Dual thermo-responsive polyrotaxane-based triblock copolymers synthesized viaATRP of N-isopropylacrylamide initiated with self-assemblies of Br end-capped Pluronic F127 with β-cyclodextrins
Jin
Wang
,
Peng
Gao
,
Lin
Ye
,
Ai-ying
Zhang
and
Zeng-guo
Feng
*
School of Materials Science and Engineering, Beijing Institute of Technology, Beijing, 100081, P. R. China. E-mail: sainfeng@bit.edu.cn; Fax: +86-01-68944630; Tel: +86-01-68912650
First published on 24th December 2010
Abstract
Dual thermo-responsive polyrotaxane (PR)-based triblock copolymers were synthesized via the atom transfer radical polymerization (ATRP) of N-isopropylacrylamide (NIPAAm) initiated with self-assemblies made from a distal 2-bromopropionyl end-capped Pluronic F127 with a varying amount of β-cyclodextrins (β-CDs) in the presence of Cu(I)Cl/N,N,N′,N′′,N′′- pentamethyldiethylenetriamine at 25 °C in aqueous medium. The structure of the copolymers was characterized in detail by means of 1H NMR, GPC, FTIR, and XRD analyses. The number of entrapped β-CDs and the degree of polymerization (DP) of attached NIPAAm oligomers appeared to be tunable. A two-step thermo-responsive transition arisen from a combination of a polypseudorotaxane middle block and poly(N-isopropylacrylamide) (PNIPAAm) flanking blocks was demonstrated by turbidity measurements. The number of entrapped β-CDs and DP of PNIPAAm are crucial for this dual thermo-responsive transition. The aggregates of one selected PR-based triblock copolymer were evidenced by TEM observations exhibiting a morphology change from core-shell particles to worm-like aggregates with increasing temperature. Furthermore, this sample can encapsulate and separate a negatively charged dye Coomassie Brilliant Blue G-250 from the aqueous solution when heated up to its deposition temperature.
Introduction
Polyrotaxanes (PRs) are a kind of unique inclusion complexes (ICs) composed of multiple cyclic molecules threaded on a polymer chain end-capped by bulky stoppers which inhibit the dethreading of threaded cyclic molecules. As typical cyclic molecules, cyclodextrins (CDs) consist of six to eight glucose units linking through α-1,4-glycosidic linkages, named α-, β-, and γ-CDs, respectively. Since the first discovery of PRs made from α-CDs with poly(ethylene glycol) (PEG) by Harada and colleagues,1 tremendous attention has been attracted in designing and preparing novel CD-based PRs because CDs are not only easily available, water-soluble and biocompatible, but also readily functionalized by a variety of synthetic strategies as well as selectively self-assembled with various polymers.2–5 Among the PRs ever reported, those showing stimuli-responsive properties in response to external stimuli, such as light, pH, and polarity, are a hot topic of extensive interest.6–11 Owing to their unique stimuli-responsive behaviors and their intriguing characteristic of free sliding and/or rotating of the threaded CDs around the polymer chains, PRs show great potential as smart materials, such as supramolecular hydrogels,12 slide-ring gels,13 biosensors,14 carriers for drug delivery,5 and insulated molecular wires.15As is well known, a large variety of bulky groups and end-capping reactions have been exploited to prepare PRs from their precursors to date, such as naphthyl,16trinitriphenyl,2fluorescein-4-isothiocyanate17 and 9-anthryl18 as the end stoppers via the coupling reactions, Huisgen cyclization of azido- and alkynyl-modified precursors,19 and enzymatic oxidative coupling of ICs made from α-CD with p-hydroxyphenylpropionate terminated PEG by using a horseradish peroxidase/H2O2 catalytic system.20 In 2006 we reported the preparation of thermo-responsive PR-based triblock copolymers flanked by poly(N-isopropylacrylamide) (PNIPAAm) blocks using photoinitiating radical telomerization.21,22 More recently, atom transfer radical polymerization (ATRP) has been successfully introduced as an end-capping technique in preparing PR-based triblock copolymers with both the number of entrapped CDs and the length of end-capping polymeric blocks adjustable to some extent.23–28 For example, initiating the hydrophilic monomer of poly(ethylene glycol) methyl ether methacrylate (PEGMA) to in situ polymerize gave rise to amphiphilic PR-based triblock copolymers showing promise as carrier for controlled release of Amphotericin B (AmB).25 As ATRP is a living/controlled radical polymerization, it can provide the polymers with predetermined molecular weight, narrow polydispersity and desired composition and molecular architecture. Significantly it is tolerant of a wide range of functional vinyl monomers and can be carried out in aqueous media under relatively mild experimental conditions compared with ionic polymerizations.27 As the resulting PR-based triblock copolymers still hold the active Br or Cl end groups, they can be further used as macroinitiators to initiate new ATRP processes or be modified by the reaction of the end-capping blocks without changing the native-CDs.29ATRP opens new avenues for preparing novel supramolecular PR-based polymers.23–26,29
PNIPAAm is one of the most extensively studied thermo-responsive polymers that possesses a very clear lower critical solution temperature (LCST) around 32 °C in aqueous solution.30,31 Below the LCST, PNIPAAm is hydrophilic and its chains are extended, while above 32 °C it becomes hydrophobic and its chains are shrunk. The mechanism of PNIPAAm undergoing discontinuous, reversible phase transition is considered due to a competitive balance between hydrophilic repulsive forces and hydrophobic attractive ones. Its LCST can be regulated by copolymerizing with more hydrophilic monomer (which raises the LCST) or more hydrophobic monomer (which lowers the LCST). Pluronic F127 is a typical poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene) (PEO100-PPO70-PEO100) triblock copolymer showing thermo-responsive character. Its aggregation occurs over a range of concentrations rather than at a unique critical micelle concentration and its critical micellization temperature decreases with increasing the concentration.32 This commercially available triblock copolymer had been investigated for drug solubilization and controlled release,33 prevention of post-surgical tissue adhesions34 and in wound covering as well.35 Interestingly, it can create site-selective ICs with α-CDs and β-CDs. For example, α-CDs selectively bind to PEO segments, while β-CDs selectively bind to PPO segment.3 Several PRs consisting of CDs with Pluronic F127 were prepared, and they either exhibited thermo-responsive behaviors11,17 or held potential to be used as biosensor or carrier for controlled drug delivery.23,25
However, as for those thermo-responsive PR-based triblock copolymers end-capped with PNIPAAm blocks ever reported,21,22 only one phase transition around their LCST in aqueous solution was observed, and the DP of their outer PNIPAAm blocks was also ill-controlled. To this end a series of dual thermo-responsive PR-based triblock copolymers were designed and synthesized by attaching PNIPAAm blocks to β-CD-Pluronic F127 IC via in situATRP. Surprisingly the morphology change of their aggregates was evidenced by TEM observations, and their encapsulation and separation capability for a negative charged dye, Coomassie Brilliant Blue G-250 in aqueous solution, was also demonstrated with increasing temperature.
