Highly water repellent aerogels based on cellulose stearoyl esters
Mari
Granström†‡
a,
Marjo Kettunen
née Pääkkö†
*b,
Hua
Jin
b,
Erkki
Kolehmainen
c,
Ilkka
Kilpeläinen
a and
Olli
Ikkala
*b
aLaboratory of Organic Chemistry, Department of Chemistry, University of Helsinki, P.O. Box 55, FI-00014, Finland
bDepartment of Applied Physics and Center for New Materials, Aalto University School of Science and Engineering (previously Helsinki University of Technology), P.O. Box 15100, Aalto, FIN-00076, Espoo, Finland. E-mail: marjo.kettunen@tkk.fi; olli.ikkala@tkk.fi
cLaboratory of Organic Chemistry, Department of Chemistry, University of Jyväskylä, P.O. Box 35, FI-400014, Finland
First published on 3rd May 2011
Abstract
Herein we combine in a novel way the physical effect of porous structure of a cellulosic aerogel with the chemical effect of long alkyl tails by a well known homogeneous green esterification method, to achieve purely bio-based and highly water repellent cellulosic materials. As an alternative for a traditional fluoro derivatized hydrophobization, here long fatty acid tails are utilized to lower the surface energy. To minimize the process emission, ionic liquid (IL) 1-allyl-3-methylimidazolium chloride is used for the esterification, due to its non-volatility and recyclability. We have shown here that low degree of substitution (DS) of the fatty acid cellulose material enables the spontaneous formation of aerogels. Additionally, the very low content of the long stearoyl tails combined with the porous aerogel structure resulted in significant increase in hydrophobicity from an aqueous contact angle of 0° up to 124°. We foresee that this approach can allow sustainable and completely bio-based coatings and insulators paving the way for a new green application potential for cellulose based materials.
Introduction
Cellulose is an attractive material being renewable and environmentally friendly, rich in functional hydroxyl groups, it exhibits hierarchical structure which has attracted growing interest in soft matter science, and it can have superior mechanical properties. However, the high hydrophilicity reduces the application potential and cellulose-based materials would greatly benefit from water repellency in daily life, biomedical, and engineering applications. Over the years, a large variety of hydrophobization methods have been introduced for cellulose.1 Surface modifications such as surfactant adsorption,2 chemical grafting,3 and surface silylation4 have been introduced. However, in these cases, the purpose has been to prevent the aggregation and to re-disperse the fibrils, rather than in their water repellency. More recently, e.g.silanes,5,6 fluorinated compounds,7–12silicone-based materials,13,14gold particles,15 and metal oxide–silane nanocoatings16 have been incorporated to render hydrophobic (contact angle, CA > 90°) and even superhydrophobic (CA > 150°) cellulose materials. However, the above materials, solvents, and processes are typically limited by their sustainability, costs and environmental concerns.Towards improved biocompatibility and biodegradability in hydrophobization, the main emphasis has usually been on proper chemical functionalization of cellulose aiming at a high degree of substitution (DS). Long chain aliphatic esters of cellulose have been shown to have potential for biodegradable films due to their natural origin and the presence of an enzymatically labile ester bond.17 The surface energy of the cellulose has been lowered by covalently attaching fatty acids on the cellulose backbone by homogeneous synthesis typically aiming at very high DS. The materials were clearly hydrophobic, however, there is still a need for more green solvents and processes.18,19 Also a heterogeneous method has been used to hydrophobize cellulose fibers with a wide range of DS of different fatty acids.20 However, in most cases the material was still hydrophilic i.e. CA < 90°. Recently, also polycaprolactone (PCL) has been grafted onto cellulose nanocrystals leading to increased hydrophobicity, but also in this case the material was still hydrophilic.21 By dipping cellulose film into alkyl ketene dimer (AKD), a common non-covalent hydrophobizing chemical, moderate hydrophobicity was achieved.22
High contact angles can be achieved not only by the surface chemical modification but also using an interplay with surface topography.23,24 Sole chemical modification of a flat surface limits the contact angle CA near 120°, even using fluorination.25 Beyond that CA, tailored surface topography at several length scales is required, as has been approached by several routes.26 A well known example deals with lotus leaves containing hierarchical roughness of micro- and nanoscale structures.27 This has spurred extensive research towards superhydrophobic behaviour and self-cleaning materials,23 where also cellulose has been utilized.9–11,13–16 Previously, also open porous structures are shown to be useful for tuning the aqueous wettability.24,28,29
Recently, we demonstrated that native cellulose nanofibrils (microfibrillated cellulose, MFC) enable low density, highly porous and mechanically robust aerogels upon proper drying.30 Such aerogels have porosity at micro- and nanoscale, where hydrogen bonds are expected to stabilize the porous dried network against collapse. Then, the porosity and structures at different length scales were shown to promote hydrophilicity of the unmodified nanocellulose as well as hydrophobicity of the nanocellulose aerogels in the modified case.31,32 This encourages us to pay special attention to surface topographies and porosity in addition to the chemical surface modification method, in order to allow high aqueous contact angles using only bio-based materials. Therefore, the present work combines spontaneous surface structuring with biological and biocompatible materials, i.e., cellulose, fatty acid and ionic liquid, to pursue highly water repellent materials. The ionic liquids are attractive as they are nonvolatile and can be re-circulated, thus minimizing the solvent emissions.33 Here, a very low DS (<0.1) is aimed to leave unreacted hydroxyl groups to form a hydrogen bond network required for the gel formation and further for stable porous aerogels. In this way the approach opposes the classic paradigm where high contact angle is achieved by high DS of low energy compound with specifically designed roughness incorporated onto the surface by additional means (e.g. by templating, post processing, or adding nanoparticles).
