Reactive nanorods based on activated ester polymers: a versatile template-assisted approach for the fabrication of functional nanorods†
Niko
Haberkorn
ab,
Katja
Nilles
a,
Philipp
Schattling
a and
Patrick
Theato
*ac
aInstitute of Organic Chemistry, University of Mainz, Duesbergweg 10-14, 55099, Mainz, Germany. E-mail: theato@uni-mainz.de
bMax Planck Institute for Polymer Research, Ackermannweg 10, 55128, Mainz, Germany
cSchool of Chemical and Biological Engineering, WCU Program of Chemical Convergence for Energy & Environment (C2E2), College of Engineering, Seoul National University, Seoul, Korea
First published on 10th November 2010
Abstract
A new route for the fabrication of polymeric nanorods with functional moieties via post-modification of reactive nanorods is described. To this end reactive nanorods with a homogenous and narrow size distribution were fabricated by utilizing an anodic aluminium oxide (AAO) template-assisted approach. The nanorods are based on activated pentafluorophenyl esters, to enable quantitative post-modification with amines under very mild reaction conditions yielding the corresponding functionalized amide. Post-modification with fluorescent dyes as well as the conversion into well-dispersed rod-shaped poly(N-isopropylacrylamide) (PNIPAM) hydrogels that exhibit a thermal-responsive phase transition was demonstrated. The platform of reactive nanorods provides the fabrication of various functional nanoobjects and may find application in research fields like drug delivery.
Introduction
One-dimensional (1D) organic nanometre-scaled objects such as rods, tubes and wires have attracted increasing attention as a result of their interesting properties1 and their potential application in different areas like optoelectronic,2 sensors,3,4 catalysis5 and drug delivery.6,7 The replication by using porous templates like macroporous silicon and anodic aluminium oxide (AAO) templates has become a well-established and versatile technique for the fabrication of such 1D organic nanostructures.8–12 In contrast to other techniques, such as electrospinning13 and direct drawing,14 the template-based approach facilitates the fabrication of nanoobjects with very defined aspect ratios. Nanoporous AAO templates, which are used as shape-defining molds, can be fabricated by a controlled electrochemical two-step anodization of aluminium.15,16 They provide hexagonally well-ordered pores with tunable pore diameters in the range of 10 to 300 nm and pore densities up to 1011 cm−2.8,17 The infiltration of low molecular weight organic materials or polymers into porous templates can be accomplished by template wetting with melts or solutions of the corresponding material or precursor. The template wetting technique was first demonstrated by Martin and coworkers and has since then been intensively investigated and extended to prepare functional nanoobjects.18–23 Based on the template wetting of functional precursor or polymeric materials, nanotubes and rods with ferroelectric,24 biodegradable25 and semiconducting26–28 properties have been reported.Results and discussion
Here we describe a new versatile route for the template-based fabrication of polymeric functional nanorodsvia post-functionalization of reactive rods (see Scheme 1). The functionalization after the completed templating process is advantageous since sensitive functional groups may decompose during direct melt wetting step or during the removal of the template, which usually requires the treatment with strong acids or bases. Further, by starting from one batch of reactive nanorods, nanoobjects with identical aspect ratios and morphologies and various different functionalities are accessible without optimizing the wetting parameters for each and every polymer.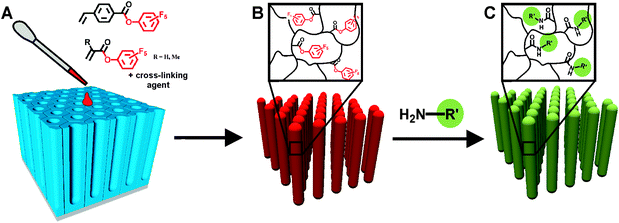 | ||
| Scheme 1 Schematic diagram of the template-assisted fabrication of reactive nanorods and their post-functionalization. (A) Infiltration of activated ester monomer, cross-linking agent and initiator into nanoporous AAO. (B) Thermal or UV-initiated polymerization and subsequent removal of the template yield cross-linked reactive polymeric nanorods. (C) Post-functionalization of the reactive rods with amine-functionalized reagents. | ||
Several reactive groups that facilitate a functionalization after the templating procedure may in principle be used, however, due to our experience with activated pentafluorophenyl esters, these have been chosen for the present study.29 Polymeric activated esters based on pentafluorophenyl esters of acrylic, methacrylic and 4-vinyl benzoic acids have found broad application for the synthesis of reactive polymers.29,30 They feature quantitative post-polymerization modification with amines under very mild reaction conditions yielding the corresponding amide.31,32 So far activated ester based polymers have been used as reactive building blocks in copolymers as well as for the preparation of reactive thin polymer films.32–34 Here we will show that polymeric activated esters are also highly suitable for the fabrication of reactive nanorods that facilitate a post-funtionalization with functional amines.
