Cyclodextrin-based polyurethanes act as selective molecular recognition materials of active pharmaceutical ingredients (APIs)†
Pu
Xiao
a,
Yves
Dudal
b,
Philippe F.-X.
Corvini
ac,
Uwe
Pieles
a and
Patrick
Shahgaldian
*a
aUniversity of Applied Sciences Northwestern Switzerland, School of Life Sciences, Gründenstrasse 40, Muttenz, CH-4132, Switzerland. E-mail: patrick.shahgaldian@fhnw.ch
bEnvolure, 2 place Pierre Viala, 34060, Montpellier cedex 2, France
cSchool of the Environment, Nanjing University, Nanjing, 210093, PR China
First published on 24th April 2011
Abstract
A series of highly cross-linked water insoluble polyurethanes have been produced using β-cyclodextrin and/or 2,2-bis(hydroxymethyl)propionic acid as monomers and toluene-2,4-diisocyanate or hexamethylene diisocyanate as cross-linkers. It is demonstrated that the binding specificities of the produced polymers for selected active pharmaceutical ingredients (APIs) are considerably different; the introduction of H-bond donor and acceptor systems and the chemical nature of the cross-linker are shown to influence the binding capabilities of the produced polymers for the studied APIs.
Molecular recognition is of great interest not only for the fundamental understanding of (bio)molecular sciences but also for its central role in various industrial fields ranging from biomedical and pharmaceutical applications to separation techniques and environmental applications, to name but a few.1
The design of synthetic systems possessing specific molecular recognition properties is, with self-assembly, the main focus of supramolecular chemistry.2,3 Since the early days of this modern area of chemical sciences, a wide range of molecules have been designed and studied for their capability of specific molecular recognition for a variety of targets. In this perspective, the use of macrocyclic systems enabling the three-dimensional positioning of multiple recognition moieties has been shown to be an excellent strategy and several families of macrocycles have been developed.3,4 Among them cyclodextrins (CDs) have attracted a considerable attention because of their favourable chemical structure,5i.e. truncated cone shape exhibiting all the primary alcohol functions at the narrow rim and all the secondary alcohols at the wide rim. Additionally, the intrinsic chiral nature of these cyclic polysaccharides has been exploited for enantiospecific recognition/separation purposes.6 CDs are produced via biocatalytic processes and are available at large amounts (annual production of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons in 2007) for a relatively low price averaging US$ 5 per kg.7 One possible approach to profit from the outstanding properties of the CDs for building mesoscopic or bulk materials is their embedment in polymers in the form of hydrogels, micro/nanoparticles, micelles, or bulk polymers.8 The mainstream of applications of CD-based polymers is their use as drug-delivery systems8 but other applications such as the preparation of specific sorbents for environmental contaminants have been explored.9–12 We recently reported the syntheses of CD-based water-insoluble polyurethane nanoparticles prepared with the three native cyclodextrins (α-, β- and γ-CDs) using toluene-2,4-diisocyanate as a cross-linker.13 Those nanomaterials have been shown to have differential binding capabilities for a series of pharmaceuticals. Mohamed et al. reported on the preparation of a series of β-cyclodextrin-based copolymer sorbents synthesized using various β-CD/diisocyanate ratios and using diisocyanates of variable molecular size and unsaturation degree;12 it has been demonstrated that these polymers possess relevant capabilities of sequestration of naphthenic acids in aqueous solutions. In our efforts to develop nanomaterials for sensing purposes, possessing not only high binding capability for a class of molecules (e.g. naphthenic acids, APIs) but also a relevant selectivity, we have decided to enhance the binding properties of the polymers by introducing in their structure additional interacting moieties. In this report, we describe a series of novel β-CD-based polymers produced using the combination of 2 different monomers and 2 different cross-linkers; the ability of the produced systems to bind three selected pharmaceuticals, namely acetaminophen (APAP), levofloxacin (LVF) and aspirin (ASP) is studied.