Experimental
Materials
The starting material 2% nanofibrillated cellulose (NFC, also denoted as MFC) aqueous gel with a hemicellulose content of 13.8% was prepared at Innventia AB (Sweden) by a combined effect of enzymatic hydrolysis, mechanical shearing and high pressure homogenization, as reported in detail elsewhere.34,35 This was further dried in a vacuum oven for the synthesis. For the wetting behaviour NFC gel was also freeze-dried to form porous native cellulose aerogels, as described in detail elsewhere,30 and see also Preparation of films and aerogels. Stearic acid, thionyl chloride, methanol, ethanol and DMF were purchased from Fluka. Thionyl chloride was distilled prior the use and DMF was dried. 1-Allyl-3-methylimidazolium chloride, [Amim]Cl, was synthesised as described previously.36Stearoyl chloride was prepared from the corresponding free fatty acid with refluxing in thionyl chloride in the presence of a catalytic amount of DMF.Synthesis of stearoyl modified cellulose
Dried NFC (0.1 g, 0.6 mmol) was dissolved in [Amim]Cl by heating at 80 °C for 12 h under argon atmosphere. Pyridine (1.3 mL, 14.8 mmol) and stearoyl chloride (1.7 g, 6.0 mmol) were added into cellulose/[Amim]Cl solution. The reaction mixture was heated to 80 °C for 24 h. Additional amounts of pyridine (5 equivalents) were added in the course of reaction in order to ensure the homogeneous reaction. The reaction mixture was allowed to cool to room temperature. The regeneration of the stearoyl modified cellulose was performed from the mixture of water and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by stirring. The precipitate was filtered off and washed first using water, followed by ethanol and finally by methanol in order to remove traces of residual reagents and [Amim]Cl. The final purification was performed by stirring the precipitate in methanol for 24 h, followed by centrifuging the sample in methanol and decanting the solution. Wet stearoyl modified cellulose gel was placed into a sinter and washed with ethanol. The obtained material was kept and stored as a gel in ethanol prior to the preparation of films and aerogels. A small amount of the sample was dried for the structural analysis. 13C CP/MAS NMR (100 MHz): δ = 62 ppm (C-6), 74 ppm (C-2, 3, 5), 84 ppm (C-4) and 104 ppm (C-1), 173 ppm (C
1) by stirring. The precipitate was filtered off and washed first using water, followed by ethanol and finally by methanol in order to remove traces of residual reagents and [Amim]Cl. The final purification was performed by stirring the precipitate in methanol for 24 h, followed by centrifuging the sample in methanol and decanting the solution. Wet stearoyl modified cellulose gel was placed into a sinter and washed with ethanol. The obtained material was kept and stored as a gel in ethanol prior to the preparation of films and aerogels. A small amount of the sample was dried for the structural analysis. 13C CP/MAS NMR (100 MHz): δ = 62 ppm (C-6), 74 ppm (C-2, 3, 5), 84 ppm (C-4) and 104 ppm (C-1), 173 ppm (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
Preparation of films and aerogels
Stearyol modified cellulose films were prepared by drop casting from a 4% (w/w) ethanol mixture. The suspension was placed onto the clean glass plate surrounded by the round “press to seal” silicon isolator (Grace JTR20-0.5, Ø 20 mm). Stearyol modified cellulose aerogels were prepared from 4% (w/w) ethanol gel by supercritical CO2 drying using a CPD 030 Critical point dryer (BAL-TEK). Before the actual drying, ethanol was exchanged to liquid CO2 in six cycles with a rate of 30 min per cycle. Thereafter, the wet gel was left in liquid CO2 overnight. The specimen chamber was heated up to the critical point of 31 °C and 73.8 bar where the liquid CO2 is transformed into gas phase, and drying the nanocellulose gel to form an aerogel. A small collapse (<10%) was observed. Similarly, the regenerated but unmodified films and aerogels were prepared. The dried nanocellulose was dissolved in the [Amim]Cl for 24 h, regenerated and purified by the same procedures as for the stearoyl treated sample. For comparison, native NFC film was prepared as a reference by drop casting and evaporating water from a 1% (w/w) aqueous sample. NFC aerogels were prepared from 2% (w/w) aqueous gel by simple freeze-drying in the vacuum oven.30 The aqueous gel was placed on a mould and then inserted into a vacuum oven, thus leading to freezing and subsequent water removal by sublimation.Characterisation
13C CP/MAS NMR experiments were run with a Bruker Avance 400 FT NMR spectrometer equipped with a standard bore 4 mm dual CP/MAS probehead working at 100.62 MHz for carbon-13 and 400.13 MHz for proton, respectively. The sample was spun in a 4 mm outer diameter zirconia rotor at 8 KHz rate at 295 °C. The spectral width was 300 ppm and the number of data points in the time domain was 2 K (giving an acquisition time of 34 ms), which was zerofilled to 8 K prior to FT giving 3.7 Hz digital resolution in the spectra. The FIDs were multiplied with an exponential window function of 10 Hz prior to FT and the chemical shifts were referenced to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O resonance of glycine at 176.03 ppm. The contact time was varied in the limits of 2–4 ms and the relaxation delay in the limits 2–10 s, respectively. The number of scans after the overnight runs varied in the limits of 5000–10
O resonance of glycine at 176.03 ppm. The contact time was varied in the limits of 2–4 ms and the relaxation delay in the limits 2–10 s, respectively. The number of scans after the overnight runs varied in the limits of 5000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
Elemental analysis of C, H and N was carried out using an Eager EA 1110 CHNS-O elemental analyzer.