For the preparation of reactive nanorods a replication technique using AAO templates was chosen. A two-step anodization process in either oxalic or phosphoric acid was used for the fabrication of AAO templates with nanometre sized pore diameters—tunable between 40 nm to 200 nm—and narrow pore size distributions. The length of the pores could precisely be varied by controlling the anodization time. Since a versatile post-modification requires the treatment with amines that are dissolved in organic solvents, the nanorods need to be resistant against the exposure of organic solvents.
Furthermore, in order to enable a complete conversion of all activated ester moieties and not only of those located at the surface, a certain swelling ability of the material during the conversion step is favorable. To match the mentioned requirements the polymerization of the activated ester monomers was conducted inside the pores in the presence of a small amount of crosslinking agent. It turned out that in the case of pentafluorophenyl acrylate (PFPA) and pentafluorophenyl methacrylate (PFPMA) the bifunctional hexamethylenediacrylate was the most efficient crosslinker, while for the polymerization of pentafluorophenyl 4-vinyl benzoate (PFPVB) divinylbenzene worked best. Both chosen crosslinkers resulted in the formation of completely crosslinked polymer rods, which did not contain any uncrosslinked moieties. The crosslinking polymerization was initiated thermally or photochemically through the addition of azobisisobutyronitrile (AIBN) or Lucirin TPO, respectively. The AAO pores were filled by either solution wetting of highly concentrated solutions of the monomer mixture or bulk wetting of the monomer mixture. Fig. 1 and S1† show a selection of scanning electron micrographs of AAO templates and the corresponding nanoobjects after partial and complete etching of the sacrificial AAO template. The length and the diameter of the nanorods correspond to those of the porous molds, even for templates with very small diameters. After the complete etching of the AAO templates, arrays of well-aligned reactive nanorods were obtained. The nanorod arrays could be fragmented into bundles (see Fig. 1D) or single dispersed nanorods (see Fig. S4†) by moderate sonication treatment. Independent of the infiltration technique used, completely filled nanorods were always obtained. Noteworthy, when the solution wetting was performed with diluted solutions (3 wt%) nanotubes could be prepared (see Fig. 1E). However, it turned out that the tubular objects tended to collapse during subsequent centrifugation and sonication steps and we therefore focus in the following on the post-modification of the mechanically more stable nanorods.
