Singular thermosensitivity of polymethyl methacrylate/poly-N-isopropylacrylamide conetworks prepared by a facile synthetic route
Inmaculada
Aranaz
,
Sergio
Carrasco
,
Myriam G.
Tardajos
,
Carlos
Elvira
,
Helmut
Reinecke
,
Daniel
López
and
Alberto
Gallardo
*
Instituto de Ciencia y Tecnología de Polímeros, CSIC, Juan de la Cierva 3, 28006, Madrid, Spain. E-mail: gallardo@ictp.csic.es; Fax: +34 915644853; Tel: +34 915618806 ext 375
First published on 18th November 2010
Abstract
A novel and facile route to prepare acrylic-based conetworks consisting of polymethacrylate and polyacrylamide chains is described. This method uses amine–succinimide coupling chemistry and sequential polymerization, and it is described using NIPA and MMA monomers as model components. The thermosensitivity in water, related to the NIPA component, has been found to be very different among the conetwork and the reference network of the crosslinked random copolymer.
Introduction
A conetwork is a network of two components, that is, interconnected chains composed of two types of polymers, polyA and polyB, as the structure I of the schematic representation in Fig. 1, which is one of the multiple structural options.1 It is a particular network topology, very different from a statistical copolymer network (structure II of Fig. 1) and, in a sense, alternative to an interpenetrated network (IPN). Conetworks show specific properties and characteristics; for instance regarding their mechanical properties. When both types of polymeric chains are incompatible, as for example some of the well-known amphiphilic conetworks,1 under appropriate conditions it is possible to prepare two co-continuous phases. In this case, both components of the conetworks can segregate, being the size of the phases in the nanometre range. This particular morphology has revolutionised the field of the contact lenses2 since co-continuous phases permit the preparation of materials that, at the same time, are able to allow hydration and ocular accommodation (due to a hydrophilic behaviour) and to allow oxygen permeation (due to a hydrophobic behaviour). On the other hand, these systems are also very interesting in the catalyst field not only due to the co-continuous phases but also due to their high interfacial area.3 Moreover, it is possible to prepare nanostructured systems with a wide range of possible applications, for instance, in the preparation of sensors.4 | ||
| Fig. 1 Simplified structural scheme of I—standard conetwork, II—bicomponent network and III—structures prepared in this work. The red and blue spheres represent two different monomeric units. | ||
Most of the conetworks are prepared by functionalizing parent polymer or oligomer blocks, followed by coupling of the two components,5 by copolymerization of macromers with the second component (structure I of the Fig. 1),6etc. We present, here, an alternative route that uses sequential polymerization and leads to conetworks where the crosslinking points are statistically distributed along both chains (structure III of the Fig. 1).
Experimental
Chemicals
N-Isopropylacrylamide, (NIPA, ACROS), was recrystallized from a diethyl ether/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) mixture. Methyl methacrylate, (MMA, Aldrich), was distilled at reduced pressure, azobisisobutyronitrile (AIBN, Aldrich) was recrystallized from ethanol. 2-Aminoethyl methacrylate hydrochloride (AEM, Polysciences), triethylamine (TEA, Aldrich), N,N′ methylene-bis-acrylamide (Bis), ethylene glycol dimethylacrylate (EGMA) and solvents were used without further purification.
5) mixture. Methyl methacrylate, (MMA, Aldrich), was distilled at reduced pressure, azobisisobutyronitrile (AIBN, Aldrich) was recrystallized from ethanol. 2-Aminoethyl methacrylate hydrochloride (AEM, Polysciences), triethylamine (TEA, Aldrich), N,N′ methylene-bis-acrylamide (Bis), ethylene glycol dimethylacrylate (EGMA) and solvents were used without further purification.
Polymerizations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 60 °C for 24 hours and using AIBN as initiator (Fig. 2). The reaction was carried out in the absence of oxygen by bubbling nitrogen for 40 min before sealing the system. The monomers and initiator concentrations were 2 and 0.015 mol L−1, respectively. The copolymer 1 was precipitated by adding triethylamine, washed in water, and dried under vacuum until constant weight.