Thermal properties were measured with a Mettler Toledo STAR system equipped with a thermogravimetric analyzer (TGA) 850 thermobalance in the temperature range from 30 °C to 800 °C at a heating rate of 10 °C min−1. Two measurements for each sample type were measured.
The thickness and roughness analysis of the films were performed with a Dektak 6M Stylus Profiler (Veeco). The stylus was a standard 12.5 μm B-type red stylus. Dektak 32 Application Software was used to calculate the average step height (ASH) (thickness) and the average roughness (Ra). Six reference samples were measured for the native and modified cellulose films. The roughness analysis was performed by scanning dimensions ranging from 1 to 3 mm.
A JEOL JSM7500F field emission electron microscope (FE-SEM) was used to characterize the morphology of the aerogels. The samples were coated with gold by sputtering at 20 mA for a minute. The size analysis of the nanostructures and the pores was carried out with Digital Micrograph 3.8.2. software (Gatan Inc.).
The determination of wetting properties of films and aerogels was carried out with a CAM 200 Optical Contact Angle Meter (KSV Instruments) measuring the static contact angle. 3 or 5 μL droplet of MilliQ water was used for aerogels and films, respectively. Five reference samples at two different positions were measured for each sample type. The water CA values were calculated based on Young–Laplace equation using the CAM software. Water CA measurements were also conducted as a function of time. Images of the deposited droplet were first recorded for every second for 2 minutes to evaluate the initial wetting, then every 10 seconds as long as the droplet was detectable. For the native NFC aerogels, the fast mode was used, where the images were recorded every 40 milliseconds.
Results and discussion
Synthesis of cellulose stearoyl esters of low degree of substitution in ionic liquids
Mixing native NFC in 1-allyl-3-methylimidazolium chloride [Amim]Cl allowed clear solutions.36 Low concentration 0.01% (w/w) was selected to allow homogeneous reaction conditions in order to promote complete dissolution and to minimize the intermolecular interactions between the cellulose chains. The previous experience with microcrystalline cellulose suggested that [Amim]Cl is a feasible reaction medium e.g. to perform acylation reaction with stearic acid in the presence of an activating agent (1-ethyl-3-(3′-dimethylaminopropyl)carbodiimide hydrochloride) yielding the DS value of 0.16.36 In the present work, a more reactive approach incorporating stearoyl chloride and pyridine was selected (Scheme 1). Only few articles on the acylation of cellulose with long aliphatic carboxylic acids in ionic liquids have been published, and they resulted in DS values between 0.12 and 1.54.37 The relative low values may be in part due to the low solubility of long chain aliphatic acid derivatives in some ionic liquids. Typically this has been regarded an undesirable phenomenon as a high degree of substitution (DS 3) has been pursued. However, in this study this behavior worked in our favour to obtain a low DS, leaving unreacted hydroxyl groups to form a hydrogen bond network required for the gel formation and further for stabile aerogels. Note that it has been observed that high DS cellulose esters require crosslinkers to form gel and thereafter aerogels.38,39 Here, crosslinkers are not needed.![Synthesis route to stearoyl modified cellulose in ionic liquid. Cellulose (1) is dissolved in [Amim]Cl (2) at 80 °C to yield cellulose–[Amim]Cl solution (3). Stearoyl chloride and pyridine are added into solution (3) to yield cellulose derivative (4). Anhydroglucopyranose unit (5) of cellulose represents the structure of cellulose chains.](/image/article/2011/PY/c0py00309c/c0py00309c-s1.gif) | ||
| Scheme 1 Synthesis route to stearoyl modified cellulose in ionic liquid. Cellulose (1) is dissolved in [Amim]Cl (2) at 80 °C to yield cellulose–[Amim]Cl solution (3). Stearoyl chloride and pyridine are added into solution (3) to yield cellulose derivative (4). Anhydroglucopyranose unit (5) of cellulose represents the structure of cellulose chains. | ||
The dried reaction product was characterized by high resolution solid state NMR (13C CP/MAS NMR) and elemental analysis to show the successful reaction between cellulose and stearoyl chloride. Characteristic NMR-shifts expected for stearoyl esters are observed (Fig. 1): the shift at 14 ppm is assigned to –CH3groups and the broad signal at 30–39 ppm is assigned to –CH2– groups of the stearoyl chains. A small peak at 173 ppm is observed, assigned to the carbonyl function of the ester bond (denoted by an arrow in Fig. 1).