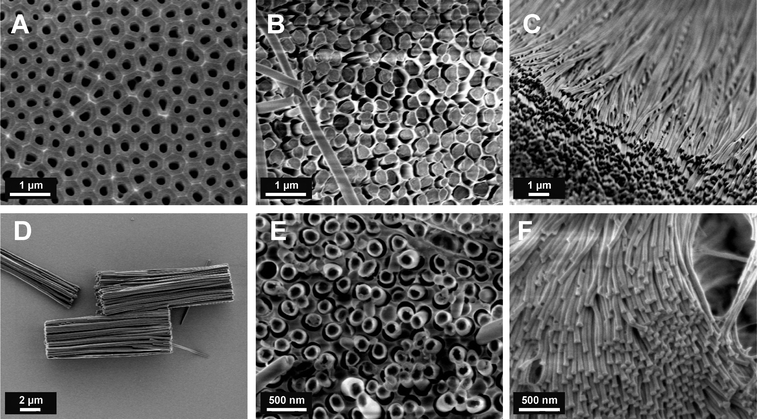 | ||
| Fig. 1 Scanning electron micrographs. (A) Unfilled AAO template; cross-linked polyPFPMA nanorods after partial (B) and complete (C) etching of the AAO template; (D) bundles of cross-linked polyPFPVB nanorods; (E) polyPFPVB nanotubes after partial etching of the AAO template; (F) array of polyPFPVB nanorods (average diameter 40 nm). | ||
One aspect that is crucial for any post-functionalization is the preservation of the activated ester groups during the wet chemical etching step of the sacrificial AAO template. Usually AAO templates are selectively etched away by treatment with aqueous KOH. However, the FT-IR spectrum of crosslinked polyPFPVB nanorods recorded after the template removal under basic conditions did not show the characteristic band of the PFP-ester at 1763 cm−1 anymore (Fig. 2A). This indicated the complete saponification of the ester, confirming that the etching under basic condition is not feasible. In contrast, the removal of the amphoteric Al2O3 mold by treatment with H3PO4 did not saponify the activated ester, as shown by FT-IR, and the reactive groups of the obtained nanorods were retained. Similarly, polyPFPA and polyPFPMA nanorods could be treated with H3PO4 without any hydrolysis of the activated ester.
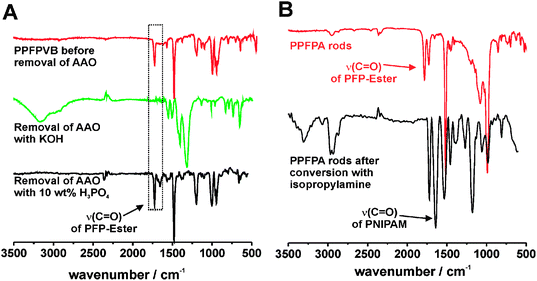 | ||
| Fig. 2 (A) ATR-FTIR spectra of a thin film of polyPFPVB (red line), polyPFPVB nanorods after etching of the template with KOH (green line) and H3PO4 (black line). (B) ATR-FTIR spectra of crosslinked polyPFPA nanorods (15 wt% crosslinker) after removal of the template (red line) and conversion with isopropylamine (black line). | ||
The feasibility of the post-modification of the reactive nanorods was first demonstrated by the conversion of polyPFPA nanorods with isopropylamine. Hence, the nanorods were dispersed in a 25 wt% solution of isopropylamine in THF for 6 h and subsequently purified by repeating cycles of centrifugation and sonication-assisted redispersion in water. Besides a good solubility of the amine and the formed pentafluorophenol in THF, the reaction medium should provide a suitable swelling of the crosslinked nanorods to allow an access to all reactive groups. The conversion of the reactive groups was recorded by FT-IR measurements (Fig. 2B). In the IR spectrum of the nanorods after treatment with isopropylamine the characteristic band of the PFP ester at 1783 cm−1 completely disappeared. The appearance of two amide bands at 1640 cm−1 and 1533 cm−1 as well as the NH valence vibration band at 3306 cm−1 in the infrared spectrum indicated the quantitative conversion yielding crosslinked poly(N-isopropylacrylamide) (PNIPAM) nanorods. Optical micrographs confirmed that the nanorods still remained in their shape and their dispersibility after the post-functionalization step. Further investigations that proved the successful preparation of cross-linked PNIPAM nanorods were based on the fact that PNIPAM is a temperature-responsive polymer, which features a lower critical solution temperature (LCST) in water. When solutions of PNIPAM are heated above the LCST, a reversible phase transition from a swollen hydrated state into a collapsed dehydrated form occurs.35,36 The