Experimental section
Materials
N-isopropylacrylamide (NIPAAm) (Acros, Belgium) was purified by recrystallization from n-hexane. β-Cyclodextrin (β-CD) (Sinopharm Chemical Reagent Company, China) was recrystallized three times before use. N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA) and Pluronic F127 were purchased from Sigma, USA. Both 2-bromopropionyl bromide and 4-dimethylaminopyridine (DMAP) were bought from Alfa Aesar, USA. Triethylamine (TEA) (VAS Chemical Reagents Company, Tianjin, China) was refluxed with p-toluenesulfonyl-chloride and distilled under vacuum. The resultant free TEA was stored over CaH2. Copper(I) chloride (Cu(I)Cl) was prepared from CuCl2, purified by stirring in acetic acid, washed with methanol and finally dried under vacuum prior to use. N,N-Dimethylformamide (DMF) was stirred with CaH2 and distilled under reduced pressure. All other solvents and reagents were of analytical grade.Synthesis of distal 2-bromopropionyl end-capped Pluronic F127 (BrP-F127-PBr)
Pluronic F127 was first converted to the corresponding ATRP macroinitiator by the end-capping reaction with a fourfold molar excess of 2-bromopropionyl bromide in CH2Cl2 according to literature.23 In brief, in a 100 ml three-neck round-bottom flask, Pluronic F127 (6.3 g, 0.5 mmol) was dissolved in distilled CH2Cl2 (15 ml). To a sample of DMAP (0.061 g, 0.5 mmol) dissolved in dry CH2Cl2 (5 ml), TEA (0.15 g, 1.5 mmol) was added. The mixture was then added dropwise to the Pluronic F127 containing flask, to which 10 ml dry CH2Cl2 containing 2-bromopropionyl bromide (0.43 g, 2 mmol) was added dropwise under nitrogen. The reaction continued for 2 h at 0 °C and for another 24 h at room temperature under stirring. Finally the mixture was filtered to remove the precipitated salt. The product was purified by precipitation into 400 ml anhydrous ether at 5 °C. The sequence was repeated three times after dissolved in CH2Cl2. The purified product was dried under vacuum, yield 84.9%. 1H NMR analysis was used to calculate the degree of esterification (>98%). 1H-NMR (DMSO-d6): δ 4.22–4.24 (t, 4H, –CH2–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–), 1.02–1.04 (d, 210H, –O–CCH3–C–O–), 1.71–1.73 (d, 3H, CH3–C–Br) ppm.
O)–), 1.02–1.04 (d, 210H, –O–CCH3–C–O–), 1.71–1.73 (d, 3H, CH3–C–Br) ppm.
Preparation of pentablock copolymer PNIPAAm-b-F127-b-PNIPAAm
A typical protocol for ATRP of NIPAAm by using BrP-F127-PBr as macroinitiator and Cu(I)Cl/PMDETA as catalyst in aqueous solution was as follows: in a sealable Pyrex reactor, PBr-F127-PBr (0.13 g, 0.01 mmol) was dissolved in 2 ml distilled water to which NIPAAm (0.113 g, 1.0 mmol) dissolved in 1 ml water was added before PMDETA (4.2 mg, 0.024 mmol) was added. The system was quenched in the liquid nitrogen to which Cu(I)Cl (2.0 mg, 0.02 mmol) was added. The reactants in the reactor were degassed three times by purging with nitrogen. The reactor was sealed under vacuum and then the reaction started and maintained at 25 °C for 8.0 h under stirring. The polymerization stopped after breaking the Pyrex reactor, the product was dialyzed using a cellulose membrane (MWCO 3500) for 48 h changing water every 12 h, and the whole content was freeze-dried. The crude product was dissolved in DMF and fractionally precipitated with anhydrous ether. The purified product was finally dried under vacuum, yield 75.7%. 1H NMR (DMSO-d6): δ 1.02–1.04 (d, 210 H + 6m H, –O–CCH3–CO– and –C(CH3)–CH3), 1.44–1.57 (d, 2m H, –CH2–C(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C–), 1.97 (s, 1 mH, –C–CH(C
O)–C–), 1.97 (s, 1 mH, –C–CH(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C–), 3.85(s, 1 mH, –N–HC(C)–C), 7.21–7.33 (d, 1 mH, –C(
O)–C–), 3.85(s, 1 mH, –N–HC(C)–C), 7.21–7.33 (d, 1 mH, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–NH–C–) ppm.
O)–NH–C–) ppm.
Synthesis of PR-based triblock copolymersviaATRP of NIPAAm
A typical protocol for the synthesis of PR-based triblock copolymerviaATRP of NIPAAm was as follows. In a sealable Pyrex reactor, an aqueous solution containing a predetermined amount of β-CDs was added to 1 ml aqueous solution of BrP-F127-PBr (0.1 g, 7.6 × 10−6 mol), followed by vigorous stirring at room temperature for 24 h to produce ICs. NIPAAm (86.0 mg, 0.76 mmol) and PMDETA (3.2 mg, 0.018 mmol) were then added to the resulting IC suspension. After quenched in the liquid nitrogen, Cu(I)Cl (1.5 mg, 0.015 mmol) was added, followed by three times of degassing using a nitrogen purge. The reactor was sealed under vacuum and the reaction started and maintained at 25 °C for 8.0 h. The polymerization stopped after breaking the Pyrex reactor. The crude product was purified through dialyzed using a cellulose membrane (MWCO 3500) for 4 d with water changing every 12 h. All the content was freeze-dried. The crude product was dissolved in DMF and fractionally precipitated with anhydrous ether. The purified product was dried under vacuum. 1H NMR(DMSO-d6): δ 1.02–1.04 (d, 210 H + 6m H, –O–CCH3–CO– and –C(CH3)–CH3), 1.44–1.57 (d, 2m H, –CH2–C(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C–), 1.97 (s, 1 mH, –C–CH(C
O)–C–), 1.97 (s, 1 mH, –C–CH(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C–), 3.85 (s, 1 mH, –N–HC(C)–C), 7.21–7.33 (d,1 mH, –C(
O)–C–), 3.85 (s, 1 mH, –N–HC(C)–C), 7.21–7.33 (d,1 mH, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–NH–C–), 4.46 (s,7 nH, –O(6)H), 4.83 (s, 7n H, H(1)), 5.66 (s, 7n H, –O(3)H), 5.75 (s, 7nH, –O(2)H) ppm.
O)–NH–C–), 4.46 (s,7 nH, –O(6)H), 4.83 (s, 7n H, H(1)), 5.66 (s, 7n H, –O(3)H), 5.75 (s, 7nH, –O(2)H) ppm.