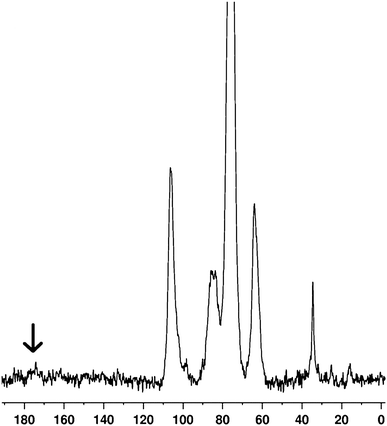 | ||
| Fig. 1 13C CP/MAS NMR spectrum of stearoyl modified cellulose showing chemical shifts typical for the anhydroglucopyranose unit of cellulose at C-6 (62 ppm), C-2, 3, 5 (74 ppm), C-4 (84 ppm), C-1 (104 ppm) and the chemical shifts from the alkyl chains are seen at 14 ppm (–CH3) and 30–39 ppm (–CH2–). | ||
More information can be extracted from the 13C CP/MAS spectrum, when comparing with the native crystalline NFC.34 There are clear changes in chemical shifts of the carbons of anhydroglucopyranose at 62 ppm (C-6), 74 ppm (C-2, 3, 5), 84 ppm (C-4) and 104 ppm (C-1). A precise signal assignment for C-2, C-3 and C-5 is not feasible due to overlapping signals.40 The C-4 signal is narrower for stearoyl modified cellulose without extensive splitting of the shift in two, as seen for native NFC. This splitting is caused by the differences in the crystallinity of native NFC.34 It is known that the more crystalline segments give rise to a chemical shift at around 87–90 ppm whereas that of the amorphous region can be seen at 80–86 ppm.41 For stearoyl modified cellulose, only one signal at 84 ppm is obtained which corresponds to an amorphous material. Also, for the native NFC, the chemical shift for C-1 from hemicellulose has been reported as a small broad signal at 102 ppm.34 This shift is absent in the stearoyl modified cellulose spectrum and only the chemical shift of anomeric C-1 from cellulose is obtained at 104 ppm. The C-6 shift of the modified cellulose is seen as a single broad signal whereas in the spectrum of the unmodified NFC, two signals are obtained.34 The chemical shifts for C-2, C-3, C-5 are observed as a single broad signal for the modified cellulose resembling the chemical shifts of pure cellulose (i.e. microcrystalline cellulose). Importantly, the above observations indicate the absence of hemicellulose in the reaction product stearoyl modified cellulose. Hemicelluloses in the native nanocellulose structure react in addition to cellulose due to the better solubility and reactivity. The subsequent stearoyl modified hemicelluloses remain in the [Amim]Cl solution even during the regeneration step and are ultimately washed away from the stearoyl modified cellulose. The reaction with hemicelluloses, in fact, decreases the available DS values for the cellulose. As observed here, ionic liquids can provide an approach also for the ‘extraction’ of hemicellulose from cellulose. This proves to be a simple method for the cellulose purification from other more soluble components such as in the current case, hemicellulose. 13C CP/MAS NMR qualitatively shows a low DS and this was further confirmed by elemental analysis, from which the DS was determined to be only 0.07. The heterogeneous nature of our starting material in the carbon amount detected by elemental analysis when the different batches were measured and the similarity of the cellulose and the substituent in terms of elemental level affect the accuracy.
The above homogeneous synthesis was selected in the present case instead of the heterogeneous one as it has previously been shown that heterogeneous modification of pulp fibers with fatty acid led only to moderate increase in hydrophobicity.20 Heterogeneous chemistry allows well established routes to derivatize cellulose to achieve short chain cellulose esters, whereas higher aliphatic esters remain more challenging due to limited reaction rates and lack of regioselectivity.42 Therefore the accessibility of the free hydroxyl groups controls both the selectivity and DS. In contrast, under homogeneous reaction conditions, the regioselectivity is determined by the reactivity differences of the three different hydroxyl groups. It is important that the substitution takes place throughout the cellulose structure and not only on the surface, to yield hydrophobically stable materials.
The present hydrophobization reaction using fatty acid under IL conditions can be carried out for any cellulose source, hence providing example of green homogeneous modification. The reason why the nanocellulose was used instead of e.g. pulp is to be able to show later the effect of porous aerogel structure on the wetting properties both at the hydrophilic stage and the hydrophobic state in every step of the process in the beginning (native cellulose)—at the interphase (dissolved and regenerated) and after modification. Only nanocellulose is able to form native cellulose aerogels.
Next the thermal stability was studied using TGA. For that aerogels of stearoyl modified cellulose were prepared based on supercritical drying (see the Experimental section) and compared with native NFC aerogels. TGA shows small but clear differences (see Fig. 2). The absorbed water is removed from the NFC aerogels near 100 °C, which is typical for highly moisture absorbing materials. However, essentially no weight loss is observed below ca. 200 °C in the stearoyl modified cellulose aerogels, indicating hydrophobicity, i.e. existence of alkyl tails. Above 200 °C, both stearoyl modified cellulose and NFC aerogels degrade roughly similarly. In the native NFC, the molecular level structure is stabilized by intermolecular hydrogen bonds between the parallel cellulose chains. In stearoyl modified cellulose, packing based on intermolecular hydrogen bonds is also possible. Derivatization of cellulose is assumed to take place exclusively at the C-6 positions due to their higher reactivity, as low DS is observed. However, the majority of C-6 hydroxyl groups remain still unsubstituted. This is expected to allow intermolecular hydrogen bondings between the C-6 and C-3 hydroxyl groups.