1) at 60 °C for 24 hours and using AIBN as initiator (Fig. 2). The reaction was carried out in the absence of oxygen by bubbling nitrogen for 40 min before sealing the system. The monomers and initiator concentrations were 2 and 0.015 mol L−1, respectively. The copolymer 1 was precipitated by adding triethylamine, washed in water, and dried under vacuum until constant weight.
 | ||
| Fig. 2 Scheme describing the synthetic procedure of the conetworks. | ||
Preparation of conetworks
After 24 hours, the networks were recovered and exhaustively washed with dioxane. Samples for ATR measurements were frozen and lyophilized. Samples for thermosensitive studies were transferred to water solutions by changing gradually the medium from dioxane to water at room temperature.
| Label | Formulation | Crosslinking degree (%) | MMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NIPA feed molar ratio NIPA feed molar ratio |
VPTT/°C | S eq in dioxane (%)b | S relaxed in dioxane (%)c |
|---|---|---|---|---|---|---|
| a Bis added to reach a nominal final crosslinking degree of 5%. b S eq: equilibrium swelling degree in dioxane. (Equilibrated for 24 h in dioxane prior to measurement.) c S relaxed: relaxed state swelling degree in dioxane. | ||||||
A–conetwork 1–1 |
Copolymer 2 + NIPA | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
29 | 531 | 320 |
B–conetwork 1–2 |
Copolymer 2 + NIPA + Bisa | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
31 | 517 | 346 |
C–network MMA–NIPA |
MMA + NIPA + 5% of Bis | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17 | 404 | 301 |
D–MMAnet |
MMA + 5% of EGDMA | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
— | 422 | 313 |
E–NIPAnet |
NIPA + 5% of Bis | 5 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
32 | 380 | 260 |
Thermosensitive studies
In vitro swelling experiments of the discs (diameter: 1 cm, height: 0.5 cm) were performed gravimetrically as a function of temperature (5–50 °C) in distilled water. The samples were allowed to swell for one day at each temperature to allow equilibrium swelling at that particular temperature. The swelling degree was determined according to the following expression: | (1) |
Instrumentation
FTIR spectra were registered in a Perkin-Elmer Spectrum One, coupled to an attenuated total reflection (ATR) device. Four scans were registered with a resolution of 4 cm−1. NMR (1H) spectra were recorded on a 300 MHz (Varian Unity 300 or Bruker 300) using deuterated chloroform at room temperature. Chemical shift values are reported in parts per million (δ) relative to tetramethylsilane (TMS). Gel permeation chromatography (GPC) analyses were carried out in a Perkin-Elmer apparatus with an isocratic pump serial 200 connected to a differential refractometric detector (serial 200a). Two ResiPore columns (Varian) were conditioned at 70 °C and used to elute the samples (1 mg ml−1 concentration) at 1 ml min−1 in HPLC-Grade DMF supplemented with 0.1% v/v LiBr. Monodisperse standard polymethylmethacrylate samples in the range of 2.9 × 103–480 × 103 obtained from Polymer Laboratories were used for the calibration. The absence of primary amino groups in copolymer 2 was tested qualitatively with ninhydrin. Samples of 1 mg ml−1 of copolymers 1 and 2 were dissolved in chloroform, 4 drops were deposited on a silica gel sheet and the sheet was submerged in a ninhydrin solution for 5 s and afterwards heated for 30 s. Purple colour indicated the presence of primary amino groups. All rheological measurements were conducted in a TA Instruments AR-1000 rheometer using a parallel plate configuration and oscillatory mode. The frequency sweeps were carried out from 0.1 to 100 rad s−1. Preliminary strain sweeps were performed to determine the linear viscoelastic region. TA rheometer Data Analysis software was used to obtain the experimental data and to calculate storage (or elastic) modulus (G′) as well as loss moduli (G″). Samples were specifically prepared for rheological measurements using Teflon disks of 40 mm diameter and were swollen to equilibrium in dioxane.Results and discussion