For the convenience of expression, the obtained PR-based triblock copolymers were designated as F-nCD-mN, where F means BrP-F127-PBr and N indicates NIPAAm, n stands for the feed molar ratio of β-CD to BrP-F127-PBr and m represents the feed molar ratio of NIPAAm to BrP-F127-PBr, respectively.
Measurements
1H NMR (400 MHz) spectra were recorded on a Bruker ARX 400 spectrometer at room temperature using DMSO-d6 as solvent and tetramethylsilane (TMS) as internal standard. FTIR spectra were measured using Shimadzu IR Prestige-21 FTIR spectrometer at room temperature in the range between 4500 and 500 cm−1, with a resolution of 2 cm−1 and 20 scans. Powder samples were prepared by dispersing the samples in KBr and compressing the mixtures to form disks. Gel permeation chromatographic (GPC) measurements were carried out at 50 °C on a Waters 2410 instrument using DMF as eluent at a flow rate of 1.0 ml min−1. LiBr (0.05 mol L−1) was added to suppress the association of the resulting PR-based triblock copolymers. All the GPC data were calibrated with polystyrene (PS) standards. The wide-angle X-ray diffraction (WXRD) measurements were carried out with powder samples using Philips X'Pert Pro diffractometer with an X'celerator detector in a reflection mode. The radiation source used was Ni-filtered, Cu-Kα radiation with a wavelength of 0.154 nm. The voltage was set to 40 kV and the current 20 mA. Samples were mounted on a sample holder and scanned from 4.5° to 60° in 2θ at a speed of 5°/min. Thermo-responsive behaviors of PR-based and blank triblock copolymers were determined by turbidity measurement using Hitachi U-2800 UV-vis spectrophotometer. The light transmittance was recorded at 500 nm of wave length after thermo-stated for 10 min with a heating rate of 1.0 °C/min. Values for the cloud point (in some occasions it corresponds to the LCST) of PR-based triblock copolymers were defined as a temperature when the transmittance is 50%. Deposition temperature (DT) was defined as a temperature when the transmittance shows an onset of rebound from its lowest point. The transmission electron microscopy (TEM) micrographs were taken with a Hitachi-700 transmission electron microscope operated at 100 kV accelerating voltage. The interaction between the dyes Rhodamine B and Coomassie Brilliant Blue G-250 and the obtained PR-based triblock copolymers in de-ionized water was traced by Hitachi U-2800 UV-vis spectrophotometer at room temperature in the range from 450–600 nm and 400–700 nm, respectively. The concentration of Coomassie Brilliant Blue G-250 and Rhodamine B aqueous solution were 4 × 10−6 mol L−1 and 4 × 10−5 mol L−1, respectively. The mixed solutions to be tested were prepared by mixing 1 ml dye solution (Coomassie Brilliant Blue G-250: 2 × 10−5 mol L−1; Rhodamine B: 2 × 10−4 mol L−1) with 4 ml selected PR-based triblock copolymer aqueous solution (0.2 wt%).Results and discussion
Preparation of PR-based triblock copolymers
The synthetic pathway for dual thermo-responsive PR-based triblock copolymers comprising a middle polypseudorotaxanes (PPR) block and two flanking PNIPAAm blocks via in situATRP is shown in Scheme 1. The composition and yields after thorough purification are summarized in Table 1. To highlight the impact of the number of entrapped β-CDs and the DP of PNIPAAm on the dual thermo-responsive behaviors, two ATRP processes were carried out at 25 °C in aqueous solution: first, the feed molar ratio of NIPAAm to BrP-F127-PBr was kept constant while that of β-CD to BrP-F127-PBr changed; second, the feed molar ratio of β-CD to BrP-F127-PBr remained unchanged while that of NIPAAm to BrP-F127-PBr varied.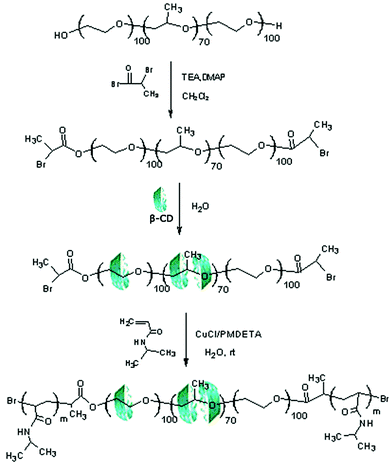 | ||
| Scheme 1 Synthetic pathway of PR-based triblock copolymersviaATRP of NIPAAm in aqueous medium. | ||
| Entry | Molar composition (Brp-F127-PBr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NIPA) NIPA) |
Molecular weight and polydispersity index | Yield (%) | |||
|---|---|---|---|---|---|---|
| Feed ratio | Found ratioa | M n(10−3) a | M n(10−3) b | M w/Mnb | ||
| a Determined by 1H NMR analysis in DMSO-d6. b Determined by GPC in DMF with LiBr (0.05mol L−1) at 1.0 ml min−1 using PS standards. | ||||||
| F-0CD-100N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112.8 112.8 |
25.9 | 188.3 | 1.26 | 75.7 |
| F-10CD–100N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 7.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127.3 127.3 |
36.4 | 193.0 | 1.19 | 30.5 |
| F-20CD–100N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.6 16.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132.1 132.1 |
46.9 | 197.6 | 1.21 | 46.2 |
| F-30CD–100N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.9 25.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127.8 127.8 |
57.0 | 217.8 | 1.22 | 49.4 |
| F-20CD-80N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.0 18.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97.7 97.7 |
44.6 | 197.1 | 1.15 | 41.3 |
| F-20CD-60N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.2 16.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89.4 89.4 |
41.7 | 196.2 | 1.23 | 36.7 |
| F-20CD-40N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.2 19.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55.6 55.6 |
41.2 | 189.4 | 1.18 | 40.3 |
When the crude products were purified by dialysis against water, the found molar ratio of entrapped β-CDs to BrP-F127-PBr was never more than 10 as determined by 1H NMR (data not shown) even though the yields were relatively higher (in the range of 55.5% for F-20CD-100N to 69.0% for F-20CD-60N). As previously reported, a competitive interaction occurred between 2-hydroxypropyl methacrylate (HPMA) and Pluronic F127 for β-CDs to be included leading to a relatively low threading ratio of β-CDs on the Pluronic F127 backbone (ca. 7.2–12.1).23 Accordingly methylated β-CDs were introduced to suppress the competitive effect of NIPAAm, but no increase in the found molar ratio was seen here. As evidenced by the following GPC curves, this low ratio of entrapped β-CDs to BrP-F127-PBr was attributed to a multimodal molecular weight distribution for each sample just purified by dialysis. As a result the dialyzed products were further purified by fractional precipitation in DMF with anhydrous ether. A nearly symmetrical and unimodal peak as shown in the GPC traces clearly indicated that the obtained PR-based triblock copolymers were thorough purified. Finally the number of entrapped β-CDs on the Pluronic F127 chain was found to nearly match the feed ratio with the mild yields in a range of 30.5% for F-10CD-100N to 49.4% for F-30CD-100N.