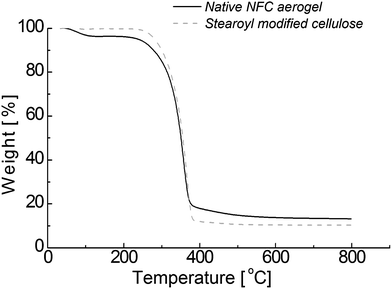 | ||
| Fig. 2 TGA curves of aerogels of stearoyl modified cellulose and NFC. | ||
Aqueous wetting properties of stearoyl modified cellulose films and aerogels
Aqueous wetting properties were next investigated for films and aerogels of stearoyl modified cellulose. First, as a reference a solid film with a thickness of 9.2 ± 0.6 μm was drop cast from an aqueous suspension of unmodified native NFC leading to an average roughness (Ra) of 547.8 ± 53.8 nm. The surface was very hydrophilic having a contact angle of only 35 ± 3.7° and wetting took place immediately (Fig. 3). Dissolved and regenerated but unmodified cellulose films were also prepared for the reference showing a similar contact angle of 36 ± 1.5°. On the other hand, a solid film with a thickness of 9.8 ± 0.7 μm was drop cast from stearoyl modified cellulose from ethanol. The clear increase in hydrophobicity of the modified film was observed, showing a contact angle of 103 ± 1°. A hydrophobic barrier is created onto the cellulose backbone as the long alkyl tails lower the surface energy. Therefore, the solid stearoyl modified cellulose film is quite hydrophobic, which is noteworthy taken the low DS. Upon esterification the average roughness (Ra) of the film is significantly increased to 1520.8 ± 77.1 which also promotes the high contact angle.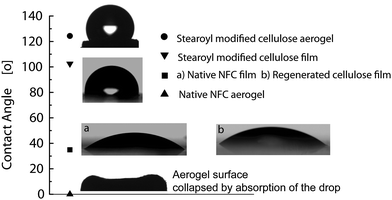 | ||
| Fig. 3 Static contact angle values. The stearoyl modified cellulose films displayed a contact angle of ca. 103° and the corresponding aerogels have a contact angle of 124°. By contrast, the native NFC film is very hydrophilic and the aerogel absorbs the droplet within milliseconds. | ||
Next aerogels were studied in order to study the effects of surface topography and porosity on the wetting. Classically, surface roughness enhances the hydrophilicity of hydrophilic surfaces as well as the hydrophobicity of hydrophobic surfaces due to the increase in available surface area. The unmodified native NFC aerogel is highly porous and consists of a network of layered structures and nanofibrils at different length scales (Fig. 4) and leads to immediate water spreading and absorption in milliseconds (Fig. 3). Therefore, the unmodified NFC aerogels showed higher aqueous wetting than the corresponding solid films due to the capillary effects connected with pores at different length scales. Also aerogels of the regenerated but unmodified cellulose made by supercritical CO2 drying were hydrophilic and absorbed the water droplet. Finally, porous aerogels from the stearoyl modified cellulose by supercritical CO2 drying showed no significant collapse during drying and porous materials were achieved. They were highly hydrophobic, resulting in contact angle values of 124 ± 1.5° (Fig. 3.). The porous structure led to ca. 20° increase in the contact angle in comparison to the corresponding solid films with the same chemical composition, indicating the contribution of the porosity and topography to the CA. Most notably, there was a remarkable change in hydrophobicity between the unmodified aerogels and the stearoyl modified cellulose aerogels with a CA change from 0° to 124°, respectively.
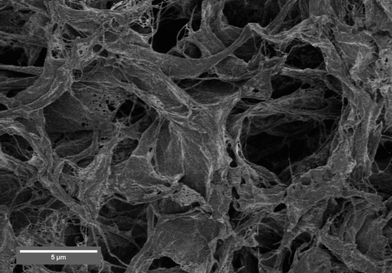 | ||
| Fig. 4 SEM image of native NFC aerogel prepared by vacuum freeze-drying from an aqueous gel. The aerogel consists of long native cellulose I nanofibrils that aggregate to porous sheet-like structures. The sheets are mutually connected to form a network skeleton and between the sheets there are micrometre range pores. | ||
The structures of the dissolved, stearoyl modified, and regenerated aerogels are shown in Fig. 5a and b. The morphology consists of fused structures that are irregular in their shape but rather uniform in the μm length scale. Structures at the smaller length scale are also observed, suggesting hierarchical morphology of the aerogels. On the other hand, the unmodified dissolved and regenerated cellulose aerogel (Fig. 5c and d) shows an order of magnitude smaller structures than the stearoyl modified cellulose aerogel. Therefore, the morphology and size of the structures are changed upon the esterification. Also, the characteristic aggregated nanofibrillar structure of the native NFC aerogels is not observed (Fig. 4) as the dissolution, regeneration and esterification lead to more amorphous cellulose.