000 tons in 2007) for a relatively low price averaging US$ 5 per kg.7 One possible approach to profit from the outstanding properties of the CDs for building mesoscopic or bulk materials is their embedment in polymers in the form of hydrogels, micro/nanoparticles, micelles, or bulk polymers.8 The mainstream of applications of CD-based polymers is their use as drug-delivery systems8 but other applications such as the preparation of specific sorbents for environmental contaminants have been explored.9–12 We recently reported the syntheses of CD-based water-insoluble polyurethane nanoparticles prepared with the three native cyclodextrins (α-, β- and γ-CDs) using toluene-2,4-diisocyanate as a cross-linker.13 Those nanomaterials have been shown to have differential binding capabilities for a series of pharmaceuticals. Mohamed et al. reported on the preparation of a series of β-cyclodextrin-based copolymer sorbents synthesized using various β-CD/diisocyanate ratios and using diisocyanates of variable molecular size and unsaturation degree;12 it has been demonstrated that these polymers possess relevant capabilities of sequestration of naphthenic acids in aqueous solutions. In our efforts to develop nanomaterials for sensing purposes, possessing not only high binding capability for a class of molecules (e.g. naphthenic acids, APIs) but also a relevant selectivity, we have decided to enhance the binding properties of the polymers by introducing in their structure additional interacting moieties. In this report, we describe a series of novel β-CD-based polymers produced using the combination of 2 different monomers and 2 different cross-linkers; the ability of the produced systems to bind three selected pharmaceuticals, namely acetaminophen (APAP), levofloxacin (LVF) and aspirin (ASP) is studied.
Four cyclodextrin-based polymers (CDPs) have been produced‡ by mixing in a solvent β-CD and a diisocyanate (aliphatic, hexamethylene diisocyanate (HDI) or aromatic, toluene-2,4-diisocyanate (TDI)) with or without an additional carboxy-containing monomer, 2,2-bis(hydroxymethyl)propionic acid (DMPA); the compositions of the four CDPs are given in Table 1 and the schematic synthetic route in Fig. 1.
| CDPs | β-CD | DMPA | HDI | TDI |
|---|---|---|---|---|
| CDP1 | 2 | — | 8 | — |
| CDP2 | 2 | 1 | 8 | — |
| CDP3 | 2 | — | — | 8 |
| CDP4 | 2 | 1 | — | 8 |
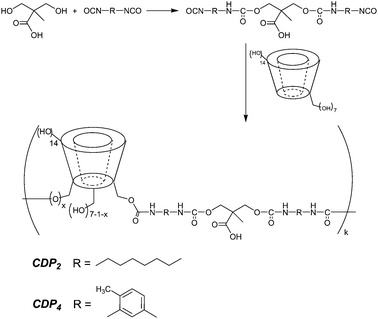 | ||
| Fig. 1 Preparation of cyclodextrin-based polymers CDP2 and CDP4. | ||
The produced polymers have been analysed using scanning electron microscopy that revealed that while CDP1, CDP2 and CDP3 do form large aggregates (Fig. S1, ESI†), CDP4 forms aggregated nanoparticulate systems with an average diameter of 100 ± 50 nm as shown in Fig. 2.§
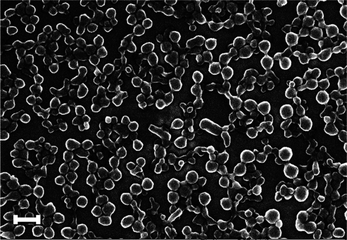 | ||
| Fig. 2 Scanning electron micrograph of CDP4 spread on a mica surface (scale bar: 200 nm). | ||
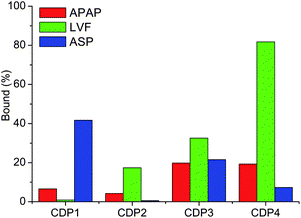
The binding capabilities of the produced CDPs of the three selected pharmaceuticals (Fig. 3), acetaminophen (APAP), levofloxacin (LVF) and aspirin (ASP), have been assessed incubating the produced CDPs with the API for 2 hours and measuring using high performance liquid chromatography (HPLC) the remaining amount of free API;¶ the results are given in Fig. 4.