As illustrated in Table 1, when the feed molar ratio of β-CD to BrP-F127-PBr was varied from 10 to 30 while that of NIPAAm to BrP-F127-PBr was kept at 100, the number of threaded β-CDs onto the Pluronic F127 chain was changed from 7.8 to 25.9. When the feed molar ratio of NIPAAm to BrP-F127-PBr was varied from 40 to 100 while that of β-CDs to BrP-F127-PBr was kept at 20, the DP of PNIPAAm blocks attached to two ends of PPRs was in the range of 27.8 to 66.1. Both the threaded number of β-CD and the DP of PNIPAAm appeared to be tunable in ATRP of NIPAAm initiated by self-assemblies of BrP-F127-PBr with a varying amount of β-CDs at 25 °C in aqueous solution.
Characterization of PR-based triblock copolymers
The 1H NMR spectra of the pure β-CD, F-0CD-100N and F-20CD-100N samples are shown in Fig. 1. Compared with the pure β-CD, the peaks of O(2)H, O(3)H and O(6)H of β-CD in F-20CD-100N were obviously broadened and somewhat shifted to the downfield due to the decrease in conformational flexibility upon forming a PR. As previously reported,17 the methyl proton peak of Pluronic F127 should shift to lower field and be broadened after forming a PR. In fact, because the methyl proton peak of Pluronic F127 located at 1.01 to 1.04 ppm was superposed with that of the end CH3 of PNIPAAm located at 1.00 to 1.06 ppm, these down-shifts and broadening were not evidently visible. It was very similar to our report on the preparation of PR-based triblock copolymersvia in situATRP of HPMA.23 Moreover, as the resulting copolymers are dissolved in DMSO, a polar aprotic solvent that may eliminate the hydrogen bonding between neighboring CDs and/or their hydrophobic interactions with PPO segment,11,17 the threaded β-CDs are mostly dispersed on the PEO segments so that the down-shift of methyl proton peak of Pluronic F127 was tiny. This was very coherent with the XRD analytical results for the sample precipitated from DMF that shows neither characteristic channel-type structure diffraction peaks nor crystal diffraction peaks of PEO. All of the other proton resonance peaks arising from β-CD and PNIPAAm were assigned in Fig. 1. The peaks c, d, e and f of protons in PNIPAAm were found both in Fig. 1B and 1C, indicating that the target PR-based triblock copolymers were successfully synthesized via in situATRP of NIPAAm initiated with self-assemblies of BrP-F127-PBr with a varying amount of β-CDs at 25 °C.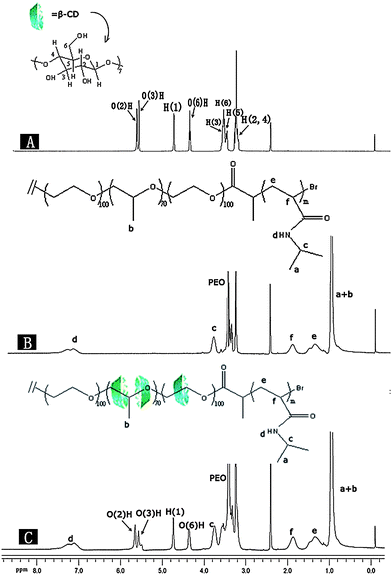 | ||
| Fig. 1 1H NMR spectra of the pure β-CD (A), F-0CD-100N (B) and F-20CD–100N (C) in DMSO-d6. | ||
As mentioned above, the methyl proton peak of Pluronic F127 was superposed with that of the end methyl of PNIPAAm. To calculate the number of incorporated NIPAAm monomer and that of β-CD entrapped on the Pluronic F127 main chain, it was necessary to subtract 6 times of the integration area of peak c in Fig. 1C from the integration area of the whole methyl proton resonance peak area of Pluronic F127 and the end methyl group of PNIPAAm (a + b). The integration area of (1)H proton resonance peak of β-CD and that of methyl proton resonance peak of the side group of PNIPAAm were employed here.
GPC curves of the as-prepared PR-based triblock copolymers are depicted in Fig. 2A and 2B. The traces exhibited a nearly symmetrical and unimodal peak with a relatively lower polydispersity index of 1.15–1.26. As for the F-nCD-100N samples (Fig. 2A), the molecular weight increased with the β-CD feed molar ratio, while regarding the F-20CD-mN species (Fig. 2B), the molecular weight rose with the feed molar ratio of NIPAAm. It further indicated that both the number of entrapped β-CDs and the DP of PNIPAAm appeared to be adjustable in this ATRP process. However, all the molecular weights determined by GPC are not coherent with those by 1H NMR. The discrepancy is most likely due to the fact that PS calibration standards were used, DMF is only a marginal solvent for polystyrene,23,24,36 as well as the threaded β-CDs may randomly locate along the Pluronic F127 chain giving rise to the greatly extended and rigid chain conformation in comparison with free PS chain, leading to overestimated molecular weights.23,25
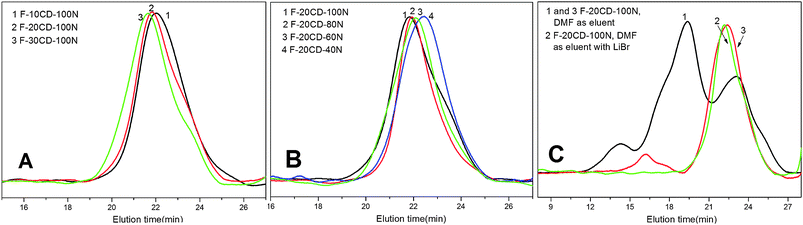 | ||
| Fig. 2 GPC traces in (A) and (B) by using DMF with LiBr (0.05%); CPG traces by using DMF with and without LiBr in (C). | ||
The GPC traces of F-20CD-100N before and after the fractional precipitation in DMF with anhydrous ether are shown in Fig. 2C. Multiple peaks were obviously seen in trace 1 while in trace 2 they became a nearly unimodal peak, indicating that the molecular weight of the crude product without fractional precipitation is polydispersed. However, in the absence of LiBr, trace 3 gave a small peak except the main peak, most likely due to the association of the resultant PR-based triblock copolymer even in pure DMF solvent.6 Adding LiBr into the fluent, its trace exhibited only one peak as presented by trace 2. Meanwhile the middle peak in trace 1 may stem from those PR-based triblock copolymers with relatively lower number of threaded β-CDs and higher DP of PNIPAAm leading to them substantially soluble in precipitation solvents. Consequently a mixing eluent of DMF with 0.05 mol L−1LiBr was used to perform the GPC measurements in this study because Li+ ion can effectively depress the hydrogen bonding of the self-aggregates.29
The FTIR spectra of powder samples of BrP-F127-PBr, PNIPAAm-b-F127-b-PNIPAAm, PR-based triblock copolymers and pure β-CD are outlined in Fig. 3. The vibration absorption peak for the amide bond of PNIPAAm appears at 1643 and 1547 cm−1, clearly indicating that the NIPAAm monomers were successfully polymerized and attached to two ends of PPRs because of a significant increase in the intensity of the amide bond stretching vibration in F-0CD-100N (b), F-10CD-100N (c), F-20CD-100N (d) and F-30CD-100N (e) compared with their common precursor BrP-F127-PBr (a). In addition, the N–H symmetric and asymmetric stretching vibration modes of amide bond in F-0CD-100 are located at 3562, 3259 and 3073 cm−1, while for the traces c, d and e, the peaks at 3562 and 3259 cm−1 are invisible because they are superposed with the peak of O–H bonds from β-CD. However, the peak at 3073 cm−1 is still clearly observable. The characteristic peaks of β-CD at 1029 and 1159 cm−1 were also found in the spectra of F-10CD-100N (c), F-20CD-100N (d) and F-30CD-100N (e). This provided direct evidence confirming that the target PR-based triblock copolymers were synthesized viaATRP of NIPAAm in the presence of Cu(I)Cl/PMDETA at 25 °C in aqueous solution.