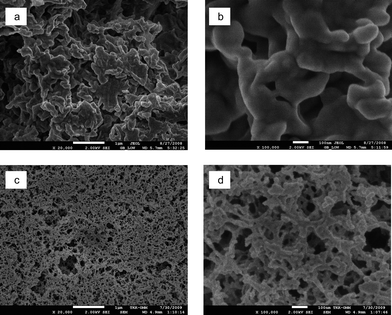 | ||
| Fig. 5 SEM images of the dissolved, stearoyl modified and regenerated cellulose aerogel (a and b) and dissolved and regenerated unmodified cellulose aerogel (c and d). The scale bar is 1 μm in (a) and (c) and is 100 nm in (b) and (d). | ||
Importantly, the present materials exhibited higher hydrophobicity with increased contact angle than the previously reported cellulose materials that have been derivatized with purely biocompatible groups. There was only moderate increase in hydrophobicity with PCL grafted cellulose nanocrystal films and TEMPO-oxidized AKA treated cellulose films, increasing the contact angle to 75° and to 94°, respectively.21,22 The contact angle values of the present solid films increased from 35° to 103° upon the stearoyl modification, whereas the heterogeneously modified stearoyl cellulose pulp with the corresponding DS did not reach the hydrophobicity level but stayed under 90° (CA from 56° to 82°).20 When homogeneous synthesis was used to modify the cellulose with fatty acids, very high DS (1.7–3) was obtained.18,19 Naturally, increased hydrophobicity with contact angles up to 115° was achieved. However, as observed here, there is no need to aim high DS to achieve high water repellency. This is important concerning the low solubility of fatty acids and reactivity toward cellulosic hydroxyl groups. As the CA of the present stearoyl modified cellulose is further increased to 124° upon aerogel formation, they start to approach the hydrophobic levels reported for fluorinated7,8 and silylated5 cellulose compounds. This is achieved due to the spontaneously formed porous structures and surface topography at different length scales. The effect of hierarchical roughness on high contact angles was also utilized in the previous (super)hydrophobization studies of cellulose, in which macroscopic cellulose fibers provided the micronscale roughness, however, the appropriate nanoroughness was achieved by additional structuring schemes, e.g. by fluorinated nanostructures,10,12 nanoscale silicone,13gold nanoparticles,15 metal oxide–silane nanocoatings16 or etching of the fibers.11 We point that very high contact angles in cellulose have been published, however then the sustainability may not be achieved.
Time dependent wetting behaviour
To obtain more insight into the wetting properties, time-dependent contact angle measurements were performed (Fig. 6). Stearoyl modified cellulose aerogels exhibit a high hydrophobicity level of CA >100° over 10 minutes, which is an uncommonly long time for such low DS, nonfluorinated samples. By contrast, the corresponding solid stearoyl modified cellulose film showed smaller hydrophobicity with an initial contact angle value of 104° and maintained hydrophobicity (>90°) for a few minutes. This qualitatively indicates the favourable interplay of surface modification and surface texture to promote the CA stability. In the case of NFC solid film, the water droplet spreads as soon as it enters the surface, showing a low contact angle of 36°. While the droplet on the stearoyl modified cellulose film did not show spreading and stayed hydrophobic, the droplet on the native NFC film continued spreading and was absorbed. As references, the dissolved, regenerated but unmodified cellulose aerogel, as well as NFC aerogel, absorb the water droplet very fast and are not plotted in Fig. 6.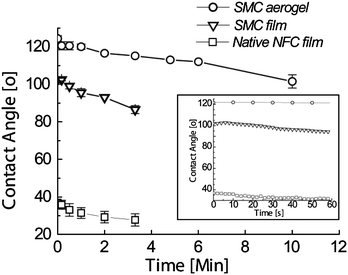 | ||
| Fig. 6 Time dependent wetting behaviour of the native NFC film, the stearoyl modified cellulose (SMC) film and the corresponding aerogel. | ||
Regarding the interpretation, two effects become relevant: spreading-absorption and evaporation.43 The decrease in CA values can be caused by the hemi-wicking phenomenon typical for textured/rough hydrophilic surfaces.44,45 It is an intermediate phase between spreading and imbibition of the drop in a wetting. The wetting is only partial and the contact angle is decreased, but remains strictly larger than zero, because of the presence of edges of the surface textures and hydrophobic. However, during long CA measurement, the decrease in the CA values of the stearoyl modified cellulose films and aerogels as a function of time can be explained mainly by the evaporation of the water from the small drop. The native NFC films showed immediate spreading of the water droplet giving rise to a very low contact angle and following absorption within minutes, not to mention the native aerogels which absorbed the droplet in milliseconds. However, at the same time the stearoyl modified cellulose films and aerogels showed still high hydrophobicity.
Despite the very low alkyl tail content, the time dependent contact angle measurements suggested superior behaviour over the previous bio-based cellulose based materials in stability.20–22 Only very low stearoyl tail coverage is sufficient to create a hydrophobic barrier and improve stability, which we expect to be due to the combined effect of the favourable homogeneous synthesis conditions, functionalizing the material throughout and not only on the surface, and the porous structure.
