952 cm−1 (f) and the asymmetric stretching vibrations for Si–O–Si around 1085 cm−1 (g) become indistinguishable (See ESI, Fig. S2†). There are two strong peaks of –CF at 1204 cm−1 (i) and shoulder around 1146 cm−1 (s). Compared with the untreated films, it indicates that the surface of polystyrene films has been modified with low-surface energy groups (–CF3 and –CF2).
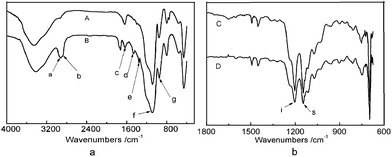 | ||
| Fig. 2 FT-IR spectra (a) of PEDS gel (A) and MPEG-g-PEDS gel (B); ATR (b) of treated films: C and D according to under dry air (RH = 15%) and humid air (RH = 50%), respectively. | ||
The surface morphology of films was characterized by scanning electron microscopy (SEM). Fig. 3 (a) shows that the diameter of MPEG-g-PEDS gel particles is ∼3 μm. After the MPEG-g-PEDS gel was mixed with polystyrene/toluene solution, the MPEG-g-PEDS gel/polystyrene/toluene solution was dried under different drying conditions. Under RH = 15%, the micro- and/or nano- conformation of gel particles can't be clearly shown because the MPEG-g-PEDS gel particles are embedded into polystyrene as shown in Fig. 3 (b). Moreover, in Fig. S3 (see ESI†), the MPEG-g-PEDS gel particles cause the roughness of films and the roughness will increase with the content of gel increasing.
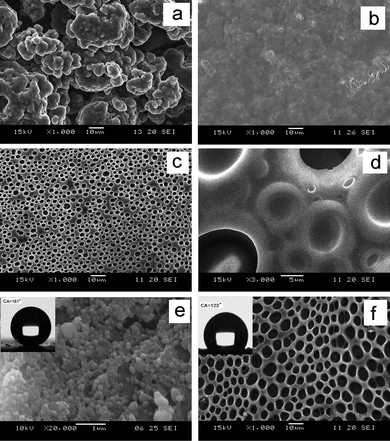 | ||
| Fig. 3 SEM of the PMEG-g-PEDS gels (a); at 50 wt% of PMEG-g-PEDS gel, the untreated film dried under RH = 15% (b) and RH = 50% (c); the magnification of sample under RH = 50% (d); the treated film dried under RH = 15% (e) and RH = 50% (f) including inters of CA, respectively. | ||
However, Fig. 3 (c) and (d) show the porous surface of MPEG-g-PEDS gel/polystyrene film dried under RH = 50%. It clearly indicates that the drying humidity has a profound effect on the surface morphology. The phenomena can be explained by vapor-induced phase separation (VIPS).8 In this theory, the influence of moisture on the topology of the untreated film largely depends on the miscibility of the solvent with water. Under humid air, water vapor was condensed on the surface of the MPEG-g-PEDS gel/polystyrene composite solution due to evaporative cooling of toluene immiscible with water. The hydrophilic MPEG-g-PEDS gel particles as nucleating agent were uniformly dispersed in mixed solution resulting in the water micro-droplets were arrayed in an ordered pattern in a very short time. Because the solid content of the mixed solutions in our work was higher than that of the literature,8 the rapid solidification made insufficient time form the perfect honeycomb-patterned porous surface. Under dry air, the evaporation of solvent gave no micro-droplets on top layer.