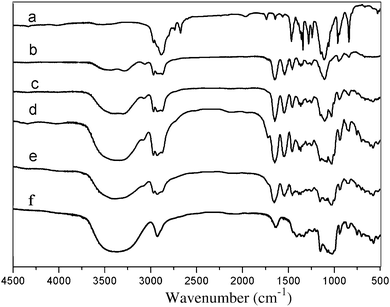 | ||
| Fig. 3 FTIR spectra of BrP-F127-PBr (a), F-0CD-100N (b), F-10CD-100N (c), F-20CD-100N (d), F-30CD-100N (e) and pure β-CD (f). | ||
The X-ray powder diffraction patterns of F-nCD-mN, BrP-F127-PBr and pure β-CD are shown in Fig. 4. As can be seen, the major peaks of pure β-CDs appear at 9.03°, 12.46°, 18.78°, 19.53° and 22.69°. The macroinitiator BrP-F127-PBr (b) and F-0CD-100N (c) show two strong diffraction peaks at 19.02° and 23.29°, arisen from the crystal structure of PEO block as previously reported.21,23 Although the diffraction peaks of crystal PEO disappear in the resulting PR-based triblock copolymers (Fig. 4d and e) as a result of the threaded β-CDs dispersing along the PEO chain to hinder its crystallizing,11,17,23 no characteristic channel-type crystal structure diffraction peaks of PRs were found in these copolymers by the XRD analysis. It strongly suggested that the entrapped β-CDs are randomly dispersed on the whole Pluronic F127 chain when the sample was precipitated from DMF with anhydrous ether giving no characteristic channel-type crystal structure.37 However, when the sample was freeze-dried from aqueous solution, the two peaks which are supposed to be the characteristic peaks of channel-type crystal structure at 11.62° and 19.03° were clearly seen as illustrated in Fig. 5. This implied that the channel-type crystal structure is destroyed by DMF and even not recovered by the precipitation with anhydrous ether. These randomly dispersed β-CDs appeared to be frozen over the Pluronic F127 backbone by the precipitation process. Nevertheless, the characteristic crystal structure can be recovered and preserved again by freeze-dried after incubated in water.
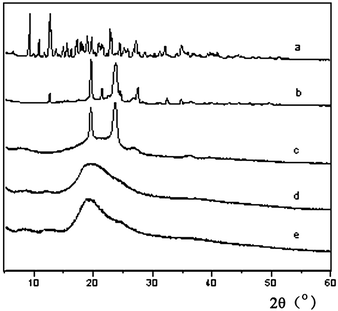 | ||
| Fig. 4 X-ray diffraction patterns of pure β-CD (a), BrP-F127-PBr (b), F-0CD-100N (c), F-20CD-100N (d) and F-30CD-100N (e). | ||
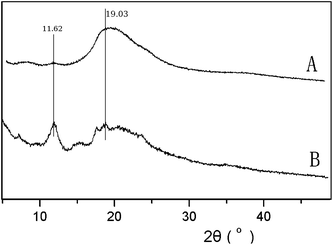 | ||
| Fig. 5 X-ray diffraction patterns of F-20CD-100N prepared by precipitate from DMF with anhydrous ether (A) and freeze-dried from aqueous solution (B). | ||
Although the majority of β-CDs move toward the PPG segment in the as-prepared PR-based triblock copolymers with increasing temperature, some β-CDs may still reside on the PEG segments.11,17 As a result, β-CDs can selectively form ICs with PPG or PEG blocks. As previously reported,38,39 when β-CDs reside on the PEO blocks of Pluronic F127, the structure of the ICs formed by β-CDs and PEO blocks is similar to that of the ICs formed by β-CDs and PPG block, so no more exact judgment whether the β-CDs stay on the PPG block or the PEO blocks can be given here. In fact, as the number of entrapped β-CDs on the Pluronic F127 chain is relatively small (a coverage ratio of 13.3% to 19.2%) according to 1H NMR results, the threaded β-CDs may locate along the chain as a whole which hinder the crystallizing of PEO blocks in the resulting PR-based triblock copolymers.