Water repellency
Finally, we address the stability of the stearoyl modified cellulose aerogels upon immersion in water (Fig. 7). Previously, it was observed the native NFC aerogels remained stable upon immersion in organic solvents but upon immersing in water for 24 h they can swell slightly and disintegrate if handled.30 Therefore, aerogel stabilization is needed. Herein, it was shown that stearoyl functionalisation led to stable water repellent aerogel material even upon water immersion. The stearoyl modified cellulose aerogels did not sink but floated on the water, maintained their dimensions and did not disintegrate even after immersing the sample for 24 h in water. Indirectly, floating suggests that air can be trapped within the aerogel structure. Secondly, stearoyl modified cellulose aerogels did not shrink and collapse after drying in ambient conditions as the unmodified native NFC aerogels do. This indicates that the alkyl tail coverage is sufficient to inhibit water absorption and the subsequent collapse upon drying. This supports the interpretation that the decrease in contact angle as a function of (long) time is caused mainly by the evaporation and following decrease in the droplet size. This observation highlights the application potential of this material as a moisture/water barrier. This property is required for example in electronic and optical applications, where dimensional instability of hydrophilic cellulose would otherwise prevent its use.46 | ||
| Fig. 7 Water immersion test. (A) Porous, 3 mm thick stearoyl modified cellulose aerogel sample was immersed in water. (B) The sample floated on the water for 24 h. (C) After 24 h the sample was lifted from water. It maintained its dimensions. (D) The shiny surface, which was in the water, indicates the hydrophobic barrier and shows how water forms droplets and thin layer on the surface instead of absorption. (E) After drying in the air, the sample did not collapse. | ||
Conclusions
We have demonstrated how the hierarchical porous aerogel structure combined with long stearoyl tails resulted in significant improvement in water repellency of cellulose upon low DS homogeneous modification. Biocompatible materials and green ionic liquid processing techniques are utilized. The concept allows stearoyl modified cellulosic aerogels after regeneration and supercritical CO2 drying with a high aqueous contact angle of 124°. Such a concept can be a sustainable alternative for fluorine-based approaches, which are frequently used for water repellency. Stability of the stearoyl modified aerogels was demonstrated by immersing the samples in water for 24 hours without disintegration or collapse after drying. The concept indicates that by tuning the structure and porosity of cellulose, even very low stearoyl tail coverage is sufficient to create a hydrophobic barrier and inhibit water absorption. We propose to develop the concept further for ecological packaging, coatings and insulation.Acknowledgements
Prof. Tom Lindstöm and Mikael Ankerfors (Innventia AB, Sweden) are acknowledged for providing the native NFC (MFC) hydrogel. We would like to express our gratitude also to Reijo Kauppinen from University of Jyväskylä, Laboratory of organic chemistry for his help in NMR and Dr Janne Halme from Aalto University School of Science and Technology for his assistance in profilometer measurements. We acknowledge Academy of Finland and TEKES. Partial funding from projects DesignCell of WoodWisdom network and Sustaincomp is also acknowledged at the final stages of the project.Notes and references
- A. G. Cunha and A. Gandini, Cellulose, 2010, 17, 875–889 CrossRef CAS.
- L. Heux, G. Chauve and C. Bonini, Langmuir, 2000, 16, 8210–8212 CrossRef CAS.
- J. Araki, M. Wada and S. Kuga, Langmuir, 2001, 17, 21–27 CrossRef CAS.
- C. Gousse, H. Chanzy, M. L. Cerrada and E. Fleury, Polymer, 2004, 45, 1569–1575 CrossRef CAS.
- M. Andresen, S.-L. Johansson, B. S. Tanem and P. Stenius, Cellulose, 2006, 13, 665–677 CrossRef CAS.
- A. G. Cunha, C. Freire, A. Silvestre, C. P. Neto, A. Gandini, M. N. Belgacem, D. Chaussy and D. Beneventi, J. Colloid Interface Sci., 2010, 344, 588–595 CrossRef CAS.
- A. G. Cunha, C. Freire, A. Silvestre, C. P. Neto and A. Gandini, J. Colloid Interface Sci., 2006, 301, 333–336 Search PubMed.
- A. G. Cunha, C. Freire, A. Silvestre, C. P. Neto, A. Gandini, E. Orblin and P. Fardim, Biomacromolecules, 2007, 8, 1347–1352 CrossRef CAS.
- C. Aulin, A. Shchukarev, J. Lindqvist, E. Malmström, L. Wåberg and T. Lindström, J. Colloid Interface Sci., 2008, 317, 556–567 Search PubMed.
- D. Nyström, J. Lindqvist, E. Östmark, A. Hult and E. Malmström, Chem. Commun., 2006, 3594–3596 RSC.
- B. Balu, V. Breedveld and D. W. Hess, Langmuir, 2008, 24, 4785–4790 CrossRef CAS.
- G. Goncalves, P. A. A. P. Marques, T. Trindade, C. P. Neto and A. Gandini, J. Colloid Interface Sci., 2008, 324, 42–46 Search PubMed.
- S. Li, H. Xie, S. Zhang and X. Wang, Chem. Commun., 2007, 4857–4859 RSC.
- S. Li, S. Zhang and X. Wang, Langmuir, 2008, 24, 5585–5590 Search PubMed.
- T. Wang, X. Hu and S. Dong, Chem. Commun., 2007, 1849–1851 RSC.
- S. Li, Y. Wei and J. Huang, Chem. Lett., 2010, 39, 20–21 CrossRef CAS.
- H. Kwatra, J. Caruthers and B. Tao, Ind. Eng. Chem. Res., 1992, 31, 2647–2651 Search PubMed.
