Recent advances in polyolefin technology
Jinliang
Qiao
*ab,
Meifang
Guo
ab,
Liangshi
Wang
ab,
Dongbing
Liu
ab,
Xiaofan
Zhang
ab,
Luqiang
Yu
ab,
Wenbo
Song
ab and
Yiqun
Liu
ab
aPolyolefin National Engineering Research Center, Beijing, 100013, China
bSINOPEC Beijing Research Institute of Chemical Industry, Beijing, 100013, China
First published on 18th March 2011
Abstract
Polyolefin resins are the major plastics consumed in the world and are the subject of many new research works. This review will discuss how polyolefin technology has been developed in the past decade, with particular attention to new products, catalysts, polymerization processes and additives around the world as well as in China.
 Jinliang Qiao | Dr Jinliang Qiao is vice president of SINOPEC Beijing Research Institute of Chemical Industry and director of the Polyolefin National Engineering Research Center. He has published more than 70 papers and filed more than 80 patents. He got his Bachelor degree from the University of Science and Technology of China in 1982, Master degree from Beijing Research Institute of Chemical Industry in 1985 and PhD from Beijing University in 1996. |
 Liangshi Wang, Xiaofan Zhang, Meifang Guo, Luqiang Yu, Dongbing Liu, Yiqun Liu and Wenbo Song |
1. New products
Due to its cleaner life-cycle of production, processing, application and recycling compared with other materials, polyolefin has been considered an ideal material for various applications, such as packaging and other disposables, agriculture, appliances, electronics, construction, communication, automotives, etc. Therefore, the consumption of polyolefin products increases very fast and polyolefin has the largest market share among thermoplastics in recent years. For example, polyolefin took about 65% of the global thermoplastic market in 2000.1 The large market share also brings polyolefin into disrepute, such as “white pollution”; therefore, polyolefin has to develop from two approaches: one is to continue to replace other materials and help the sustainable development of our society; the other is to improve polyolefin itself to make it more friendly to the environment and human beings. Through these two approaches, polyolefin can help save energy in materials manufacturing, forming, recycling, and product weight reduction, and reduce hazardousness, dangerousness, emission and waste. Both new polyolefin and modified polyolefin developed based on new catalyst, new polymerization process, new additive and polymer physics have promoted sustainable development of our society. The new polyolefins, such as soft polyolefin, new PE pipe grades and metallocene-PP have replaced many other materials; the modified polyolefin resins, such as low-soluble-fraction PP for film and transparent container, low VOC (volatile organic compounds) impact PP, phthalate free PP, anti-static polyolefin and anti-bacterial polyolefin with low cost have benefited our health and environment. The reactor-made high performance polyolefin resins, such as reactor-made high melt strength PP and special resins for automobile bumper have replaced some polyolefin compounds.1.1 New polyolefin pipe grades
PE pipe has been recognized as the most suitable pipe for water and gas conveyance in cities. As was confirmed in the earthquake of Kobe of Japan in 1995, PE pipe can be much better than steel pipe in earthquake. In fact, no PE pipes were broken in that Kobe earthquake. The PE pipe is not only suitable for gas and cold water supply, but also feasible for hot water application. PE-RT pipe, a non-crosslinked PE pipe, grows very fast in recent years to replace crosslinked PE pipes for hot water transportation.Several PE manufacturers can produce PE-RT resins, such as Dow chemical company, Lyondellbasell and SK Corporation. New generation PE-RT with specifically designed molecular structures and controlled side-chain distribution, produced by different polymerization processes, has better high-temperature long term hydrostatic strength. For example, DOWLEX 2388 is an ethylene-octene copolymer produced by Dow's constrained geometry catalyst and solution process. PE-RT pipe has many advantages, such as non-crosslinking, high flexibility, excellent weldability, and long-term application at elevated temperature. PE-RT is a kind of environment friendly material with high performance and low cost.
1.2 Soft PP and olefin elastomer
To replace PVC (polyvinyl chloride), SBS (styrene-butadiene-styrene block copolymer), SIS (styrene-isoprene-styrene block copolymer), SEBS (hydrogenated styrene-butadiene-styrene copolymer), TPVs (thermoplastic vulcanizates), etc., several soft PPs and olefin elastomers including low modulus impact PP, stereoblock PP4 and olefin block copolymers (OBCs)5etc. have been developed in recent years, and among them OBCs with the trade name INFUSE are the most attractive.The INFUSE OBCs were developed and manufactured by Dow Chemical Company using a shuttling polymerization process, which will be introduced in detail in section 2.1 of this review. OBCs’ applications include flexible molded goods, profile extruded products, hoses and tubes, elastic fibers and films, foams, adhesives and tapes. OBCs comply with all requirements for food contact as set forth by FDA and EU. OBCs maintain excellent flexibility at much higher temperature than traditional olefin elastomers and have exceptional processibility, a similar permanent set to SEBS and similar compression set to TPV at 70 °C. Compared with traditional thermoplastic elastomers including flexible PVC, EVA (ethylene-vinyl acetate copolymer), SEBS, SBS and SIS, OBCs have many advantages, such as lighter weight and lower cost. Compared with other polyolefin based elastomers including TPV, polyolefin elastomer (POE), polyolefin plastomer (POP) and amorphous poly-alpha-olefin (APAO), OBCs also have advantages, such as better compression set and improved processibility.
1.3 Transparent PP with low soluble fraction
The transparent PP film and container have been used more and more widely in food contact applications, which often require heating with food by microwave oven. Compared with glass or other transparent materials, transparent PP can help save energy during the formation process, transportation and application. However, transparent PP usually contains a high level soluble fraction, which is detrimental to human health when the soluble components dissolve into food. Several kinds of transparent PP with a low soluble fraction have been developed in recent years.The random PP copolymer made by metallocene catalyst has a much lower soluble content compared with traditional random PP copolymer because of its uniform co-monomer distribution. It can be used for both food contacting films and food containers.
Propylene/butene-1 random copolymer usually contains less soluble fraction than traditional propylene/ethylene random copolymer. SINOPEC has commercialized propylene/butene-1 random copolymers by using new additives and its high isotacticity catalyst recently. The propylene/butene-1 random copolymers, both BOPP (biaxially orientated polypropylene) film and transparent container grades with less soluble content, are extremely suitable for Chinese food packing since Chinese food usually contains oil. The propylene/butene-1 random copolymers are not only suitable for Chinese food packaging, but also can benefit Chinese PP manufacturers since butene-1 is even cheaper than ethylene in China. The PP manufacturers in the refinery with lower cost monomer can also easily produce propylene/butene-1 random copolymers. Therefore, propylene/butene-1 random copolymers could be a kind of high performance polypropylene with low cost in China.
BOPP film is widely used for food packaging; therefore, BOPP resin with low soluble content is required by the food packing industry. However, high speed BOPP resin must contain more than 4% soluble fraction according to traditional theory. Based on a fundamental research breakthrough in the so called “973” program,6SINOPEC has developed a technology to produce high speed BOPP resin with soluble fraction of less than 2%, which is extremely suitable for food packaging.
1.4 Low VOC impact PP
Impact PP grades with high melt flow index (MFI) are usually produced via a controlled-rheology process, where PP with low MFI is produced in a reactor first and then peroxide is used to reduce PP's molecular weight. This process inevitably produces a lot of VOCs in the final PP resin. VOCs can give plastic products unpleasant smell like that in new cars. In order to reduce VOCs, the key point is to produce high MFI impact PP in the reactor directly with the help of external-donors. Among the processes developed is SINOPEC's unique asymmetric external donor (ASD) process,7 which will be introduced in section 3.2 of this review.1.5 Phthalate free PP
Phthalate has been found to be harmful to health, e.g. reducing male semen; therefore, materials containing phthalate have been restricted around the world. Unfortunately, PP catalysis commonly uses phthalate as an internal donor. The use of phthalate containing PP is likely to be prohibited in the near future, especially in food and toy industries.Several phthalate free PP catalysts have been commercialized in recent years and make phthalate free PP available, which will be reviewed in section 2.2 hereunder. For example, Lyondellbasell and SINOPEC can produce various PP grades without phthalate based on their phthalate free PP catalysts.