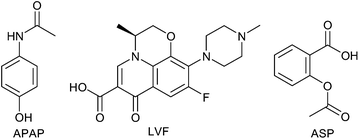 | ||
| Fig. 3 Chemical formulas of the studied pharmaceuticals. | ||
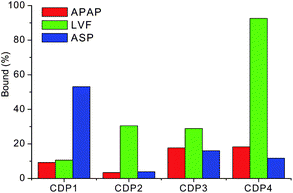 | ||
| Fig. 4 Relative amounts (in percentage) of polymer-bound APAP, LVF and ASP onto different polymers in water solutions. | ||
For the fluoroquinolone LVF, it can be seen that CDP4 binds more than 93% of the API in solution while this value drops down to 11%, 30% and 29% for CDP1, CDP2 and CDP3, respectively. The relative selectivity of CDP4 for LVF can be explained by different factors. First, in addition to the presence of the CD cavity that has been shown to be suitable for the hydrophobic inclusion of LVF,13 the presence of aromatic groups in the polymer allows π–π stacking with the guest molecule. This is confirmed by the comparison with the amount of bound LVF to CDP2 (30%) that has been produced using the same mixture of monomers but using a non-aromatic cross-linker. One could also assume that the addition in the polymer of a H-bond donor and acceptor such as DMPA increases the possibility of this polymer to form H-bonds with its guest. Because of a number of H-bond acceptors (N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–C) in the structure of LVF, it is postulated that the higher affinity observed for CDP4 also arises from the presence of the carboxyl functions within the polymer structure. This is confirmed when comparing the results obtained with CDP4 with those of CDP3 that has the same composition as CDP4 lacking the carboxyl groups of DMPA. The effect of the polymer concentrations on the polymer–pharmaceutical interaction has been investigated (Fig. S2, ESI†). As expected, the percentage of bound pharmaceuticals increases with increasing the concentration of polymers.
O, C–O–C) in the structure of LVF, it is postulated that the higher affinity observed for CDP4 also arises from the presence of the carboxyl functions within the polymer structure. This is confirmed when comparing the results obtained with CDP4 with those of CDP3 that has the same composition as CDP4 lacking the carboxyl groups of DMPA. The effect of the polymer concentrations on the polymer–pharmaceutical interaction has been investigated (Fig. S2, ESI†). As expected, the percentage of bound pharmaceuticals increases with increasing the concentration of polymers.
In the case of ASP, the aliphatic cross-linker based polymer lacking the carboxyl group (CDP1) exhibits the highest binding capability with 53% of bound ASP in the studied conditions while the values are of 4%, 16% and 12% for CDP2, CDP3 and CDP4, respectively. This relative selectivity could be explained by the more hydrophobic nature of the API and its limited ability to form H-bonding as compared to LVF. Moreover, the aliphatic cross-linker based polymer is expected to have a higher accessibility of the β-CD inclusion sites compared with that of aromatic cross-linker based polymer,12 the inclusion of the ASP molecule is then facilitated. The results show the same trend that the data reported by Wilson et al. show where the aliphatic crosslinker based polymer displayed higher binding capability toward naphthenic acids than that of aromatic one.12 Regarding APAP, the binding capabilities of CDP3 and CDP4 with 17.7% and 18.3% of bound APAP, respectively, are higher than those of CDP1 and CDP2 with 9% and 3%. These results indicate that the polymers containing the aromatic cross-linker (TDI) display higher binding capabilities than those containing the aliphatic cross-linker (HDI). This could be explained by the fact that the π–π interaction plays a major role in the interaction between APAP and the produced polymers. However, none of the studied polymers shows a higher binding capability to APAP than that to the other two studied pharmaceuticals.
In order to confirm the specificity of the produced CDPs, the binding experiments have been performed with equimolar mixtures of the APIs in aqueous conditions. The CDPs were incubated with mixed solutions at a concentration of 10−4 mol L−1 for each pharmaceutical used (APAP, LVF and ASP) for 2 h; the results are presented in Fig. 5.
 | ||
| Fig. 5 Sorption of APAP, LVF and ASP onto different polymers in the mixed water solution (polymers: 5 mg; pharmaceutical solution: 1.0 × 10−4 mol L−1, 1.5 mL; contact time: 2 h). | ||
The results obtained using mixtures of APIs confirm those obtained with pure APIs, CDP4 shows the highest binding capability for LVF (82%), while it binds only weakly to APAP (19%) and ASP (7%) confirming the specificity of this CD-based polyurethane for LVF. It can also be seen that, as predicted, ASP is preferentially bound onto CDP1 with a percentage of bound 42%, while those of APAP (7%) and LVF (1%) are substantially lower. LVF is also preferentially bound onto CDP2 (17.4%) compared with APAP (4.2%) and ASP (0.6%) but the binding capability of CDP2 is substantially lower than for CDP4. The four produced CDPs do not show preferential binding efficiency to APAP in the mixed solution. The difference of APAP (20%), LVF (33%) and ASP (21%) bound onto CDP3 is not significant, even though CDP3 exhibits the highest binding capability to APAP compared to other CDPs.