Synthetic procedure
The conetworks have been synthesized by sequential polymerization as indicated in Fig. 2. In the design of the synthetic procedure, we have kept the structural homology between both types of polymerizable functions, methacrylate and acrylamide. Thus, in the first step, a methacrylate is used as crosslinker precursor and copolymerized with MMA, and in the second step, only acrylamide functions are involved. This structural homology should optimize the homogeneity of the crosslinking.7 On the other hand, a simple amine–succinimide chemistry that works easily in gentle conditions is used to convert the precursor to acrylamide. The more challenged point has been to find proper solvents for the polymerizations. On one hand, the solubility of the cationic hydrochloride of ethylamine methacrylate led us to use methanol with a small amount of water as solvent. On the other hand, a solvent with intermediate polarity such as dioxane—able to solubilize both homopolymers, polyMMA and polyNIPA, as well as to swell the networks—was chosen for the second polymerization as well as for the preparation of the other networks used as reference systems.In Fig. 3, the proton NMR spectra of the copolymers 1 and 2 with a nominal 5 molar% of the amine and acrylamide respectively are shown. In the upper part (spectrum of 1), the incorporation of both methacrylates is confirmed by the signals indicated in the figure. Main signals correspond to the methyl methacrylate units. The integration of the signals is in good agreement with the feed composition (5 molar%). After the derivatization (bottom spectrum) of the amine with the acryloyl succinimide, the protons of the acrylamide double bond arise at 6.3 and 5.7 ppm correlatively to the shifting of the ethyl proton at 4 ppm and the disappearing of the amine protons at 8.7 ppm, confirming the complete conversion of 1 into 2.
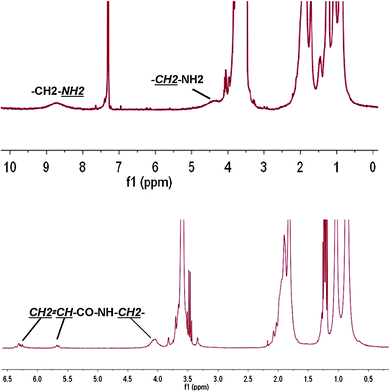 | ||
| Fig. 3 Proton NMR spectra of the copolymers 1 (up) and 2 (down). | ||
The degree of substitution was qualitatively determined by using the ninhydrin test. Copolymer 1 showed a purple colour while the same amount of copolymer 2 showed almost no colour which indicated a high degree of substitution (data not shown.) Copolymer 2 showed a number average molecular weight (Mn) of 45 kDa with a polydispersity index of 2.5.
The second polymerization of Fig. 2 yielded network formation, that is, an insoluble gel was obtained. Two different conetworks with final MMA/NIPA molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were prepared as indicated in Table 1. For comparative purposes, the three extra reference networks indicated in Table 1 were prepared as well by a single polymerization step. It has to be noted that the nominal MMA/NIPA ratios of conetwork A and network C are the same, that is, both have equimolar amounts of MMA and NIPA.
2 were prepared as indicated in Table 1. For comparative purposes, the three extra reference networks indicated in Table 1 were prepared as well by a single polymerization step. It has to be noted that the nominal MMA/NIPA ratios of conetwork A and network C are the same, that is, both have equimolar amounts of MMA and NIPA.