1.6 Reactor-made high performance PP
A large amount of modified PP or PP compounds are used in automobile industry and household appliances because virgin PP resins could not meet the requirements of these industries in the past. Secondary processing apparently means more energy use and more pollution; therefore, it is necessary to develop high performance PP resins directly from the reactor to replace modified PP. In recent years, reactor-made high melt strength PP and high performance impact PP have been developed and made a market success.High melt strength PP (HMSPP) has been used in PP foaming, thermoforming and many other applications. However, HMSPP manufacturing is a costly process. The first commercial HMSPP was produced by irradiation process and then followed by reactive extrusion process. In recent years, reactor-made HMSPP is available by different technologies, such as Dow's metallocene catalyst technology, Lyondellbasell's Spherizone process and SINOPEC's ASD process. With those new technologies, HMSPP will replace more other materials. For example, foamed PP made of HMSPP will replace more and more foamed polystyrene.
The automobile industry consumes a lot of PP compounds, such as in bumpers. Now the PP manufacturers can supply reactor-made PP resin that can satisfy the automobile industry's requirement for bumpers. It only needs a new mould to overcome the shrinkage difference between PP resins and PP compounds. SINOPEC has developed a special impact PP resin for car bumpers and successfully replaced some PP compounds in China.
1.7 Functional polyolefin with lower cost
Functional polyolefins account for only a small market share, one reason for this is due to their high cost. For example, anti-bacterial polyolefin can benefit human health, but it is not very popular in the market because of the expensive anti-bacterial additives used. In order to reduce the cost, SINOPEC has developed a “compound additive” technology platform for high efficiency additives including α-PP nucleating agent, β-PP nucleating agent, LLDPE nucleating agent, laser marking pigment, anti-bacterial agent, etc. With the help of this technology, some high performance polyolefins with lower cost have been commercialized, which will be introduced in section 4 of this review.2. Catalyst development
The history of polyolefin is actually the history of catalyst development. The catalyst, from Ziegler–Natta catalyst and Phillips catalyst to single-side catalyst, allows us to control the polyolefin purity, isotacticity and isotacticity distribution. It was the breakthroughs in catalyst technology that eliminated the process for the removal of the atactic fraction and catalyst residues to make the polymerization process simpler and cleaner. It was also the breakthroughs in catalyst technology that expanded the product properties envelope of polyolefins to make polyolefin’s market share increase continuously.2.1 PE catalysts
After LDPE, a non-catalyzed polyolefin developed by the Imperial Chemical Industries (ICI) in the 1930s, HDPE, LLDPE and PP became major plastics with the development of Ziegler–Natta catalysts8 and chromium catalysts9 in the 1950s. After more than 50 years of development, now there are four types of PE catalysts, Z–N catalysts, chrome catalysts, metallocene single-site catalysts and non-metallocene single site catalysts.An emulsion process for Z–N catalyst preparation was developed by Borealis recently. The process was originally developed for PP catalysis and then found to be also suitable for PE catalysis. The catalyst particles have an extremely low surface area, perfect spherical particle shape and a narrow particle size distribution. The polymer particles produced with this catalyst are compact and perfectly spherical with a narrow particle size distribution, reduced porosity, high bulk density and intra-particle homogeneity.11 This catalyst also leads to a polymer with advanced properties, such as a low level of fines etc.12,13
N-series PP catalyst (BASF Lynx 1000 series based on the same patent technology) developed by SINOPEC BRICI delivers PP with higher isotacticity and better stability. Recently SINOPEC BRICI extended the N-catalyst technology to PE catalyst as BCE and BCS catalysts for slurry and gas-phase processes. The BCE catalyst is a new generation high activity PE catalyst for slurry process.14–16 The catalyst can be applied to CX, Hostalen, Innovene and Phillips loop processes to produce HDPE, especially high added value grade, such as PE-100+ pipe grade and PE powder for CPE (chloride polyethylene) grades. The BCE catalyst has narrower particle size distribution; therefore, PE resin produced with the BCE catalyst has a lower fine content and higher polymer bulk density. The BCE catalyst also has better copolymerization ability, which can decrease the content of soluble low molecular weight PE. The PE production process with BCE catalyst consumes less energy, less steam and has a long operation cycle with easy and stable operation. The BCS catalyst is a slurry injection catalyst for gas phase process to produce LLDPE resin. The BCS catalyst has such advantages as high activity, excellent co-polymerization, good flowability, good particle size distribution of both catalyst and PE, and low fine content.17
From the industrial point of view, increasing the supported catalyst's activity and reducing catalyst cost are still the most important issues. Recently Albemarle developed a technology, ActivCat technology that can increase the productivity of conventional MAO/silica metallocene catalysts by 200%.28,29SINOPEC BRICI developed a MgCl2 modified silica carrier for metallocene catalyst that can increase activity of a supported catalyst by more than 50% compared with traditional silica supported catalysts.30
Borealis developed an emulsion-based single-site catalyst preparation process as described in Fig. 1.31 This process enables catalyst particles to have an inherently perfect spherical shape and unique intra and inter-particle homogeneity. The catalyst has higher catalyst activities and its morphology is shown in Fig. 2. The catalyst particles are very compact and have a low surface area.
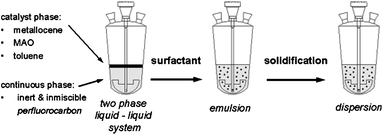 | ||
| Fig. 1 The preparation process for emulsion-based single-site catalyst. | ||
Single reactor bimodal HDPE technology is another PE catalyst hot research field. Univation has developed the PRODIGY Catalyst for bimodal HDPE in a single gas phase reactor.32 The PRODIGY Bimodal Catalyst system has two single site catalysts, a metallocene catalyst and a post-metallocene catalyst for HMW and LMW components. Another representative single site catalyst, as reviewed in 1998 by McKnight and in 2003 by Gibson, is an ansa-cyclopentadienyl-amido group IV catalyst called constrained geometry catalyst (CGC).33 In the 1990s, Dow Chemical Company commercialized the CGC technology to produce polyolefin plastomers and elastomers with long-chain branches in a solution process.
The pyridyl amine-base catalyst was developed by Dow Chemical Company in conjunction with Symyx high throughput technologies in 2004. The catalyst as shown in Fig. 3 is a non-metallocene single site catalyst. It allows copolymerization of propylene with various olefin comonomers over a broad range of compositions in an isotactic fashion and with high molecular weight. Dow commercialized the propylene/ethylene copolymer under the trademark VERSIFY. The VERSIFY plastomers and elastomers have a very narrow molecular weight distribution, a broad and unique composition distribution and unique regio-errors.3
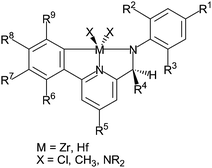 | ||
| Fig. 3 Pyridyl amine-based catalysts. | ||
Chain shuttling catalyst technology is another of Dow's innovations on single site catalysts. Chain shuttling is defined as the passing of a growing polymer chain between catalyst sites, such that portions of a single polymer molecule are synthesized by at least two different catalysts. The chain shuttling agent (CSA) is a component that facilitates this transfer, such as a metal alkyl complex. The chain shuttling mechanism can be described in Fig. 4.5
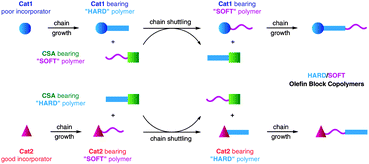 | ||
| Fig. 4 Depiction of the likely chain shuttling mechanism in a single reactor, dual-catalyst approach. | ||
Cat1 (solid circles) and Cat2 (solid triangles) represent catalysts with high and low monomer selectivity respectively, whereas the CSA (solid squares) facilitates the chain shuttling reaction. Cat1 produces a segment of hard polymer with low comonomer content. Shuttling occurs when this segment is exchanged with the CSA bearing a soft copolymer with higher comonomer content. Further chain growth at Cat1 then extends the soft copolymer chain with a hard segment, thus giving a block copolymer. Pyridyl amine-based catalyst is used as Cat2 to produce the segment of soft polymer and salicylaldiminato-type catalyst (known as FI catalyst) is used as Cat 1 for the segment of hard polymer.