Dual thermo-responsive behaviors
The optical transmittance changes of the samples containing 0.30 wt% F-0CD-100N, 0.30 wt% F-10CD-100N, 0.30 wt% F-20CD-100N, and 0.15 wt% F-30CD-100N are illustrated in Fig. 6. Their cloud point values are summarized in Table 2. As previously reported, incorporating a hydrophilic segment into PNIPAAm may enhance its cloud point or LCST,30,31 the cloud point of the PNIPAAm-F127-PNIPAAm triblock copolymer was found to be ca. 37.1 °C, which is higher than that of PNIPAAm (approximately at 32 °C).40,41 Because there are about 200 PEG repeat units and ca. 112.8 NIPAAm repeat units in F-0CD-100N, it is quite reasonable for F-0CD-100N to show a cloud point at ca. 37.1 °C. As can be seen in Fig. 6 and Table 2, with increasing the number of entrapped β-CD in the PR-based triblock copolymers, their cloud points are raised from 37.7 °C to 41.2 °C. According to the literature,11,17,23 β-CDs can selectively form ICs with PEG block or PPG block, the β-CDs are distributed along the whole Pluronic F127 main chain at low temperature in aqueous media but move toward the PPG segment with increasing temperature. As a result the aggregation and shrinkage of the PPG segment is restricted due to the hydrophilic enhancement after β-CDs threaded onto the PPG segment. The more the number of β-CDs threaded on the PPG segment, the higher the coverage ratio of the PPG segment and the stronger the restricting effect on the aggregation of PPG at elevated temperatures. Evidently, this provided an opportunity for tailoring the cloud points of PNIPAAm end-capping polyrotaxanes exhibiting the potential to be used as stimuli-responsive smart materials for drug controlled release and biosensors.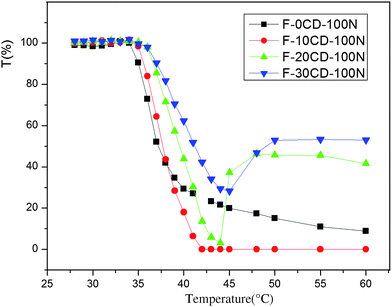 | ||
Fig. 6 Temperature dependence of optical transmittance of aqueous solution of 0.30 wt% F-0CD-100N (-■-), 0.30 wt% F-10CD-100N ( ), 0.30 wt% F-20CD-100N ( ), 0.30 wt% F-20CD-100N ( ) and 0.15 wt% F-30CD-100N ( ) and 0.15 wt% F-30CD-100N ( ). ). | ||
| Entry | Name | Compositiona | Coverage ratio (%)b | Cloud point (°C) | DTc (°C) |
|---|---|---|---|---|---|
| a Determined by 1H NMR analysis in DMSO-d6. b Coverage ratio was defined as the ratio of the number of repeat unit covered by β-CD to that of the whole repeat unit. Coverage = 2(CD/F127(mol mol−1))/(PEG and PPG repeat units). c DT stands for deposition temperature, its value was defined as a temperature when the transmittance showing an onset of rebound from its lowest point. d No deposition in the range of testing temperatures. e No visible phase transition in the heating range. | |||||
| 1 | F-0CD-100N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112.8 112.8 |
0 | 37.1 | —d |
| 2 | F-10CD-100N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.8 7.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127.3 127.3 |
5.8 | 37.7 | —d |
| 3 | F-20CD-100N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.6 16.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132.1 132.1 |
12.3 | 39.5 | 43.8 |
| 4 | F-30CD-100N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.9 25.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127.8 127.8 |
19.2 | 41.2 | 45.0 |
| 5 | F-20CD-80N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.0 18.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97.7 97.7 |
13.3 | 41.0 | 43.0 |
| 6 | F-20CD-60N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.2 16.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89.4 89.4 |
12.0 | 39.2 | 42.0 |
| 7 | F-20CD-40N | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.2 19.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55.6 55.6 |
14.2 | —e | —e |
To gain insight into the impact of the DP of PNIPAAm on the thermo-responsive behavior, the optical transmittance changes of the species containing 0.30 wt% F-20CD-100N, 0.10 wt% F-20CD-80N, 0.10 wt% F-20CD-60N, and 0.10 wt% F-20CD-40N are displayed in Fig. 7. Their cloud point values are shown in Table 2. Interestingly, all of them exhibited visual thermo-responsive transition except for F-20CD-40N, clearly indicating that the DP or molecular weight of PNIPAAm is crucial for their thermal responsibility. Furthermore, the traces of F-20CD-100N and F-20CD-60N were almost superposed, likely due to the same number of entrapped β-CDs on the Pluronic F127 main chain as determined by 1H NMR. As for F-20CD-40N, there was no visible phase transition to occur possibly due to too short PNIPAAm segments attaching to two terminals of the resultant PR-based triblock copolymer.
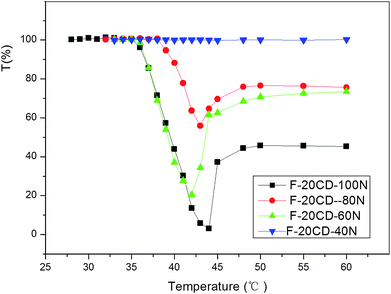 | ||
Fig. 7 Temperature dependence of optical transmittance of aqueous solution of 0.30 wt% F-20CD–100N (-■-), 0.10 wt% F-20CD-80N ( ), 0.10 wt% F-20CD-60N ( ), 0.10 wt% F-20CD-60N ( ) and 0.10 wt% F-20CD-40N ( ) and 0.10 wt% F-20CD-40N ( ). ). | ||
The photos of aqueous solutions of 0.3 wt% F-0CD-100N (1), 0.3 wt% F-10CD-100N (2), 0.3 wt% F-20CD-100N (3), and 0.15 wt% F-30CD-100N (4) at 30 °C (A), 37 °C (B) and 45 °C (C) are presented in Fig. 8, respectively. As can be seen in Fig. 8A, all the aqueous solutions were transparent at 30 °C with a 100% transmittance. As temperature elevated up to 37 °C, the solutions became turbid (Fig. 8B). When temperature continued to rise to 45 °C (C), the solution of F-0CD-100N still showed a bit of transparency, but the solution of F-10CD-100N became opaque. At the same time, sediments were formed in the solutions of F-20CD-100N and F-30CD-100N, leaving a transparent solvent on the upper layer, although some of sediments were still suspended in the solvent like snowstorm giving a hazy sky (Fig. 8C). In fact, a precipitation also occurred in the solution of F-10CD-100N as depicted in Fig. 8C, but the precipitates were very tiny and just suspended in the solvent like milk, while F-20CD-100N and F-30CD-100N gave rise to larger precipitates to deposit onto the bottom. Consequently, the number of entrapped β-CDs on the Pluronic F127 chain not only affects the cloud point of these PR-based triblock copolymers, but also endows them the deposition properties, leading to a dual thermo-responsive character. The optical transmittance changes around deposition temperature (DT) are shown in Fig. 6 and 7, and the corresponding DT values are summarized in Table 2. To our knowledge, this was the first observation that a PR exhibited a dual thermo-sensitivity due to a unique combination of two thermo-responsive components of a middle β-CD-Pluronic F127 IC block and outer PNIPAAm blocks.