responsive behavior of crosslinked PNIPAM hydrogels is accompanied by deswelling and swelling, which results in a shrinkage or expansion of the hydrogel. Therefore, the temperature-responsive behavior of water dispersed PNIPAM nanorods with a crosslinker content of 2 wt% was investigated by optical microscopy and turbidity measurements. For better visualization and size analysis, a whole bundle of post-functionalized PNIPAM nanorods was selected for optical microscopy measurements. Fig. 3A–C visualize the reversible temperature-dependent shape variation of the nanorod bundle. At 15 °C (below LCST) the nanopatterned PNIPAM hydrogel is swollen in water and exhibited a more than 50% extended length (and also diameter) in comparison to the dried nanorods. By heating up to 60 °C the nanorods considerable shrunk, which indicated the phase-transition into the deswollen hydrophobic state. Noteworthy, when the nanorods were prepared with an increased amount of cross-linker (>15 wt%), no significant thermal responsive shape change could be observed. The swelling and subsequent shrinking are completely reversible and can be repeated for numerous cycles. The curve of the temperature-dependent length variation is illustrated in Fig. 3D. Due to its small size and large surface, the nanorods exhibited a rapid response rate compared to macroscopic PNIPAM hydrogels, which may require up to several hours for complete swelling. The slight hysteresis is probably caused by a limited heat transfer of the heating set-up. The shape of the curve in Fig. 3D has a turning point at about 35 °C, which is in agreement with the turbidity measurements of water-dispersed PNIPAM nanorods (see Fig. 3E). Due to deswelling of the water-dispersed nanorods above the LCST, the optical transmittance decreased to 50%. Compared to the sharp LCST behavior of non-crosslinked PNIPAM the thermal responsive transition is significantly broadened, which has, however, also been reported for other PNIPAM microgels.37 Noteworthy, even though there are numerous reports about thermo-responsive PNIPAM microgels38 not a lot of work has been devoted to rod-shaped PNIPAM microgels. These mesoscopic shape-anisotropic particles exhibit a very large surface to volume ratio and are thus of interest for drug release applications.
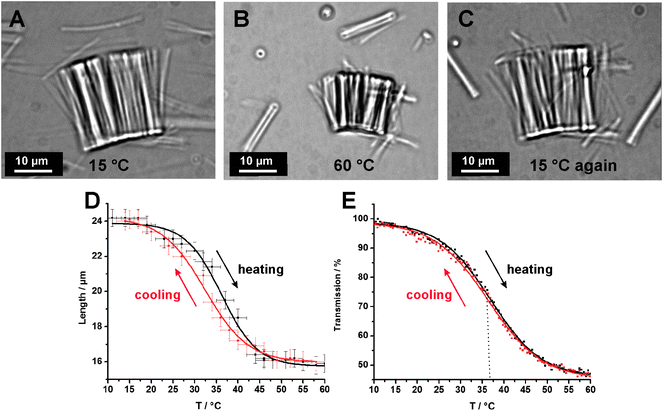 | ||
| Fig. 3 (A–C) Optical micrographs of a bundle of cross-linked polyPFPA nanorods after conversion into PNIPAM nanorods. (D) Plot of the length variation (along the axis of the nanorods) of PNIPAM nanorods dispersed in waterversus the temperature (heating rate 10 °C min−1). (E) Temperature-dependent turbidity measurement curve of an aqueous dispersion of PNIPAM nanorods. | ||
In further experiments the post-modification of reactive polyPFPVB nanorods with amine-functionalized fluorescence dyes, such as piperazinyl-4-chloro-7-nitrobenzofuran (pip-NBD) and sulforhodamine 101 cadervarine (S101C), was demonstrated. For this purpose, the polyPFPVB nanorods were dispersed in a dilute solution of pip-NBD in THF or S101C in a THF/DMSO mixture, respectively. The residual non-covalently attached fluorescent dye molecules were removed by centrifugation and redispersion cycles in THF and water.