In 2006, Dow announced ethylene–octene INFUSE™ olefin block copolymers (OBCs), which are produced using the chain shuttling catalyst technology. This technology employs two catalysts, one forming high-density PE with higher melting points and the second forming an elastomer with high incorporation of octene in the ethylene chain. A chain shuttling agent, diethyl zinc, forces an exchange of catalysts as the polymer chain grows leading to block polymers that combine the attributes of high-density PE and the olefin elastomer. Compared to ethylene–octene random copolymers made by metallocene catalysts, the block architecture of OBCs imparts a higher crystallization rate and higher melting temperature while maintaining a lower glass transition temperature and a more highly order crystalline morphology. Because of these structural differences, the physical and mechanical properties of OBCs are different from random copolymers, resulting in better performance in many applications.
2.2 PP catalysts
Since the first isotactic PP formed with TiCl3-AlR3 catalyst was developed in 1954,38 PP catalysts have been the main research focus for both the PP industry and academia. There are three major series of PP catalyst systems now: traditional Ziegler–Natta catalysts, metallocene catalysts and non-metallocene single-site catalysts.2.2.1.1 Internal donors. In the early 1980s, a MgCl2 supported catalyst system with a new internal donor, alkylphthalate, was developed. This new catalyst could afford much higher catalytic activity and isotacticity, so that de-ashing and removal of the atactic polymer could be avoided.41 This catalyst is called the fourth-generation Z–N catalyst, so far it is still widely used in the polypropylene industry. Since then, many further studies on electron donors have been done. Many internal donors have been developed, such as 1,3-dione, isocyanate, 1,3-diether, malonic ester, succinate, 1,3-diol ester, glutaric acid ester, diamine, 1,4-diol, 1,5-diol ester, phthalate esters, cycloalkyl esters and the use of a variety of binary electron donor complex etc. The catalytic properties differ a lot when different kinds of compound are used as the internal donor. At present, the catalysts using 1,3-diether, succinate or 1,3-diol ester as the internal donor have been commercialized or are in the stage of application promotion.42 The catalysts with these three types of internal donors are all phthalate free and so are the polypropylenes made with these catalysts.
① 1,3-Diethers as internal donors. In 1989, a PP catalyst with 1,3-diether as the internal donor was developed by the Himont company. This new internal donor provided a PP catalyst with extremely high activity and isotacticity without the need for any external donor, which breaks the restrictions of the last two generations of catalyst that the PP catalyst must contain both internal and external donors.43 It could be considered as the fifth generation of PP Z–N catalyst. Compared with the conventional catalyst, the diether catalyst has higher activity, higher hydrogen sensitivity, and narrower molecular weight distribution. Moreover, the obtained polymer exhibits high isotacticity and low oligomer content. Diether catalyst has been successfully applied to Montell Spheripol process. At present, Basell has developed a series of PP catalyst containing diether compound as the internal donor. PPs obtained with these catalysts have narrow molecular weight distribution, which is suitable for melt spinning process. Due to the fact that the Z–N catalyst with 1,3-diether as an internal donor has excellent hydrogen sensitivity, it is also suitable for the multi-reactor polymerization process, which can broaden the molecular weight distribution of polypropylene in existing operating conditions.
② Succinates as the internal donors. Succinate as internal donor was also developed by Basell. In 2003, Basell began to commercialize their new Z–N polypropylene catalysts with succinate as internal electron donor.43 The catalytic activity increased and the polymer molecular weight distribution became wider compared with the fourth generation catalyst using the phthalate. The stiffness and processing performance of the polypropylene resins made by this series of catalysts are greatly improved. The new type of catalyst can be applied to most of the PP production processes, including bulk, gas phase and slurry processes.
③ 1,3-Diol esters as the internal donors. In 2002, a new series of PP catalysts were developed by SINOPEC BRICI with 1,3-diol esters as the internal donors.44,45 Such catalysts are characterized by a high catalytic activity and better stereospecificity and the obtained polymers have a wide molecular weight distribution. The hydrogen sensitivity can be varied by changing the type and position of substituent of the 1,3-diol ester compound. In addition, the PP performance has also been greatly enhanced. The industrial application shows that the catalyst with 1,3-diol esters has unique advantages and PP made with this catalyst can be used in many applications.
2.2.1.2 External donors. The external donors also have great influence on polypropylene catalyst activity, hydrogen sensitivity and stereospecificity. It is necessary that the internal and external donors have proper match to each other.46 At present, the most commonly used catalyst in the PP industry is the fourth generation catalyst, containing phthalate as the internal electron donor and the organic siloxane compound as external donor. In the development of organic siloxane, many studies have shown the great effect of the structures of alkyl and alkoxy on catalyst performance.46–48
Proto and Sacchi found that the performance of organic siloxane was affected by the alkoxy number, size, and groups connected with the silicon atoms.48,49 According to their study, small alkoxy groups, such as methoxy and ethoxy were ideal for suitable organic siloxane. For the non-alkoxy groups, the effect of organic siloxane size on polypropylene isotacticity was in sequence: methyl < butyl < isobutyl = phenyl < isopropyl.50 Currently the most commonly used external donors in PP industry are methyl cyclohexyl dimethoxy silane (CHMDMS), diisobutyl dimethoxy silane (DIBDMS), dicyclopentyl dimethoxy silane (DCPDMS) and diisopropyl dimethoxy silane (DIPDMS). The effect on the stereospecificity was in the sequence: DCPDMS > DIPDMS > CHMDMS > DIBDMS; the effect on the hydrogen sensitivity was in sequence: DIBDMS > CHMDMS > DIPDMS > DCPDMS.47 Among the known organic siloxanes, the catalyst using DCPDMS as an external donor showed the highest stereospecificity, but the worst hydrogen sensitivity. Therefore, when PP with a broad molecular weight distribution is produced by using two external donors, one must be fixed on DCPDMS, another is usually an external donor with high hydrogen response and moderate stereospecificity properties.50,51
In recent years, scientists also have tried to develop new types of external electron donors. A series of PP catalysts using amino-silanes as external donors have attracted much attention.52,53 The catalyst with amino silane can broaden the polypropylene molecular weight distribution without reducing its isotacticity compared with the organic siloxane catalyst. It is also reported that the polypropylene produced by the catalyst containing a ring-amino-silane as external donor not only has a high crystallinity, but also a wide molecular weight distribution.54 When diethyl amino-silane is used as external donor, the catalyst exhibits high activity, high stereospecificity and good hydrogen response.55
Kemp developed an external donor, the calixarene, which is a macrocyclic oligomer formed by condensation reaction of phenol and formaldehyde.56,57 Using calixarene as external donor, the catalyst stereoregularity could be improved significantly while the catalytic activity changed slightly. When alkyl group substituted calixarene was used as an external donor, the PP isotacticity could be further enhanced while the catalytic activity decreased. In addition, if calixarene substituted siloxane was used as external donor, the catalyst stereoselectivity significantly increased while the activity changed little.