Networks characterization
The networks have been analyzed by ATR (see Fig. 4). The ester and amide groups of reference monocomponent networks D and E based on polyMMA and polyNIPA show distinctive signals at 1725 and 1630 cm−1 respectively, which have been used to do a qualitative compositional analysis of the bicomponent networks A, B and C. The spectra of conetwork A and the network C are very similar, which is in agreement with the mentioned unit equimolarity present in both systems. The conetwork B, as it should be expected, is richer in NIPA. | ||
| Fig. 4 ATR spectra of the 5 networks prepared in this work. | ||
Rheological studies have been carried out to further characterize the aforementioned networks. All of them showed higher storage moduli (G′) than loss moduli (G″) along the experiments (data not shown) which is characteristic of solid-like systems and indicates the formation of the networks. G′ of both conetworks (A and B) has a similar value to network D (MMA crosslinked with 5% EGDMA) being this value lower than the value of networks C and E (crosslinked random copolymer and NIPA crosslinked with 5% Bis).
Interestingly, the storage moduli (G′) of conetworks A, B and network C were independent of the frequency while a slight downturn at low frequencies was observed in the other networks as it can be seen in Fig. 5. This result points to a rather heterogeneous structure for networks E and D in contrast to more homogeneous A, B and C networks, in accordance with previous results in the literature.8,9 The frequency dependence would be due to the relaxation of elastically ineffective polymer chains coming from cyclizations, pendant chain ends, formation of microgels, etc.
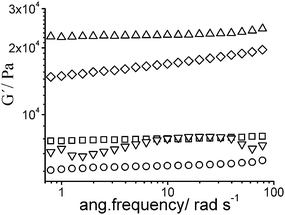 | ||
| Fig. 5 Rheological behaviour of networks. Variation of storage moduli with the frequency. (Frequency sweep) Network A (□), network B (○), network C (△), network D (▽) and network E (◇). | ||
Thermosensitivity in water
Studies of thermosensitivity of other MMA/NIPA systems can be found in literature. Zhang and Zhuo prepared standard statistical networks obtained by simultaneous copolymerization of NIPA and MMA.10 These hydrogels showed the well-known decrease in transition temperature associated to the incorporation of hydrophobic units along the chain as it has been reported for copolymers of N-butyl acrylamide.11–14 Zhang et al. have also prepared polyNIPA/polyMMA/silica hybrid capsules by inverse Pickering emulsion polymerization.15 Some of these non-conventional systems exhibited thermosensitivity.Fig. 6 and 7 show the influence of network topology of the samples prepared in this work with the material performance, that is, in their thermosensitivity. In these figures, the swelling behaviour in water for the different gels versus the temperature has been depicted. The most relevant result is the singular thermosensitivity behaviour of the conetwork A compared to the ‘isomeric’ network C. Both systems exhibit the same amount of MMA and NIPA unit, but their topology is very different as well as their thermosensitivity. The C system, which is formed by crosslinked statistical copolymer chains of MMA and NIPA, exhibits the mentioned decrease in the transition temperature associated with the incorporation of hydrophobic units along the chain. However, conetwork A, as well as conetwork B, which are composed of pure polyMMA and polyNIPA chains crosslinked in knots statistically distributed along the chains, exhibit a VPTT (29 and 31 °C, respectively) much closer to the pure NIPA-network (32 °C, see Fig. 6) and more than 10 degrees higher than network C (17 °C). The conetwork topology reduces the MMA influence to the possible interchain interactions since the intrachain interactions have been removed. In this case, the proximity of the neighbour NIPAm units allows the transition to occur similarly to pure polyNIPAm, i.e.hydrogen bonding between the polymer and the water molecules below the transition, and intra–intermolecular hydrophobic interactions between the polymer side chain groups above the transition.16
 | ||
| Fig. 6 Swelling degree versus temperature for systems A and C. Swelling degree obtained as indicated in the Experimental section. | ||
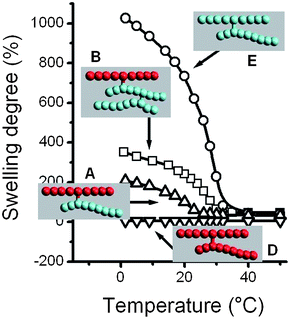 | ||
| Fig. 7 Swelling degree versus temperature for systems A, B, D and E. Swelling degree obtained as indicated in the Experimental section. | ||
Regarding the swelling degree in the expanded state below the VPTT, conetworks A and B have shown a clear influence of the MMA content (swelling degree at 1 °C of 207, 352 and 1026% for the A, B and E systems, respectively). Since the crosslinking degree and the network elasticity are similar for all the networks, these differences must be attributed to the increase in hydrophobicity as the MMA content increases from E (0 molar% of MMA) to B and A (0.33 and 0.5% respectively). These data are in agreement with the studies of Zhang and Zhuo mentioned above.13 These authors found in the NIPA/MMA statistical networks a correlative decrease in the equilibrium swelling degree in the expanded state with the increase of MMA content.