Fig. 4 shows the optical micrograph image (Fig. 4A) and the corresponding confocal laser scanning micrograph (CLSM) images (Fig. 4B) of an array of four nanorods after the post-functionalization with pip-NBD. Fig. 4C and D illustrate the CLSM images of a bundle and a single isolated polyPFPVB nanorod after their modification with S101C. Even though it is difficult to comment on the radial distribution of immobilized dye molecules, because it is beyond the resolution limit of the CLSM, the images reveal that the reactive nanorods can be successfully functionalized with fluorescence dyes with a homogenous distribution along their axis.
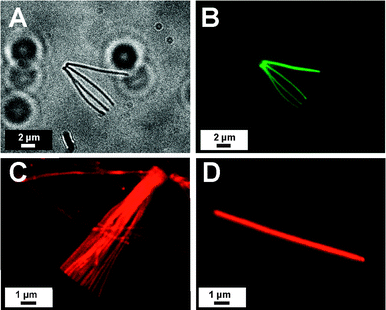 | ||
| Fig. 4 Optical micrograph (A) and corresponding CLSM image (B) of post-functionalized polyPFPVB nanorods with pip-NBD (λex = 488 nm). Fluorescence microscope images of a bundle of polyPFPVB nanorods (C) and an individual nanorod (D) after functionalization with sulforhodamine 101 cadervarine (λex = 543 nm). | ||
Experimental
Materials
All reagents were commercially available and used without further purification unless otherwise stated. The activated ester monomers pentafluorophenyl acrylate (PFPA), pentafluorophenyl methacrylate (PFPMA) and pentafluorophenyl 4-vinyl benzoate (PFPVB) were synthesized according to the literature.30,31Piperazinyl-4-chloro-7-nitrobenzofuran (pip-NBD) was synthesized as reported.39Instrumentation
An Oriel Instruments mercury lamp (500 W) with a 365 nm line filter was used for UV-irradiation. IR spectra were recorded at a Bruker Vector 22 FT-IR spectrometer using an ATR unit. Temperature-dependent turbidity measurements of an aqueous dispersion of nanorods were performed with a Jasco V-630 photo-spectrometer and a Jasco ETC-717 peltier element at a concentration of 3 mg mL−1. The cloud point curves were recorded by optical transmittance at 632 nm. The heating rate was 1 °C min−1. SEM images were recorded using a Zeiss Leo 1530 at an acceleration voltage of 3.0 kV for AAO membranes and 0.7 kV for polymeric samples. TEM was measured using a Philips EM420 120 kV.Fluorescence measurements were performed by using an inverted laser scanning microscope (Leica TCS SL) with an immersion objective (40×). A drop of the nanorod dispersion was dropped onto a glass plate and dried. An argon laser (λex = 488 nm) was used for the excitation of pip-NBD. The sulforhodamine 101 cadaverine (S101C) was imaged by excitation with the 543 nm line of a He/Ne laser.
Fabrication of AAO
The templates were prepared by a two-step anodization process. Experimental details are given in the ESI†.Fabrication of reactive nanorods
AAO templates were filled by solution wetting at room temperature with mixtures of PFPA and PFPMA respectively, hexamethylenediacrylate (2–15 wt%) and an initiator. In the case of the solid PFPVB, a highly concentrated mixture with divinylbenzene (10 wt%) in dichloromethane was prepared. For the thermal-initiated polymerization with AIBN (1.0 wt%), the samples were pressed between two glass plates and heated to 80 °C for 12 h. UV-initiated polymerization with Lucirin TPO (5 wt%) was performed by UV-irradiation for 20 min. Subsequently, the remaining polymer film on top of the template was removed with a razor blade and the template was selectively etched away with phosphoric acid (10 wt%). The nanorods were purified by three cycles of centrifugation and sonication-assisted redispersion in deionized water.Post-functionalization of reactive nanorods
Cross-linked PPFPA nanorods were stirred in a solution of isopropylamine in tetrahydrofuran (50 wt%) for 8 h. After the reaction, the nanorods were isolated by centrifugation/redisperison cycles in DI water. For the post-functionalization with fluorescent dyes polyPFPVB rods were stirred in a solution of pip-NBD in THF (w/v 0.5%) and sulforhodamine 101 cadervarine in a mixture of DMSO/THF (v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/v 0.05%), respectively, for 18 h. Non-covalently attached residual dyes were removed by subsequent centrifugation/redisperison cycles.