Himont also reported a kind of catalyst using 1,3-diether as an external donor and phthalate as the internal donor. This catalyst system has similar activity and hydrogen response to the catalyst using 1,3-diether as an internal donor.58
2.2.1.3 Catalyst preparation process. PP catalyst preparation process can be simply divided into two categories: one is preparing MgCl2 support first and then reacting the support with TiCl4 and the electron donor, such as Basell's spherical catalyst; the other is dissolving a magnesium compound first and then co-precipitating MgCl2 with titanium in the presence of TiCl4, such as the Mitsui Company's TK catalyst. Catalyst preparation process is also important for catalyst performance. Although the final components of the solid catalyst made from different processes are almost same, their microstructures can be quite different, such as catalyst active center number and distribution, so that the performance of catalysts can differ a lot.
In recent years, some new catalyst preparation processes have been developed and among them the most noteworthy is the emulsion process developed by the Borealis Company.59Catalysts prepared by this method have good morphology and narrow particle size distribution. PP produced by these catalysts has narrow particle size distribution, low fine content, and high bulk density. Although the catalysts prepared by this method have a small surface area and low porosity, the catalytic activity is still very high. This result introduces a new PP catalyst research direction that is completely different from the traditional one. In order to enhance catalyst activity, traditionally catalyst scientists always concentrate on increasing the catalyst surface area and porosity. Now the PP catalyst with small surface area and low porosity can also have high activity.
In 1995, Coates and Waymouth60 synthesized a non-bridged metallocene catalyst (2-Ph-Ind)2ZrCl2 with both meso and racemic structures that can be converted to each other (Fig. 5). It was found that the raceme mainly resulted in preparing isotactic polypropylene, while the meso structure made random polypropylene segments and the segment length was determined by the conversion rate between raceme and meso and monomer insertion rate. By changing the polymerization conditions, PP with various structures can be prepared. By changing the substituents on the phenyl group of the ligand, the research team developed a number of other metallocene PP catalysts with similar properties.61
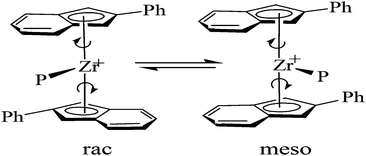 | ||
| Fig. 5 The structure of rac- and meso-(2-Ph-Ind) 2ZrCl2. | ||
Ewen and coworkers found that the CS-symmetrical metallocene catalyst, as shown in Fig. 6, can be used for producing highly syndiotactic PP, which is a significant breakthrough in the development of syndiotactic polypropylene.62 This finding not only produced a new kind of plastic, but also provided proof for the double active site polymerization mechanism of the metallocene catalyst.
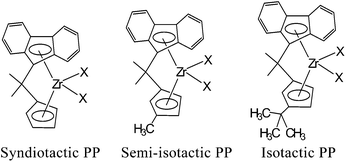 | ||
| Fig. 6 CS-symmetrical metallocene catalysts. | ||
The zirconocene catalysts rac-Et(Ind)2ZrCl21 and the silicon-bridged metallocene2 (R1 and R2 are hydrogen) are the two most thoroughly studied catalysts (Fig. 7). When R1 is methyl (complex 2), the molecular weight of polypropylene can be significantly increased. When R2 is naphthyl, propylene polymerization activity of catalyst 2 is as high as 8.8 × 108 g PP/mol M h, and polymer molecular weight is 920000, with a melting point of 161 °C, isotactic pentad ratio (mmmm) of 99.1%.63 So far 2-methyl-4-aryl-indenyl-based catalysts developed by the Spaleck research team are the most successful metallocene catalysts for producing isotactic polypropylene.
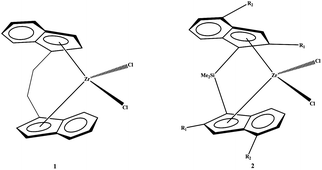 | ||
| Fig. 7 Ethyl- and dimethyl silicone-bridged metallocene catalysts. | ||
In recent years, one of the main research focuses on metallocene catalysts is new supports to further reduce the amount of MAO and further improve the metallocene catalyst activity.64 Grace has developed a support technology named IOLA, which was prepared by spray drying a reactant of SiO2 and clay.65IOLA is a micro spherical particle with very rough surface, plenty of caves and pores inside. These micro particles were ionized clay particles enabling the Lewis acid uniformly dispersed throughout the particles and interacted easily in the catalyst. In the pre-polymerization stage, IOLA acts as an activator for the catalyst, and in polymerization stage, it acts as a support for the catalyst. Thus IOLA has dual functions as both support and activator, eliminating the need for expensive MAO or borane as a cocatalyst, dramatically reducing the production cost of single-site catalysts.
Compared with metallocene polyethylene catalysts, the development of metallocene polypropylene catalysts is relatively slow. However, the metallocene polypropylene has shown its advantages; therefore, the metallocene polypropylene catalysts are expected to develop as good as metallocene polyethylene catalysts in the future.
In 1998, the Fujita group of the Mitsui Chemicals Company developed a novel type of salicylaldiminato group IV transition metal complexes, known as FI catalysts, which showed excellent catalytic performance on olefin polymerization.67 Such catalysts showed high polymerization activity for ethylene polymerization. But for propylene polymerization, the stereoselectivity was highly depended on the central metal and cocatalyst. With MAO as the cocatalyst the salicylaldiminato zirconium catalyst is very difficult to make high stereoselectivity polypropylene. However, under certain polymerization conditions, the salicylaldehyde titanium catalyst can prepare high regularity syndiotactic polypropylene.68,69 It is also found that with iBu3Al/Ph3CB (C6F5)4 as cocatalyst, high molecular weight atactic polypropylene could be prepared when using the salicylaldiminato titanium catalyst, while moderate to high molecular weight isotactic polypropylene can be prepared by zirconium or hafnium salicylaldiminato catalyst with the same cocatalyst.70,71
3. Polymerization process
3.1 PE process
In the past 10 years, the main PE processes almost remain unchanged. However, some progress in modification of existing processes has made PE process technology move forward. In HDPE processes, the main advances were enlarging the device capacity, using multi-reactors to manufacture multi-modal PE products and simplifying the processes. For example, the Chevron Phillips company had improved its loop process in following aspects: (1) use larger scale reactor to realize the economic benefits; (2) reduce the number of devices; (3) improve the equipment's efficiency and capacity; (4) simplify the installation, such as using aself-supporting reactor with fewer steel structures; (5) improve the catalyst feed system; (6) improve process control technology. The investment cost of the improved devices was reduced by 50% compared to the old one designed in 1990. In order to produce bimodal and multi-modal HDPE products smoothly, the improvement of Hostalen process was mainly focused on the reactor cooling system, gas emission system and de-waxing separation part.In the LDPE process, the main progress lies in three areas: improving product quality, increasing productivity and reducing costs. For example, the capacity of single-tube CTR technology has been increased to 400,000 tons per year. In high-pressure autoclave LDPE field, LyondellBasell has developed the Lupotech A process that has a special monomer and initiator injection system to control the reaction pressure and temperature distribution, so as to obtain the higher conversion rate. This process can be used to produce adhesives and sealants that contain up to 40% vinyl acetate.