Also, it has to be mentioned that the swelling between the ‘isomeric’ A and C networks at 1 °C is very similar although the trends of both profiles are quite different. The points below zero are experimentally unreachable in distilled water, but from the work mentioned above it can be suggested that the hypothetical equilibrium swelling degree should not be far from the last point at 1 °C.
Conclusions
PolyMMA–polyNIPA conetworks composed of pure polyMMA and polyNIPA chains crosslinked in knots statistically distributed along the chains have been readily prepared using easy and gentle chemistry. The resulting conetworks have shown singular thermosensitivity, very different from the ‘isomeric’ network obtained by simultaneous copolymerization of MMA and NIPA, being their VPTT close to the one of pure polyNIPA. Comparing with the standard statistical networks obtained by simultaneous copolymerization, the incorporation of MMA in the conetworks decreases the swelling degree but it maintains the transition very close to the pure polyNIPA. Moreover, the rheological data indicate that both conetworks present good properties as gels.References
- G. Erdodi and J. P. Kennedy, Prog. Polym. Sci., 2006, 31, 1–18 CrossRef CAS.
- P. C. Nicolson and J. Vogt, Biomaterials, 2001, 22, 3273–3283 CrossRef CAS.
- N. Bruns and J. C. Tiller, Nano Lett., 2005, 5, 45–48 CrossRef CAS.
- M. Hanko, N. Bruns, S. Rentmeister, J. C. Tiller and J. Heinze, Anal. Chem., 2006, 78, 6376–6383 CrossRef CAS.
- C. He, G. Erdodi and J. P. Kennedy, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 1474–1481 CrossRef CAS.
- N. Bruns, M. Hanko, S. Dech, R. Ladisch, J. Tobis and J. C. Tiller, Macromol. Symp., 2010, 291–292, 293–301 CrossRef CAS.
- H. Reinecke and A. Gallardo, Macromol. Theory Simul., 2009, 18, 25–29 CrossRef CAS.
- H. Tobita and A. E. Hamielec, Polymer, 1990, 31, 1546–1552 CrossRef CAS.
- E. Fernández, D. López, E. López-Cabarcos and C. Mijangos, Polymer, 2005, 46, 2211–2217 CrossRef CAS.
- X. Z. Zhang and R. X. Zhuo, Mater. Lett., 2002, 52, 5–9 CrossRef CAS.
- A. S. Hoffman, P. S. Stayton and V. Bulmus, et al., J. Biomed. Mater. Res., 2000, 52, 577–586 CrossRef CAS.
- Y. H. Bae, T. Okano and S. W. Kim, Pharm. Res., 1991, 8, 531–537 CrossRef CAS.
- Y. H. Bae, T. Okano and S. W. Kim, Pharm. Res., 1991, 8, 624–628 CrossRef CAS.
- R. Yoshida, K. Sakai, T. Okano and Y. Sakurai, J. Biomater. Sci., Polym. Ed., 1994, 6, 585–598 CAS.
- K. Zhang, W. Wu, K. Guo, J. Chen and P. Zhang, Langmuir, 2010, 26, 7971–7980 CrossRef CAS.
- Y. H. Bae, T. Okano and S. W. Kim, J. Polym. Sci., Part B: Polym. Phys., 1990, 28, 923–936 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