1, w/v 0.05%), respectively, for 18 h. Non-covalently attached residual dyes were removed by subsequent centrifugation/redisperison cycles.
Conclusions
In conclusion, it could be demonstrated that preparation of reactive nanorodsvia a templating approach provides a new versatile platform for the fabrication of functional rods via post-modification with various amines. The modification of reactive nanorods with fluorescent dyes as well the conversion into well-dispersed rod-shaped PNIPAM hydrogels, which exhibit a thermal-responsive phase transition, could be demonstrated for the very first time. As such, future experiments utilizing this reactive platform for the preparation of very well-defined functional nanoobjects are expected to have an impact on different research areas, including drug delivery applications.Acknowledgements
The Electron Microscopy Center at the Max-Planck Institute for Polymer Research in Mainz, in particular G. Glasser, is acknowledged for access to SEM measurements. Dr H. Götz is acknowledged for the help with CLSM measurements. This research was supported by the DFG (IRTG 1404) and by the WCU (World Class University) program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-10013).References
- M. Aryal, K. Trivedi and W. W. Hu, ACS Nano, 2009, 3, 3085 CrossRef CAS
.
- N. Haberkorn, M. C. Lechmann, B. H. Sohn, K. Char, J. S. Gutmann and P. Theato, Macromol. Rapid Commun., 2009, 30, 1146 CrossRef CAS
.
- C. Martin, Acc. Chem. Res., 1995, 28, 61 CrossRef CAS
.
- A. Gitsas, B. Yameen, T. D. Lazzara, M. Steinhart, H. Duran and W. Knoll, Nano Lett., 2010, 10, 2173 CrossRef CAS
.
- M. Steinhart, Z. Jia, A. Schaper, R. Wehrspohn, U. Gosele and J. Wendorff, Adv. Mater., 2003, 15, 706 CrossRef CAS
.
- R. Dersch, M. Steinhart, U. Boudriot, A. Greiner and J. Wendorff, Polym. Adv. Technol., 2005, 16, 276 CrossRef CAS
.
- A. Greiner, J. H. Wendorff, A. L. Yarin and E. Zussman, Appl. Microbiol. Biotechnol., 2006, 71, 387 CrossRef CAS
.
- M. Steinhart, T. Shimazu, T. Shimazu and M. Steinhart, Adv. Polym. Sci., 2008, 220, 123 CAS
.
- J. Hulteen and C. Martin, J. Mater. Chem., 1997, 7, 1075 RSC
.
- A. Huczko, Appl. Phys. A: Mater. Sci. Process., 2000, 70, 365 CrossRef CAS
.
- A. Li, F. Muller, A. Birner, K. Nielsch and U. Gösele, J. Appl. Phys., 1998, 84, 6023 CrossRef CAS
.
- S. Ono, M. Saito, M. Ishiguro and H. Asoh, J. Electrochem. Soc., 2004, 151, B473 CrossRef CAS
.
- A. Greiner and J. Wendorff, Angew. Chem., Int. Ed., 2007, 46, 5670 CrossRef CAS
.
- S. Harfenist, S. Cambron, E. Nelson, S. Berry, A. Isham, M. Crain, K. Walsh, R. Keynton and R. Cohn, Nano Lett., 2004, 4, 1931 CrossRef CAS
.
- H. Masuda and K. Fukuda, Science, 1995, 268, 1466 CrossRef CAS
.