The LLDPE process was mainly focused on further enlarging capacity, bimodal LLDPE technologies, controlling comonomer distribution and easy-processing metallocene LLDPE. To reduce the cost of the production, the capacity of Unipol gas-phase polyethylene reactor can reach 600,000 tons per year. Because of the increase in the reactor diameter, heat transfer must be strengthened in engineering, so are the materials transfer, the predicting of reactor agglomeration and the technology of reducing the transition product.72 The Spherilene technology with a dual-reactor recently took advantages of the Lupotech G technology to simplify the catalyst system and optimize exhaust gas removal system. The Westlake Company developed a technology to control comonomer distribution in polyethylene chain by adding some special compound during polymerization, which can improve the performance of LLDPE resin.73 The Evolue process now can use metallocene catalyst and a dual-reactor to produce bimodal metallocene polyethylene. NOVA Chemicals have adopted the Sclairtech dual-reactor and non-metallocene single-site catalyst to produce high-performance LLDPE products.74
3.2 PP process
Since the PP industry started in 1957, the advances in catalyst technology and the demand for improving product performance have always been the two main driving forces to promote the development of propylene polymerization processes.2 The typical current PP processes are shown in Fig. 8.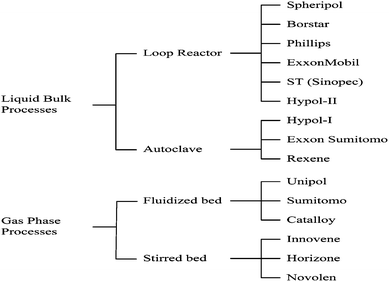 | ||
| Fig. 8 Some typical current polypropylene processes. | ||
Since the loop reactor has lots of advantages like high space-time yield, full use of reactor volume, simple structure, less investment in construction, excellent heat and mass transfer and seldom fouling in the reactor etc., it is regarded as the best reactor for propylene homo-polymerization. This leads the loop reactor process to be the most important manufacturing process for polypropylene now. Actually, the autoclave process has been abandoned.1,75,76
In order to improve the economics of plant operation, the scale of polypropylene plants have become larger and larger, and the largest production line is now over 450 kt/a. In order to meet the requirement for high quality polypropylene products, APC (advanced process control) technology has also been applied universally.77,78
Further improving polypropylene performance, such as polymer flexibility, rigidity and melt strength, is an important target in the PP industry. Among current polypropylene processes, some processes are designed for this purpose, such as the Catalloy process developed by BASELL.79,80 The Catalloy process is based on reactor particle technology (RGT), in which broader MWD, higher isotactic index, random copolymer with up to 15 wt% comonomer, multi-phase polyolefin alloy containing more than 70 wt% rubber can be produced. The most important advantage of this copolymerization technology is that the low modulus PP resins can be produced.
The commercial Spherizone process started in 2003 by the Basell Company was the most important breakthrough in PP production processes in the last 10 years.81,82 The core technology of the Spherizone process lies in the multi-zone circulating reactor (MZCR), which contains two different regions, i.e. Riser and Downcomer. Compared with the multi-reactor technology, the residence time of the particles circulating around in MZCR is much shorter than that in the multi-reactor, so that the particles uniformity is improved significantly.
Flexible control of catalyst properties is another way to achieve high performance polypropylene. It can be reached by Asymmetric External Donor (ASD) technology developed by SINOPEC Beijing Research Institute of Chemical Industry (BRICI) in 2006.7 The aim of ASD technology is to improve the overall performance of broad molecular weight distribution PP. Through regulating the amount and/or species of external donor in different polymerization stages, the catalyst performance, such as stereoregulating effect, hydrogen sensitivity and co-polymerization behavior, can be controlled in different polymerization stages by using ASD technology. The technology has been widely applied in a series of PP products, such as the high performance BOPP resin, PPH pipes, high melt strength polypropylene and high melt index impact polypropylene with low VOC.
4. Additives
Additives are indispensable components for polyolefin resins, which can improve the resins' processibility and other important end-use properties. There are tens of different kinds of additives used in polyolefin resins, in which nucleating agent is one of the most noteworthy additives. Some progress of PP nucleating agent will be introduced in this section.4.1 PP nucleating agent
Nucleating agent is an important additive for semi-crystalline polyolefin, especially for polypropylene. Some important physical and mechanical properties of PP resin can be greatly improved when low levels (generally between about 0.01 and 0.5 wt%) of commercial high-performance nucleating agent are added in the resin formula. The research concerning nucleating agent is very active and there are hundreds of patents filed in this area. Some review articles or books have been published in the last decade on various aspects of polymer nucleating agents.83–86Asahi Denka Corp.87 reported using pulverization apparatus to produce finely pulverized phosphoric acid aromatic ester metal salt in a European patent application. By controlling pulverization time, the average major-axis length of nucleator can be adjusted between 0.1–5 microns and the resulting nucleator has enhanced dispersibility. Their examples show that the nucleator particle size can reach 0.3 microns after jet-milling 30 min and ball-milling for another 2.5 h. Particle size below 0.1 micron is not preferred due to higher pulverization cost and lowering of fluidity.
Xinzhong et al.88 disclose a method for producing ultrafine organic phosphate nucleating agent. Said method includes the following steps: dissolving organic phosphate nucleating agent in organic solvent, adding surfactant, adding water to the above-mentioned solution under stirring, then collecting the described ultrafine organic phosphate nucleating agent from the reaction product. Its average particle size is less than 0.3 micrometres.
Libster et al.89,90 disclosed a novel dispersion method to improve the nucleation efficiency of a water soluble nucleator, bicycloheptane dicarboxylate salt, commercially known as HPN-68 from Milliken. Their concept is based on using microemulsion technology as a transport vehicle. The nucleating agent must be dispersed in a microemulsion to decrease its size from micro to nanoscale, which was then introduced to the target PP using a mixer. By the end of the melt mixing, when the water phase had evaporated, only the nucleator and the surfactant remained in the matrix. SEM results showed a four-fold decrease in the PP spherulite size due to the improved dispersion of HPN-68 within the matrix via microemulsion compared to conventional nucleator incorporation. DSC results show that only 50 ppm HPN-68 is sufficient to achieve the highest TC, similar to the one obtained by adding 300 ppm dispersed nucleator powder, i.e. 20% nucleating agent is required to achieve the same nucleating effect.
Xinzhong et al.91 disclosed a non-metallocene compound for catalyzing and nucleating polyolefin. This non-metallocene compound (as shown in Fig. 9) can be supported on magnesium halide to catalyze olefin polymerization. After catalysis, the compound is deactivated to become the nucleating agent for crystallizing polyolefin to raise the crystallization performance, mechanical performance and optical performance of polyolefin greatly.
 | ||
| Fig. 9 The chemical structure of non-metallocene catalysts. | ||
Yi et al.92 developed a method of exploiting a nucleation agent as support for metallocene catalyst. The metallocene compound can be well supported on 1,3,2,4-dimethyl benzylidene sorbitol (DMBS), a sorbitol based nucleating agent. Propylene polymerization with the supported catalyst resulted in i-PP polymers with granular morphology. The role of a catalyst support ensures a good dispersion of the nucleation agent in the formed i-PP matrix. The i-PP/DMBS composition exhibits a much higher crystallization temperature (TC) than the neat i-PP (129.5 vs. 113.3 °C), as well as much finer spherulites, a typical representation of crystallization with the assistance of nucleation agent.
4.2 Improving efficiency of polyolefin additives with elastomeric nanoparticles
Apart from a few polyolefin additives such as antioxidantsetc., which can be molten and well mixed with the resin matrix via melt compounding with polyolefins, most additives need to be well dispersed in polyolefin matrix so as to function well at low addition levels. Some powerful additives are usually expensive and the improvement of their efficiency means the reduction of additive loading and reduction of polyolefin resin cost, which is thus very attractive for resin producers and converters.Rigid nanoparticles such as nanosilica, layered silicate etc. are well known and have been used as polymer fillers for many years. The elastomeric nanoparticles (ENPs) mentioned here are flexible powder made from rubber latex through Narpow® technology invented by SINOPEC BRICI a decade ago. These ENPs are used as toughening agent for thermoplastic93–96 and thermosetting resins.97–99 The Narpow® technology uses rubber latex and irradiation sensitive as raw material and fully vulcanizes the latex particles through irradiation and finally spray-dries the vulcanized latex to obtain ENP products.100,101 It can be inferred from this manufacturing process that the surface part of ENP has a higher cross-linking degree than the interior due to a higher concentration of irradiation sensitivity near the surface part as well as more reactions with excited molecules and ions in water produced by the irradiation. Such a unique structure assures that ENPs maintain good elastic properties and at the same time the agglomerates of ENPs can still be easily broken apart and well dispersed in a plastic matrix.