In conclusion, four water insoluble CD-based polymers have been prepared and used as sorbents for binding three selected pharmaceuticals in aqueous solutions. The results indicate that CDP1 (produced using an aliphatic cross-linker in the absence of carboxyl moieties) and CDP4 (aromatic cross-linker with the carboxyl group) possess preferential binding efficiency to ASP and LVF, respectively. This approach of such structure/property relationships is expected to be of interest for the design of new CD-based sorbents for specific detection of organic contaminants in water. The work is underway to produce a wider series of polymers to improve the binding capability/specificity of the CDPs for APIs.
The financial support of the Swiss Commission for Technology and Innovation (CTI) is gratefully acknowledged. The authors would like to thank M. Alessandro Cumbo for his assistance for SEM experiments and the French National Institute for Agronomy Research (INRA) for financial support (YD).
Notes and references
- J. M. Lehn, J.-L. Atwood, E. D. Davies, D. D. Macnicol and F. Vogtle, Molecular Recognition: Receptors For Molecular Guests, Pergamon, Tarritown, 1996, vol. 2 Search PubMed.
- J. W. Steed, D. R. Turner and K. Wallace, Core Concepts in Supramolecular Chemistry and Nanochemistry, John Wiley & sons, Chichester, 2007 Search PubMed.
- Modern Supramolecular Chemistry: Strategies for Macrocycle Synthesis, ed. F. Diederich, P. Stang and R. R. Tykwinski, Wiley-VCH, Weinheim, 2008 Search PubMed.
- S. Higson and F. Davis, Macrocycles: Construction, Chemistry and Nanotechnology Applications, John Wiley & sons, Chichester, 2011 Search PubMed.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1754 CrossRef CAS.
- P. Shahgaldian and U. Pieles, Sensors, 2006, 6, 593–615 CrossRef CAS.
- T. Loftsson and D. Duchêne, Int. J. Pharm., 2007, 329, 1–11 CrossRef CAS.
- F. van de Manakker, T. Vermonden, C. F. van Nostrum and W. E. Hennink, Biomacromolecules, 2009, 10, 3157–3175 CrossRef CAS.
- S. Berto, M. Bruzzoniti, R. Cavalli, D. Perrachon, E. Prenesti, C. Sarzanini, F. Trotta and W. Tumiatti, J. Inclusion Phenom. Macrocyclic Chem., 2007, 57, 637–643 CrossRef CAS.
- S. D. Mhlanga, B. B. Mamba, R. W. Krause and T. J. Malefetse, J. Chem. Technol. Biotechnol., 2007, 82, 382–388 CrossRef CAS.
- B. Sancey, G. Trunfio, J. Charles, P. M. Badot and G. Crini, J. Inclusion Phenom. Macrocyclic Chem., 2010 DOI:10.1007/s10847-010-9841-1.
- M. H. Mohamed, L. D. Wilson, J. V. Headley and K. M. Peru, Phys. Chem. Chem. Phys., 2011, 13, 1112–1122 RSC.
- P. Xiao, Y. Dudal, P. F. X. Corvini and P. Shahgaldian, Polym. Chem., 2011, 2, 120–125 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, scanning electron micrographs of CDP1, CDP2 and CDP3, experimental procedures for the HPLC experiments and concentration dependent binding experiments. See DOI: 10.1039/c1py00114k |
| ‡ Briefly, for the syntheses of CDP2 and CDP4, DMPA is mixed in DMSO with a diisocyanate (HDI or TDI). After 2 hours of reaction at 65 °C, β-CD is added and reacted at 65 °C for 3 h. The mixture is then poured into acetone and washed with water and ethanol. CDP1 and CDP3 have been prepared using the same conditions but in the absence of DMPA. The produced polymers, CDPs, are urethane cross-linked cyclodextrins produced by the reaction of the isocyanate groups of diisocyanate and the hydroxyl groups of DMPA and β-CD. |
| § Scanning electron microscopy imaging was carried out using a Zeiss Supra 40VP system equipped with a InLens detector using an accelerating voltage of 20 kV. 1 µL of a CDP suspension in water was submitted to ultrasonic treatment for 30 min and subsequently spread on a freshly cleaved mica slide. |
| ¶ 5 mg polymers were added to 1.5 mL pharmaceutical (1 × 10−4 mol L−1) aqueous solutions and the suspensions were shaken at 25 °C for 2 h. Subsequently 600 µL suspension were separated by centrifugation to remove the polymers and the concentration of pharmaceuticals in the supernatant was measured by HPLC, cf. ESI†. |
| This journal is © The Royal Society of Chemistry 2011 |