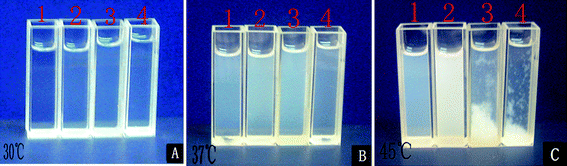 | ||
| Fig. 8 Photos of aqueous solutions of 0.3 wt% F-0CD-100N (1), 0.3 wt% F-10CD-100N (2), 0.3 wt% F-20CD-100N (3), and 0.15 wt% F-30CD-100N (4) at 30 °C (A), 37 °C (B) and 45 °C (C). | ||
Significantly, as the temperature dropped to ambient temperature, the sediments as described above dissolved again. This means that the copolymers can be reusable. As a consequence the sedimentation of F-20CD-mN and F-30CD-100N aqueous solutions at relative higher temperatures (43.8 °C and 45.0 °C) make them promising candidates for separation and accumulation media for small molecules similar to a bioenrichment process. Taking account this dual thermo-responsive character, the as-prepared PR-based triblock copolymers showed the great potential to be used not only as thermo-responsive smart materials for the drug controlled release and biosensors but also as separation and accumulation media for small molecules.
TEM observations
Evidently the attachment of PNIPAAm to the two ends of β-CD-Pluronic F127 IC not only improves the solubility of the resulting PRs but also fosters their thermo-responsive aggregations. The TEM images of F-20CD-100N and F-0CD-100N at different temperatures are shown in Fig. 9. At 27 °C, both F-20CD-100N and F-0CD-10N were self-assembled into nano-sized particles ca. 50–60 nm in diameters. However, as temperature elevated up or approached to their cloud points, the aggregation of two samples headed to different directions clearly due to the presence of threaded β-CDs. As for F-20CD-100N, the PNIPAAm segments become hydrophobic and begin to shrink at 37 °C, while the majority of β-CDs move towards the central PPO segment to inhibit its aggregation and shrinkage due to the hydrophilic enhancement after β-CDs are threaded onto the PPG segment.11,17 As a result worm-like aggregates were formed with a diameter of ca. 100 nm and length of ca. 400 nm as presented in Fig. 9A′. When the solution of the PR-based triblock copolymer was further elevated to higher temperatures, the movement of entrapped β-CDs toward the PPG segment became predominant leading to sediments. As for F-0CD-100N, because no β-CDs were entrapped on the main chain and the PPO core would not be broken, the small particles would merge into the vesicles as shown in Fig. 9B′. The mechanism of the impact of entrapped β-CDs on the aggregate changes is our ongoing study.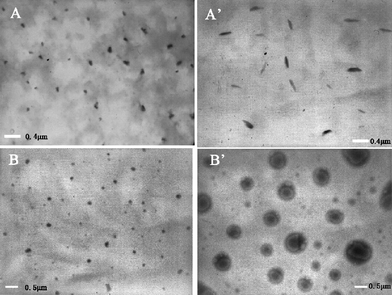 | ||
| Fig. 9 TEM images of F-20CD-100N at 27 °C (A) and 37 °C (A′); F-0CD-100N at 27 °C (B) and 37 °C (B′) from 0.10 wt% aqueous solution. | ||
Encapsulation and separation of Coomassie Brilliant Blue G-250
As mentioned above, the PR-based triblock copolymers with a feed molar ratio of β-CD more than 10 can deposit from their aqueous solutions as temperature elevates. It was postulated whether this kind of supramolecular copolymers has a potential application in separation and accumulation of small molecules. Thereby, a test was carried out toward Coomassie Brilliant Blue G-250 (a negatively charged dye) and Rhodamine B (a positively charged dye) by using F-20CD–100N as a typical PR-based triblock copolymer sample. As shown in Fig. 10, both of them gave rise to a homogenous solution of F-20CD-100N at a lower temperature. While it elevated up to deposition temperature of F-20CD-100N, at about 46 °C, Coomassie Brilliant Blue G-250 was encapsulated into the selected triblock copolymer, separated from the solution and settled to the bottom (Fig. 10A). However, Rhodamine B cannot be encapsulated by the sediments of this copolymer, and the solution still remained the color of Rhodamine B and the sediments are white (Fig. 10B).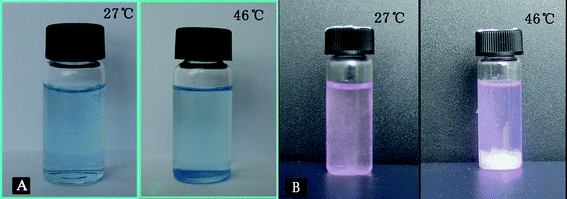 | ||
| Fig. 10 Photos of the encapsulation and separation test of Coomassie Brilliant Blue G-250 (A) and Rhodamine B (B) by 0.2 wt% F-20CD-100N aqueous solution. | ||
The separation and accumulation ability of the selected PR-based triblock copolymer was further determined by UV-vis spectrophotometric analysis of interactions of Coomassie Brilliant Blue G-250 and Rhodamine B with F-20CD-100N. As shown in Fig. 11, both the absorption peaks of Rhodamine B and PR-Rhodamine B dilute aqueous solutions were located at 554 nm, indicating that there is no interaction between Rhodamine B and the selected copolymer sample. Meanwhile there was also no significant change in the absorption strength of the filtrate solution of the mixture above DT (Fig. 11B), showing no separation and accumulation capability of F-20CD-100N for Rhodamine B. However, the absorption peak of dilute aqueous solution of this selected copolymer with Coomassie Brilliant Blue G-250 gave rise to a red-shift from 590 nm for the pure dye aqueous solution to 614 nm for the mixing aqueous solution measured at room temperature, possibly due to the no-covalent interaction of Coomassie Brilliant Blue G-250 with F-20CD-100N. Furthermore, a significant drop was noted in the absorption strength of the mixing solution of F-20CD-100N and Coomassie Brilliant Blue G-250 after the mixture was settled above DT and the sediments were filtered (Fig. 11A), clearly suggesting that a great deal of Coomassie Brilliant Blue G-250 was encapsulated in the sediments of the selected PR-based triblock copolymer and separated from the solution. A detailed investigation of the encapsulation and separation ability/efficiency of these PR-based triblock copolymers for small molecules is underway.