It has been found in recent years that ENP can significantly improve the efficiency of some plastic additives, which means a similar or better performance can be achieved at lower loading of additive when ENPs are compounded with the corresponding additive. The compound nucleating agent introduced in this review is one of the examples that ENPs can improve the efficiency of PP additives.102
The compound nucleating agents can be obtained by mixing commercial nucleating agents, such as sodium 2,2′-methylene-bis-(4, 6-di-t-butylphenylene) phosphate (NA-11) and styrene-butadiene rubber ENPs. The nucleating effects of pure NA-11 and the compound were compared in different PP matrices as shown in Fig. 10. The crystallization temperature, flexural modulus and heat distortion temperature curves all show that the compound nucleating agent has better properties even at lower loading. Typically ca. 25% of NA-11 concentration in the compound nucleating agent can achieve the same or even better nucleating effect as compared to pure NA-11. This technology has been successfully applied to some high impact heco-PP grades and high crystalline homo-PP grades in SINOPEC in recent years. And more than 100,000 tons of high performance PPs with lower cost have been produced by using this compound nucleating agent in recent years.
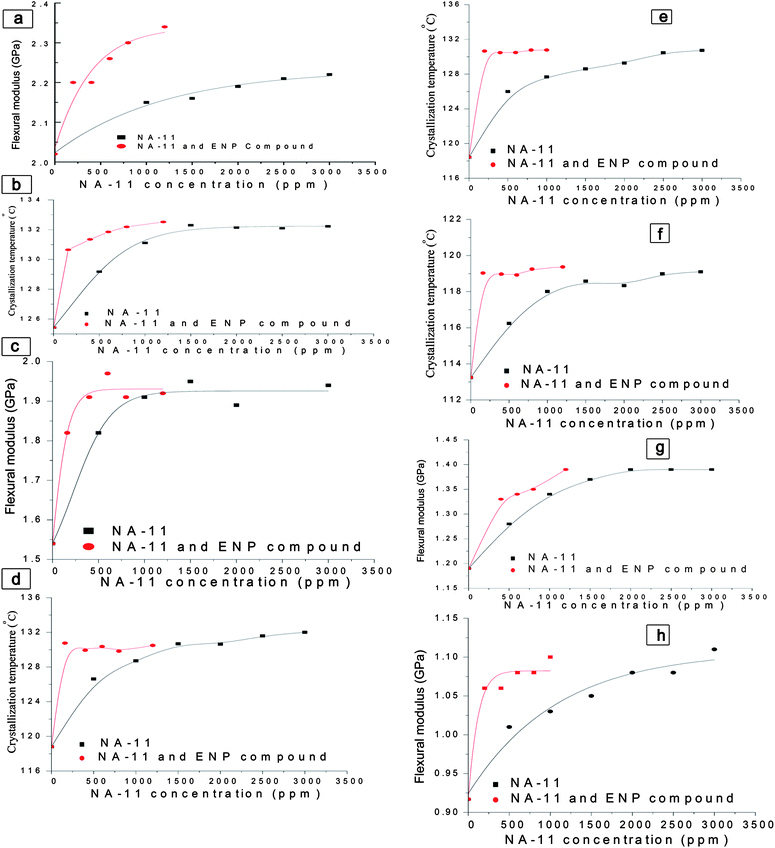 | ||
| Fig. 10 Comparison between nucleating effect of NA-11 and NA-11/ENP compound in different PP matrices. (a) and (b) in high isotactic PP matrix; (c) and (d) in regular homo-PP matrix; (e) and (h) in heco-PP matrix; (g) and (f) in raco-PP matrix. | ||
Even though the direct observation of nucleator in PP matrix is difficult due to its low concentration, some indirect experimental results as shown in the PLM micrographs (Fig. 11) indicate that the crystal size is smaller and the nuclei density is higher in PP sample nucleated with compound nucleator. These results can be preliminarily used as evidence to prove that ENPs can promote the dispersion of nucleator in PP matrix and enhance the nucleating efficiency accordingly.
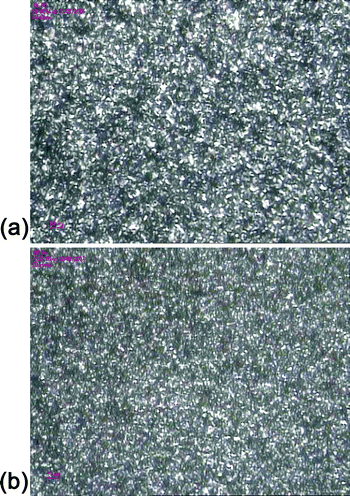 | ||
| Fig. 11 PLM micrographs of homo-PP modified by 0.4‰ NA-11 (a) pure NA-11 (b) NA-11/ENP compound. | ||
5. Polyolefin development in china
China is now a major polyolefin consumer and producer in the world. Mainland China consumed about 28 million tons and produced about 16 million tons of polyolefins in 2009. China's polyolefin industry uses monomers from 3 sources, ethylene plant, refinery and MTO/MTP plant. Based on coal, the MTO/MTP-polyolefin industry has grown very fast in China recently. There are 1.52 million tons/a polyolefin capacity based on MTO/MTP at present and more capacity is still in construction in China. It is difficult for polyolefin manufacturers to avoid fierce competition in China because polyolefin capacity increases much faster than consumption does in China and nearby countries recently. To meet the challenge, major polyolefin producers such as SINOPEC have targeted development and production of both sustainable products with environmental and safety benefits and speciality high performance polyolefin.The polyolefin is so important in China that even the fundamental research of polyolefin has been supported by “973” program for 10 years from 1999 to 2010. “973” program, the National Basic Research Program, is China's on-going national keystone basic research program organized by the Ministry of Science and Technology. The program was granted more than 10 million USD to support the scientists from universities, Chinese Academy of Sciences and SINOPEC working together on basic polymer science, such as basic polymer science in random polyolefin copolymer and impact PP. The “973” program on polyolefin has had great achievements and some of them have been introduced in this review.
The polymer scientists in China, including university professors, researchers from Chinese Academy of Sciences and industry, are still carrying on studies on the basic science and technology of polyolefins, such as co-monomer distribution on different macromolecules, anti-static, printability, and special requirements for washing machine to prevent the barrel from going mouldy. The polyolefin industry in China will continue to develop in the future.
6. Abbreviations
| LDPE | low density polyethylene |
| HDPE | high density polyethylene |
| LLDPE | linear low density polyethylene |
| PP | polypropylene |
| VOC | volatile organic compounds |
| PE | polyethylene |
| PE-RT | polyethylene of raised temperature resistance |
| PVC | poly(vinyl chloride) |
| SBS | poly(styrene-b-butadiene-b-styrene) |
| SIS | poly(styrene-b-isoprene-b-styrene) |
| SEBS | poly(styrene-b-ethylene butylene-b-styrene) |
| TPVs | thermoplastic vulcanizates |
| OBCs | olefin block copolymers |
| FDA | Food and Drug Administration |
| EU | European Union |
| POE | polyolefin elastomer |
| POP | polyolefin plastomer |
| APAO | amorphous poly-alpha-olefin |
| BOPP | biaxially orientated polypropylene |
| MFI | melt flow index |
| ASD | asymmetric external donor |
| HMSPP | high melt strength polypropylene |
| ICI | Imperial Chemical Industries |
| Z-N | Ziegler–Natta |
| CPE | chloride polyethylene |
| MAO | methylaluminoxane |
| IUPAC | International Union of Pure and Applied Chemistry |
| HMW | high molecular weight |
| LMW | low molecular weight |
| CGC | constrained geometry catalyst |
| FI | salicylaldiminato-type catalyst |
| CSA | chain shuttling agent |
| CHMDMS | methyl cyclohexyl dimethoxy silane |
| DIBDMS | diisobutyl dimethoxy silane |
| DCPDMS | dicyclopentyl dimethoxy silane |
| DIPDMS | diisopropyl dimethoxy silane |
| Cp | cyclopentadienyl |
| APC | advanced process control |
| RGT | reactor particle technology |
| MWD | molecular weight distribution |
| MZCR | multi-zone circulating reactor |
| SEM | scanning electron microscope |
| DMBS | 1,3,2,4-dimethyl benzylidene sorbitol |
| ENP | elastomeric nano particles |
| USD | United States dollar |
References
- P. Galli and G. Vecellio, Prog. Polym. Sci., 2001, 26, 1287–1336 Search PubMed.