- C. Vaca-Carcia, S. Thiebaud, M. E. Borredon and G. Conzzelino, J. Am. Oil Chem. Soc., 1998, 75, 315–319 CrossRef CAS.
- L. Crépy, L. Chaveriat, J. Banoub, P. Martin and N. Joly, ChemSusChem, 2009, 2, 165–170 CrossRef CAS.
- C. S. R. Freire, A. J. D. Silvestre, C. P. Neto, M. N. Belgacem and A. Gandini, J. Appl. Polym. Sci., 2006, 100, 1093–1102 CrossRef CAS.
- Y. Habibi and A. Dufresne, Biomacromolecules, 2008, 9, 1974–1980 CrossRef CAS.
- H. Fukuzumi, T. Saito, T. Iwata, Y. Kumamoto and A. Isogai, Biomacromolecules, 2009, 10, 162–165 CrossRef CAS.
- X. Zhang, F. Shi, J. Niu, Y. Jiang and Z. Wang, J. Mater. Chem., 2008, 18, 621–633 RSC.
- D. Quéré, Annu. Rev. Mater. Res., 2008, 38, 71–99 CrossRef CAS.
- T. Nishino, M. Meguro, M. Nakamae and Y. Ueda, Langmuir, 1999, 15, 4321–4323 CrossRef CAS.
- X. Feng and L. Jiang, Adv. Mater., 2006, 18, 3063–3078 CrossRef CAS.
- W. Barthlott and C. Neinhuis, Planta, 1997, 202, 1–8 CrossRef CAS.
- Y. Li, W. Cai, B. Cao, G. Duan and F. Sun, Nanotechnology, 2006, 17, 238–243 CrossRef CAS.
- N. Shirtcliffe, G. McHale, M. I. Newton, C. C. Perry and P. Roach, Chem. Commun., 2005, 3135 RSC.
- M. Pääkkö, J. Vapaavuori, R. Silvennoinen, H. Kosonen, M. Ankerfors, T. Lindström, L. A. Berglund and O. Ikkala, Soft Matter, 2008, 4, 2492–2499 RSC.
- M. Kettunen, R. J. Silvennoinen, N. Houbenov, A. Nykänen, J. Ruokolainen, J. Sainio, V. Pore, M. Kemell, M. Ankerfors, T. Lindström, M. Ritala, R. H. A. Ras and O. Ikkala, Adv. Funct. Mater., 2010, 21, 510–517 Search PubMed.
- H. Jin, M. Kettunen, A. Laiho, H. Pynnönen, J. Paltakari, A. Marmur, O. Ikkala and R. H. A. Ras, Langmuir, 2010, 27, 1930–1934 Search PubMed.
- O. El Seoud, A. Koshella, L. C. Fidale, S. Dorn and T. Heintze, Biomacromolecules, 2007, 8, 2629 CrossRef CAS.
- M. Pääkkö, M. Ankerfors, H. Kosonen, A. Nykänen, S. Ahola, M. Österberg, J. Ruokolainen, J. Laine, P. T. Larsson, O. Ikkala and T. Lindström, Biomacromolecules, 2007, 8, 1934–1941 CrossRef CAS.
- M. Henriksson, G. Henriksson, L. A. Berglund and T. Lindström, Eur. Polym. J., 2007, 43, 3434–3441 CrossRef CAS.
- M. Granström, J. Kavakka, A. King, J. Majoinen, V. Mäkelä, J. Helaja, S. Hietala, T. Virtanen, S.-L. Maunu, D. S. Argyropoulos and I. Kilpeläinen, Cellulose, 2008, 15, 481–488 CrossRef.
- S. Barthel and T. Heinze, Green Chem., 2006, 8, 301–306 RSC.
- C. Tan, B. M. Fung, J. K. Newman and C. Vu, Adv. Mater., 2001, 13, 644–646 CrossRef CAS.
- F. Fischer, A. Rigacci, R. Pirard, S. Berthon-Fabry and P. Achard, Polymer, 2006, 47, 7636–7645 CrossRef CAS.
- S. Berlioz, S. Molina-Boisseau, Y. Nishiyama and L. Heux, Biomacromolecules, 2009, 10, 2144–2151 CrossRef CAS.
- F. Horii, A. Hirai and R. Kitamura, Polym. Bull., 1982, 8, 163–170 Search PubMed.
- K. J. Edgar, C. M. Buchanan, J. S. Debenham, P. A. Rundquist, B. D. Seiler, M. C. Shelton and D. Tindall, Prog. Polym. Sci., 2001, 26, 1605–1688 CrossRef CAS.
- L. Wågberg, Nord. Pulp Pap. Res. J., 2000, 15, 598–606 Search PubMed.
- J. Bico, C. Tordeux and D. Quéré, Europhys. Lett., 2001, 55, 214–220 CrossRef CAS.
- C. W. Extrand, S. I. Moon, P. Hall and D. Schmidt, Langmuir, 2007, 23, 8882–8890 Search PubMed.
- M. Nogi, S. Iwamoto, A. N. Nakagaito and H. Yano, Adv. Mater., 2009, 20, 1595–1598 Search PubMed.
Footnotes |
| † These authors have equal contribution to the paper. |
| ‡ Present address: BASF SE, GCI/R-M311, 67056 Ludwigshafen, Germany. |
| This journal is © The Royal Society of Chemistry 2011 |