After the MPEG-g-PEDS gel/polystyrene films were soaked in the FTEOS/water/ethanol solution for 24 h, the MPEG-g-PEDS gel in polystyrene film would be hydrolyzed and condensed to nano-silica. The surface of treated film (RH = 15%) is conglutinated with the silica nanoparticles as shown in Fig. 3 (e). According to the above operation, Fig. 3 (f) shows the surface of treated film (RH = 50%) which has a perfect honeycomb-pattern, and the porous wall is composed of polystyrene (see ESI, Fig. S4 and S5†). The wettability of the resulting surface is evaluated by water CA measurement. The CA of untreated film decreases with the increase in the weight content of hydrophilic MPEG-g-PEDS gel, and the CAs of untreated films dried under RH = 50% are less than those under RH = 15% (see ESI, Table S1†). An insert in Fig. 3 (e) shows the shape of a water droplet on silane fluorinated polystyrene films (RH = 15%) with SiO2 nano-spheres. The CA reaches a maximum 161° at 50 wt% of MPEG-g-PEDS, which reveals the super-hydrophobic nature of the resulting surface. When the water drop that sits on the peak has ‘air pockets’ along its contact with the substrate. The CA of a drop is given by the Cassie-Baxter model:9
| cosθa = f2cosθt−f1 | (1) |
Here,θq and θt describe the CA on a rough surface and a smooth surface with uniform surface chemistry, respectively; f2 is the projective area fraction of the peaks on the horizontal surface; f1 is the projective area fraction of air pockets, and f1 + f2 = 1. It is easy to deduce from eqn (1) that increasing f1 increases θq, that is, the fraction of air in the surface is important for the super-hydrophobicity. According to eqn (1), the values of θq and θt are 161° and 115°,10 respectively. So the value of f1 is estimated to be 0.90, which indicates that the fraction of air in the surface is very high. This is because the fluorinated modification silica nano-particles as extrusive part touch with water droplets. As shown in Fig. 3 (f), the treated films (RH = 50%) comprised honeycomb-patterned walls as extrusive parts touching the water droplets. According to eqn (1), here, θq and θt are about 123.0° and 95.3° (see ESI, Table S1†), respectively. The result f2 = 0.501 is obtained. Moreover, the projective area fraction of honeycomb-patterned wall is calculated as being 0.507 by Sigmascan software. Hence, the two results are very close.
In summary, the preparation of super-hydrophobic silane fluorinated polystyrene film with MPEG-g-PEDS gel requires only mild conditions, namely room temperature. The good fluidity and good film-forming of the high solid content coating solution are due to the micron level of MPEG-g-PEDS gel particles. So the MPEG-g-PEDS gel/polystyrene film can be obtained by the simple coating route or the dip-coating route. The humidity has a profound effect on the surface morphology of the gel film. After the hydrolysis and condensation of MPEG-g-PEDS gel and fluorinated modification on the MPEG-g-PEDS gel/polystyrene film dried under RH = 15%, the CA reaches 161° at 50 wt% of MPEG-g-PEDS gel. However, the porous surface of film is obtained under RH = 50%. With potential applications, the MPEG-g-PEDS gel is also dispersed in other matrix resins such as polyurethane, polyether and polyacrylate, etc.
Notes and references
- W. Barthlott and C. Neinhuis, Planta, 1997, 202, 1 CrossRef CAS; A. Nakajima, K. Hashimoto, T. Watannabe, K. Takai, G. Yamauchi and A. Fujishima, Langmuir, 2000, 16, 7044 CrossRef CAS; C. Neinhuis and W. Barthlott, Ann. Bot., 1997, 79, 667 CrossRef; P. M. Barkhudarov, P. B. Shah, E. B. Watkins, D. A. Doshi, C. J. Brinker and J. Majewski, Corros. Sci., 2008, 50, 897 CrossRef CAS; A. Nakajima, K. Hashimoto and T. Watannabe, Monatsh. Chem., 2001, 132, 31 CrossRef CAS; W. Barthlott, C. Neinhuis and P. Walzel, Langmuir, 2005, 21, 956 CrossRef; T. Sun, L. Feng, X. Gao and L. Jiang, Acc. Chem. Res., 2005, 38, 644 CrossRef CAS.
- X. Gao and L. Jiang, Nature, 2004, 432, 36 CrossRef CAS; F. Shi, J. Niu, J. L. Liu, F. Liu, Z. Q. Wang, X. Q. Feng and X. Zhang, Adv. Mater., 2007, 19, 2257 CrossRef CAS; Z. G. Guo and W. M. Liu, Plant Sci., 2007, 172, 1103 CrossRef CAS; Z. Yoshimitsu, A. Nakajima and T. Watanabe, Langmuir, 2002, 18, 5818 CrossRef; A. Lafuma and D. Quéré, Nat. Mater., 2003, 2, 457 CrossRef CAS.
- L. Feng, Y. Song and L. Jiang, Angew. Chem., Int. Ed., 2003, 42, 800 CrossRef CAS; B. T. Qian and Z. Q. Shen, Langmuir, 2005, 21, 9007 CrossRef CAS; G. Gu, H. Dang, Z. Zhang and Z. Wu, Appl. Phys. A: Mater. Sci. Process., 2006, 83, 131 CrossRef CAS; A. V. Rao, M. K. Manish and D. P. Amalnerkar, J. Non-Cryst. Solids, 2003, 330, 187 CrossRef CAS; T. Khorasamim, H. Mirzadeh and W. Kemaniz, Appl. Surf. Sci., 2005, 242, 339 CrossRef CAS.