- P. Galli and G. Vecellio, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 396–415 CrossRef CAS.
- P. S. Chum and K. W. Swogger, Prog. Polym. Sci., 2008, 33, 797–819 Search PubMed.
- V. Busico, in Stereoselective Polymerization with Single-site Catalysts, ed. L. S. Baugh and J. A. M. Canich, CRC Press, Boca Raton, 2008, ch. 8, pp. 203–229 Search PubMed.
- D. J. Arriola, E. M. Carnahan, P. D. Hustad, R. L. Kuhlman and T. T. Wenzel, Science, 2006, 312, 714–719 CrossRef CAS.
- China Petroleum & Chemical Corp., US Pat., 7 611 776, 2009.
- China Petroleum & Chemical Corp., WO Pat., 2007/124680, 2007.
- K. Ziegler, DE Pat., 973 626, 1953; K. Ziegler, US Pat., 3 037 972, 1954.
- Phillips Petroleum Company, US Pat., 2 825 721, 1953.
- Norio Kashiwa, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 1–8 CrossRef.
- M. Abboud, P. Denifl and K. Reichert, Macromol. Mater. Eng., 2005, 290, 1220–1226 Search PubMed.
- Borealis Tech Oy, CN Pat., 02830031.9, 2005.
- Borealis Tech Oy, CN Pat., 200810108732.7, 2008.
- Z. F. Guo, W. Chen, J. Zhou and H. Yang, Chin. J. Chem. Eng., 2009, 17, 530–534 Search PubMed.
- Z. F. Guo, D. B. Yin, J. L. Zhou and B. Y. Ji, Petrochem. Technol., 2008, 37, 937–940 Search PubMed.
- Y. Zhang, Z. F. Guo and J. L. Zhou, Petrochem. Technol., 2008, 37, 281–285 Search PubMed.
- L. Yang, Proceedings of PEPP 2008 conference, Zurich, 2008 Search PubMed.
- B. M. Weckhuysen and R. A. Schoonheydt, Catal. Today, 1999, 51, 215–221 CrossRef CAS.
- E. Groppo, C. Lamberti, S. Bordiga, G. Spoto and A. Zecchina, Chem. Rev., 2005, 105, 115–183 CrossRef CAS.
- M. P. McDaniel, K. S. Collins, E. A. Benham and T. H. Cymbaluk, Appl. Catal., A, 2008, 335, 252–261 Search PubMed.
- M. P. McDaniel, K. S. Collins, E. A. Benham and T. H. Cymbaluk, Appl. Catal., A, 2008, 335, 180–186 Search PubMed.
- M. P. McDaniel, K. S. Collins and E. A. Benham, J. Catal., 2007, 252, 281–295 Search PubMed.
- M. P. McDaniel and K. S. Collins, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 845–865 Search PubMed.
- W. Kaminsky, H. Sinn and R. Woldt, Proceedings of IUPAC Internat. Symp. on Macromolecules, Firenze, 1980 Search PubMed.
- W. Kaminsky, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 3911–3921 CrossRef CAS.
- G. G. Hlatky, Chem. Rev., 2000, 100, 1347–1376 CrossRef CAS.
- J. R. Severn, J. C. Chadwick, R. Duchateau and N. Friederichs, Chem. Rev., 2005, 105, 4073–4147 CrossRef CAS.
- C. Knight, Proceedings of PEPP 2008 conference, Zurich, 2008 Search PubMed.
- A. Motto, Proceedings of PEPP 2007 conference, Zurich, 2007 Search PubMed.
- China Petroleum & Chemical Corp., CN Pat., 200710176589, 2007.
- M. Bartke, M. Oksman, M. Mustonen and P. Denifl, Macromol. Mater. Eng., 2005, 290, 250–255 Search PubMed.
- J. H. Oskam, Proceedings of PEPP 2008 conference, Zurich, 2008 Search PubMed.
- A. L. McKnight and R. M. Waymouth, Chem. Rev., 1998, 98, 2587–2598 CrossRef CAS; V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283–315 CrossRef CAS.
- L. K. Johnson, C. M. Killian and M. Brookhart, J. Am. Chem. Soc., 1995, 117, 6414–6415 CrossRef CAS.
- C. M. Wang, S. Friedrich, T. R. Younkin, R. T. Li, R. H. Grubbs, D. A. Bansleben and M. W. Day, Organometallics, 1998, 17, 3149–3151 CrossRef CAS.
- B. L. Small, M. Brookhart and A. M. A. Bennett, J. Am. Chem. Soc., 1998, 120, 4049–4050 CrossRef CAS; G. J. P. Britovsek, V. C. Gibson, B. S. Kimberley, P. J. Maddox, S. J. McTavish, G. A. Solan, A. J. P. White and D. J. Williams, Chem. Commun., 1998, 849–850 RSC.
- S. Matsui, M. Mitani, J. Saito, Y. Tohi, H. Makio, H. Tanaka and T. Fujita, Chem. Lett., 1999, 28, 1263–1264 CrossRef.
- Montecatini, It. Pat., 535 712, 1954; Montecatini, It. Pat., 537 425, 1954.
- E. Albizzati, U. Giannini, G. Collina, L. Noristi and L. Resconi, in Polypropylene Handbook, ed. R. A. Phillips, M. D. Wolkowicz and E. P. Moore, Hanser, New York, 1996, Ch. 2, pp. 12–13 Search PubMed.
- K. Soga and T. Shiono, Prog. Polym. Sci., 1997, 22, 1503–1546 CrossRef CAS.
- Montedison S.P.A., Eur. Pat., 45 977, 1982.
- M. Gao and H. Li, Petrochem. Technol., 2007, 36, 535–546 Search PubMed.
- G. Cecchin, G. Morini and A. Pelloconi, Macromol. Symp., 2001, 173, 195–209 Search PubMed.
- H. T. Liu, J. Ma, C. Ding, M. Z. Gao and B. Mao, Petrochem. Technol., 2006, 35, 127–131 Search PubMed.
- M. Z. Gao, H. T. Liu, J. Wang, C. X. Li, J. Ma and G. S. Wei, Polymer, 2004, 45, 2175–2180 Search PubMed.
- M. L. Ferreira and D. E. Damiani, J. Mol. Catal. A: Chem., 1999, 150, 53–69 Search PubMed.
- X. Bai, M. Gao and T. Li, Petrochem. Technol., 2004, 34, 617–620 Search PubMed.
- A. Proto, L. Oliva and C. Pellecchia, Macromolecules, 1990, 23, 2904–2907 Search PubMed.
- M. C. Sacchi, F. Forlini, I. Tritto, R. Mendichi, G. Zannoni and L. Noristi, Macromolecules, 1992, 25, 5914–5918 Search PubMed.