- H. Masuda, H. Yamada, M. Satoh, H. Asoh, M. Nakao and T. Tamamura, Appl. Phys. Lett., 1997, 71, 2770 CrossRef CAS
.
- T.-Y. Kim and S.-H. Jeong, Korean J. Chem. Eng., 2008, 25, 609 Search PubMed
.
- V. Cepak and C. Martin, Chem. Mater., 1999, 11, 1363 CrossRef CAS
.
- M. Steinhart, R. Wehrspohn, U. Gösele and J. Wendorff, Angew. Chem., Int. Ed., 2004, 43, 1334 CrossRef CAS
.
- M. Steinhart, J. H. Wendorff and R. B. Wehrspohn, ChemPhysChem, 2003, 4, 1171 CrossRef CAS
.
- S. Schlitt, A. Greiner and J. H. Wendorff, Macromolecules, 2008, 41, 3228 CrossRef CAS
.
- R. O. Al-Kaysi, T. H. Ghaddar and G. Guirado, J. Nanomater., 2009, 2009, 436375 Search PubMed
.
- M. Zhang, P. Dobriyal, J. Chen, T. Russell, J. Olmo and A. Merry, Nano Lett., 2006, 6, 1075 CrossRef CAS
.
- C.-C. Wang, Q.-D. Shen, S.-C. Tang, Q. Wu, H.-M. Bao, C.-Z. Yang and X.-Q. Jiang, Macromol. Rapid Commun., 2008, 29, 724 CrossRef CAS
.
- S. L. Bechara, A. Judson and K. C. Popat, Biomaterials, 2010, 31, 3492 CrossRef CAS
.
- N. Haberkorn, J. S. Gutmann and P. Theato, ACS Nano, 2009, 3, 1415 CrossRef CAS
.
- R. Palacios, P. Formentín, T. Trifonov, M. Estrada, R. Alcubilla, J. Pallarés and L. Marsal, Phys. Status Solidi RRL, 2008, 2, 206 Search PubMed
.
- H.-S. Wang, L.-H. Lin, S.-Y. Chen, Y.-L. Wang and K.-H. Wei, Nanotechnology, 2009, 20, 075201 CrossRef
.
- P. Theato, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6677 CrossRef CAS
.
- M. Eberhardt, R. Mruk, R. Zentel and P. Théato, Eur. Polym. J., 2005, 41, 1569 CrossRef CAS
.
- K. Nilles and P. Theato, Eur. Polym. J., 2007, 43, 2901 CrossRef CAS
.
- M. I. Gibson, E. Froehlich and H.-A. Klok, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4332 CrossRef CAS
.
- F. D. Jochum and P. Theato, Macromolecules, 2009, 42, 5941 CrossRef CAS
.
- D. Kessler and P. Theato, Macromolecules, 2008, 41, 5237 CrossRef CAS
.
- D. Kuckling, Colloid Polym. Sci., 2009, 287, 881 CrossRef CAS
.
- H. Schild, Prog. Polym. Sci., 1992, 17, 163 CrossRef CAS
.
- J. Rubio-Retama, N. E. Zafeiropoulos, C. Serafinelli, R. Rojas-Reyna, B. Voit, E. L. Cabarcos and M. Stamm, Langmuir, 2007, 23, 10280 CrossRef CAS
.
- D. Kuckling, J. Hoffmann, M. Plotner, D. Ferse, K. Kretschmer, H. Adler, K. Arndt and R. Reichelt, Polymer, 2003, 44, 4455 CrossRef CAS
.
- R. Nudelman, O. Ardon, Y. Hadar, Y. Chen, J. Libman and A. Shanzer, J. Med. Chem., 1998, 41, 1671 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Fabrication of AA templates, SEM images of unfilled and filled AAO membranes, optical microscope images of rods before and after conversion, phase contrast microscope images of aqueous dispersions of nanorods. See DOI: 10.1039/c0py00314j |
| This journal is © The Royal Society of Chemistry 2011 |