- J. Feng, B. Y. Huang and M.q. Zhong, J. Colloid Interface Sci., 2009, 336, 268 CrossRef CAS; M. Yu, G. T. Gu and W. D. Meng, et al. , Appl. Surf. Sci., 2007, 253, 3669 CrossRef CAS; W. A. Daoud, J. H. Xin and X. M. Tao, J. Am. Ceram. Soc., 2004, 87, 1782 CrossRef CAS; N. Abidi, E. Hequet and S. Tarimala, et al. , J. Appl. Polym. Sci., 2007, 104, 111 CrossRef CAS; Y. H. Liu, X. K. Wang, J. B. Luo and X. C. Lu, Appl. Surf. Sci., 2009, 255, 9430 CrossRef CAS.
- W. X. Hou and Q. H. Wang, J. Colloid Interface Sci., 2007, 316, 206 CrossRef CAS.
- C. J. Lee, G. S. Kim and S. Hyun, J. Mater. Sci., 2002, 37, 2237 CrossRef CAS; A. P. Rao, G. M. Pajonk and A. V. Rao, J. Mater. Sci., 2005, 40, 3481 CrossRef CAS; D. Sharad Bhagat, Y. H. Kima, K. H. Suh, Y. S. Ahn, J. G. Yeo and J. H. Han, Microporous Mesoporous Mater., 2008, 112, 504 CrossRef; J. L. Gurav, D. Y. Nadargi and A. V. Rao, Appl. Surf. Sci., 2008, 255, 3019 CrossRef CAS.
- K. Okada, S. Yasoham and S. Hayashi, J. Eur. Ceram. Soc., 1998, 18, 1879 CrossRef CAS; K. Kamiya, A. Katayama, H. Suzuki and K. Nishida, J. Sol-Gel Sci. Technol., 1999, 14, 95 CrossRef CAS; M.-A. Einarsrud, E. Nilsen and A. Rigacci, J. Non-Cryst. Solids, 2001, 285, 1 CrossRef CAS; C. Xu, J. Shen and B. Zhou, J. Non-Cryst. Solids, 2009, 355, 492 CrossRef; P. B. Wagha, R. Begag1, G. M. Pajonkb, A. V. Raoa and D. Haranatha, Mater. Chem. Phys., 1999, 57, 214 CrossRef CAS ; Us. Pat., 5795557.
- N. Zhao, Q. D. Xie, L. H. Weng, S. Q. Wang and X. Y. Zhang, Macromolecules, 2005, 38, 8996 CrossRef CAS; O. Karthaus, N. Maruyama, X. Gieren, M. Shimomura, H. Hasegawa and T. Hashimoto, Langmuir, 2000, 16, 6071 CrossRef CAS; J. X. Chen and J. A. Gardenlla, Macromolecules, 1998, 31, 9328 CrossRef CAS; A. Nakajima, K. Abe, K. Hashimoto and T. Watanabe, Thin Solid Films, 2000, 376, 140 CrossRef CAS.
- G. Mxhale, N. J. Shirtcliffe and M. I. Newton, Langmuir, 2004, 20, 1014; M. Yavuz, A. Demirel and M. Frank, Langmuir, 2005, 21, 5073 CrossRef; Y. Chen, B. He, J. Lee, A. Neelesh and N. A. J. Patankar, J. Colloid Interface Sci., 2005, 316, 458 CrossRef; N. A. J. Patankar, Langmuir, 2003, 19, 1249 CrossRef CAS.
- M. Miwa, A. Nakjiama, A. Fujishima, K. Hashimoto and T. Watannabe, Langmuir, 2000, 16, 5754 CrossRef CAS; T. Nishino, M. Meguro, K. Nakamae and M. Matsushita, Langmuir, 1999, 15, 4321 CrossRef CAS; H. M. Shang, Y. Wang, S. J. Limmer, T. P. Chou, K. Takahashi and G. Z. Cao, Thin Solid Films, 2005, 472, 37 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental materials, details and instrumentation, Fig. S1–S5, Formula S1, Table S1. See DOI: 10.1039/c0py00389a |
| This journal is © The Royal Society of Chemistry 2011 |