- ExxonMobil Chemical Patents Inc., US Pat., 6 686 433, 2004.
- Fina Technology, Inc., WO Pat., 2 006 121 746, 2006.
- Toho Catalyst Co., Ltd., WO Pat., 2 006 129 773, 2006.
- H. Ikeuchi, T. Yano, S. Ikai, H. Sato and J. Yamashita, J. Mol. Catal. A: Chem., 2003, 193, 207–215 Search PubMed.
- Basell North America Inc., Eur. Pat., 0 926 164, 1999.
- Ube Industries, Ltd., KR. Pat., 20 050 055 693, 2005.
- R. A. Kemp, D. S. Brown, M. Latman and J. Li, J. Mol. Catal. A: Chem., 1999, 149, 125–133 CrossRef CAS.
- Equisatar Chemicals, CA. Pat., 2 523 247, 2004.
- Himont Inc., CN. Pat., 1 042 157, 1990.
- Borealis Technology, Eur. Pat., 1 397 401. 2004.
- G. W. Coates and R. M. Waymouth, Science, 1995, 267, 217–219 CrossRef CAS.
- C. D. Tagge, R. L. Kravchenko, T. K. Lal and R. M. Waymouth, Organometallics, 1999, 18, 380–388 CrossRef CAS.
- J. A. Ewen, M. J. Elder, R. L. Jones, S. Curtis and H. N. Cheng, in Catalystic Olefin Polymerization, Studies in Surface Science and Catalyst, ed. T. Keii and K. Soga, Elservier, New York, 1990, vol. 56, p. 439 Search PubMed.
- W. Spaleck, F. Kuber, A. Winter, J. Rohrmann, B. Bachmann, M. Antberg, V. Dolle and E. F. Paulus, Organometallics, 1994, 13, 954–963 CrossRef CAS.
- W. Wang and L. Wang, J. Polym. Mater., 2003, 20, 1–8 Search PubMed.
- W. R. Grace & Co.-Conn., SG. Pat., 88 794, 2002.
- S. D. Ittel, L. Johnson and M. Brookhart, Chem. Rev., 2000, 100, 1169–1203 CrossRef CAS; V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283–316 CrossRef CAS.
- Mistui Chemicals, Inc., Eur. Pat., 874 005, 1998.
- R. Furuyama, J. Saito, S. Ishii, M. Mitani, S. Matsui, Y. Tohi, H. Makio, N. Matsukawa, H. Tanaka and T. Fujita, J. Mol. Catal. A: Chem., 2003, 200, 31–42 CrossRef CAS.
- J. Tian and G. W. Coates, Angew. Chem., Int. Ed., 2000, 39, 3626–3629 CrossRef CAS.
- J. Saito, M. Onda, S. Matsui, M. Mitani, R. Furuyama, H. Tanaka and T. Fujita, Macromol. Rapid Commun., 2002, 23, 1118–1123 CrossRef CAS.
- A. V. Prasad, H. Makio, J. Saito, M. Onda and T. Fujita, Chem. Lett., 2004, 33, 250–251 CrossRef CAS.
- F. G. Stakem, International Polyolefins Conference, Houston, 2009 Search PubMed.
- Eastman Chemical Company, US Pat., 6 191 239, 2001.
- Plastics, Technology, 2006, 52(2), 25 Search PubMed.
- D. Bigiavi, M. Covezzi, M. Dorini, R. T. Lenoir, R. B. Lieberman, D. Malucelli, G. Mei, G. Penzo, in Polyporpylene Handbook, ed. N. Pasquini, Hanser Publishers, Munich, 2nd edn, 2005, ch. 6, pp. 361–378 Search PubMed.
- X. H. Yu, in Polypropylene, Theory, Process and Technology, ed. D. Y. Hong, Sinopec Press, Beijing, 2002, ch. 6, pp. 370–376 Search PubMed.
- X. J. Guo, Techno-Economics in Petrochemicals, 2007, 23, 31–37 Search PubMed.
- Amoco Corp., US Pat., 5 504 166, 1996.
- Montell Technology Company-US5733987, PCT Pat., 92/21706, 1992.
- Basell Poliolefine Italia S.R.L. [IT/IT], PCT Pat., 2008/015113, 2008.
- M. Covezzi, Chem. Eng. Sci., 2001, 56, 4059–4067 Search PubMed.
- Montel Technology Company bv, Netherlands, US Pat., 5 698 642, 1997.
- M. K. Watanabe, K. Okada and S. Yamazaki, Adv. Polym. Sci., 2005, 191, 137–186 Search PubMed.
- R. F. Becker, E. Burgin, L. P. J. Burton and S. E. Amos, in Polypropylene Handbook, ed. N. Pasquini, Hanser, Munich, 2nd edn, 2005, ch. 4, 280–283 Search PubMed.
- D. Libster, A. Aserin and N. Garti, Polym. Adv. Technol., 2007, 18, 685–695 Search PubMed.
- J. Kurja and N. A. Mehl, in Plastics Additives Handbook, ed. H. Zweifel, Hanser, 5th edn, 2000, ch. 18, 949–969 Search PubMed.
- Asahi Denka Kogyo Kk [JP], EP Pat., 1 209 190A1, 2002.
- East China University of Technology, CN Pat., 1 687 201, 2005.
- D. Libster, A. Aserin and N. Garti, J. Colloid Interface Sci., 2006, 299, 172–181 Search PubMed.
- D. Libster, A. Aserin and N. Garti, J. Colloid Interface Sci., 2006, 302, 322–329 Search PubMed.
- East China University of Technology, CN Pat., 1 974 611, 2006.
- Q. Yi, X. Wen, J. Dong and C. C. Han, Macromol. React. Eng., 2007, 1, 307–312 Search PubMed.
- M. L. Zhang, Y. Q. Liu, X. H. Zhang, J. M. Gao, F. Huang, Z. H. Song, G. S. Wei and J. L. Qiao, Polymer, 2002, 43, 5133–5138 Search PubMed.
- Y. Q. Liu, X. H. Zhang, J. M. Gao, F. Huang, B. H. Tan, G. S. Wei and J. L. Qiao, Polymer, 2004, 45, 275–286 Search PubMed.
- X. H. Zhang, Y. Q. Liu, J. M. Gao, F. Huang, Z. H. Song, G. S. Wei and J. L. Qiao, Polymer, 2004, 45, 6959–6965 Search PubMed.
- W. F. Dong, Y. Q. Liu, X. H. Zhang, J. M. Gao, F. Huang, Z. H. Song, B. H. Tan and J. L. Qiao, Macromolecules, 2005, 38, 4551–4553 Search PubMed.
- F. Huang, Y. Q. Liu, X. H. Zhang, G. S. Wei, J. M. Gao, Z. H. Song, M. L. Zhang and J. L. Qiao, Macromol. Rapid Commun., 2002, 23, 786–790 Search PubMed.
- H. Y. Ma, G. S. Wei, Y. Q. Liu, X. H. Zhang, J. M. Gao, F. Huang, B. H. Tan, Z. H. Song and J. L. Qiao, Polymer, 2005, 46, 10568–10573 Search PubMed.
- Y. Q. Liu, Z. Q. Fan, H. Y. Ma, Y. G. Tan and J. L. Qiao, Wear, 2006, 261, 225–229 Search PubMed.
- China Petrochemical Corp., US Pat., 6 423 760, 2000.
- Y. Q. Liu, X. H. Zhang, G. S. Wei, J. M. Gao, F. Huang, M. L. Zhang, M. F. Guo and J. L. Qiao, Chinese J. Polym. Sci., 2002, 20, 93–98 Search PubMed.
- M. X. Liang, Master's Thesis, SINOPEC Beijing Research Institute of Chemical Industry, 2008.
| This journal is © The Royal Society of Chemistry 2011 |