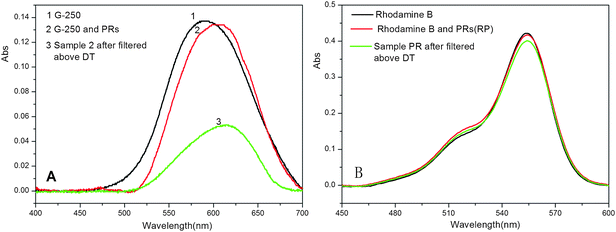 | ||
| Fig. 11 UV-vis spectra of Coomassie Brilliant Blue G-250 (G-250) and PR-G-250 (A), and Rhodamine B and PR-Rhodamine B (B) aqueous solution. | ||
Conclusions
Dual thermo-responsive polyrotaxane-based triblock copolymers were synthesized via in situATRP of NIPAAm initiated with self-assemblies made from a distal 2-bromopropiomyl end-capped Pluronic F127 with a varying amount of β-CDs using Cu(I)Cl/PMDETA as catalyst at 25 °C. Their GPC traces exhibited a narrow molecular weight distribution with a polydispersity index range of 1.15–1.26. Combining the thermo-response components of β-CD-Pluronic F127 ICs and PNIPAAm led to the resulting copolymers showing a decrease and rebound process of optical transmittance of aqueous solution with increasing temperature. Their thermo-responsive aggregates were also revealed by TEM with a great change of morphology as a response to the changes of temperature. Interestingly, they can encapsulate and separate Coomassie Brilliant Blue G-250 from the solution when heated up to their deposition temperature. This dual thermo-responsive finding offers the opportunity to use the PR-based triblock copolymers not only as carriers for drug controlled release and biosensors, but also as separation and accumulation media for small molecules.Acknowledgements
The authors acknowledge the support from the Natural Science Foundation of China (No. 20974015) and the Doctoral Program Foundation of Ministry of Education of China (No. 20091101110029).References
- A. Harada and M. Kamachi, Macromolecules, 1990, 23, 2821–2823 CrossRef CAS.
- A. Harada, Coord. Chem. Rev., 1996, 148, 115–133 CrossRef CAS.
- G. Wenz, B. H. Han and A. Muller, Chem. Rev., 2006, 106, 728–817.
- J. Araki and K. Ito, Soft Matter, 2007, 3, 1456–1473 RSC.
- J. Li and X. J. Loh, Adv. Drug Delivery Rev., 2008, 60, 1000–1017 CrossRef CAS.
- T. Ikeda, N. Watabe, T. Ooya and N. Yui, Macromol. Chem. Phys., 2001, 202, 1338–1344 CrossRef CAS.
- P. L. Nostro, J. R. Lopes and C. Cardelli, Langmuir, 2001, 17, 4610–4615 CrossRef.
- G. Sun, D. Wu, Y. Liu, C. He, T. S. Chung and S. H. Goh, Polymer, 2005, 46, 3355–3362 CrossRef CAS.
- K. M. Huh, T. Ooya, S. Sasaki and N. Yui, Macromolecules, 2001, 34, 2402–2404 CrossRef CAS.
- K. M. Huh, H. Tomita, T. Ooya, W. K. Lee, S. Sasaki and N. Yui, Macromolecules, 2002, 35, 3775–3777 CrossRef CAS.
- H. Fujita, T. Ooya and N. Yui, Macromol. Chem. Phys., 1999, 200, 706–713 CrossRef CAS.
- J. Li, X. Li, Z. Zhou, X. Ni and K. W. Leong, Macromolecules, 2001, 34, 7236–7237 CrossRef CAS.
- Y. Okumura and K. Ito, Adv. Mater., 2001, 13, 485–487 CrossRef CAS.
- T. Ooya, M. Eguchi and N. Yui, J. Am. Chem. Soc., 2003, 125, 13016–13017 CrossRef CAS.
- M. J. Frampton and H. L. Anderson, Angew. Chem., Int. Ed., 2007, 46, 1028–1064 CrossRef CAS.
- A. Harada, J. Li, T. Nakamitsu and M. Kamachi, J. Org. Chem., 1993, 58, 7524–7528 CrossRef CAS.
- H. Fujita, T. Ooya and N. Yui, Macromolecules, 1999, 32, 2534–2541 CrossRef CAS.
- M. Okada, Y. Takashima and A. Harada, Macromolecules, 2004, 37, 7075–7077 CrossRef CAS.
- S. Loethen, T. Ooya, H. S. Chio, N. Yui and D. H. Thompson, Biomacromolecules, 2006, 7, 2501–2506 CrossRef CAS.
- Z. G. Xie, D. D. Hou, L. Ye, A. Zhang and Z. G. Feng, Front. Mater. Sci. China, 2007, 1, 395–400 Search PubMed.
- H. Q. Yu, Z. G. Feng and A. Y. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3717–3723 CrossRef CAS.
- H. Q. Yu, Z. G. Feng, A. Y. Zhang, D. D. Hou and L. G. Sun, Polymer, 2006, 47, 6066–6071 CrossRef CAS.
- X. W. Zhang, X. Q. Zhu, X. M. Tong, L. Ye, A. Y. Zhang and Z. G. Feng, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5283–5293 CrossRef CAS.
- X. M. Tong, X. W. Zhang, L. Ye, A. Y. Zhang and Z. G. Feng, Polymer, 2008, 49, 4489–4493 CrossRef CAS.
- X. W. Zhang, X. Q. Zhu, F. Y. Ke, L. Ye, E. Q. Chen, A. Y. Zhang and Z. G. Feng, Polymer, 2009, 50, 4343–4351 CrossRef CAS.
- L. X. Ren, Y. M. Chen, F. Y. Ke, D. H. Liang and J. Huang, Macromolecules, 2008, 41, 5295–5300 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS.
- J. Wang, L. Ye, A. Y. Zhang and Z. G. Feng, J. Mater. Chem., 2011 10.1039/c0jm02803g.
- X. M. Tong, X. W. Zhang, L. Ye, A. Y. Zhang and Z. G. Feng, Soft Matter, 2009, 5, 1848–1855 RSC.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163–249 CrossRef CAS.
- H. Senff and W. Richtering, Colloid Polym. Sci., 2000, 278, 830–840 CrossRef CAS.
- M. Bohorquez, C. Koch, T. Trygtad and N. Pandit, J. Colloid Interface Sci., 1999, 216, 34–40 CrossRef CAS.
- M. Yokoyama, Crit. Rev. Ther. Drug Carrier Syst., 1992, 9, 213–248 CAS.
- A. Steinleitner, H. Lambert, C. Kazensky and B. Cantor, Obstet. Gynecol., 1991, 77, 48–52 CAS.
- R. M. Nalbandian, R. L. Henry and H. S. Wilks, J. Biomed. Mater. Res., 1972, 6, 583–590 CrossRef CAS.
- J. V. Weaver, I. Bannister, K. L. Robinson, X. Bories-Azeau, S. P. Armes, M. Smallridge and P. Mchenna, Macromolecules, 2004, 37, 2395–2403 CrossRef CAS.
- M. Okada, H. Okumura, M. Kamachi and A. Harada, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 4839–4849 CrossRef CAS.
- E. I. Popova and I. N. Topchieva, Russ. Chem. Bull., 2001, 50, 620–625 Search PubMed.
- I. N. Topchieva and K. Karezin, J. Colloid Interface Sci., 1999, 213, 29–35 CrossRef CAS.
- H. H. Lin and Y. L. Cheng, Macromolecules, 2001, 34, 3710–3715 CrossRef CAS.
- M. D. C. Topp, P. J. Dijkstra, H. Talsma and J. Feijen, Macromolecules, 1997, 30, 8518–8520 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
