Polythiophene–block–poly(γ-benzyl L-glutamate): synthesis and study of a new rod–rod block copolymer†‡
Zong-Quan
Wu
ab,
Robert J.
Ono
a,
Zheng
Chen
a,
Zicheng
Li
a and
Christopher W.
Bielawski
*a
aDepartment of Chemistry and Biochemistry, The University of Texas at Austin, 1 University Station, A5300, Austin, TX 78712, USA. E-mail: bielawski@cm.utexas.edu
bSchool of Chemical Engineering, Hefei University of Technology, Hefei, Anhui 230009, P. R. China
First published on 28th October 2010
Abstract
Coupling of ethynyl terminated poly(3-hexylthiophene) with azide terminated poly(γ-benzyl L-glutamate) afforded the respective block copolymer in good yield and high purity; this material was found to self assemble into hierarchal structures in solution and in the solid state.
Efforts directed toward conjugated polymers (CPs) have experienced tremendous growth in recent years due to advancements in applicable technologies, including: organic photovoltaics (OPVs),1 light emitting devices (OLEDs),2 and field-effect transistors (OFETs).3 An outstanding challenge is the ability to control the patterning of active materials on length scales mandated by the charge separation and shuttling processes of the particular application, generally in the 10–100 nm range. A promising solution is to merge homopolymers with desirable self-assembling properties with those that exhibit useful electronic properties into block copolymers that afford controlled, highly ordered morphologies.4
Over the past decade, a broad range of rod–coil block copolymers, where the former block is typically a CP and the latter is used to guide self assembly, have been developed.5 In contrast, analogous rod–rod block copolymers have been relatively less studied.6 While this deficiency is at least partially due to synthetic challenges in accessing such materials, they offer the possibility of affording unique polymer morphologies. Additionally, the effect of replacing flexible, coil-type segments with rigid-rods on the self-assembly, and ultimately on the solid state structures, of semiconducting block copolymers is relatively unknown. We surmise that the tendencies for rigid-rod segments to self-organize7 will ultimately lead to morphologies that are distinct from those obtained from more conventional rod–coil block copolymers.
Many polypeptides are known to exhibit rod-like structures and have been used to achieve morphological control in macromolecules.8–10 Poly(γ-benzyl L-glutamate) (PBLG), in particular, is a polypeptide that can be readily prepared via the ring opening polymerization (ROP) of its corresponding N-carboxyanhydride (NCA),11 and is known to form well-defined rod-like α-helical secondary structures in solution as well as in the solid-state.12 Moreover, the use of PBLG as a directing component in block copolymers is well established. For instance, Mezzenga reported a series of PBLG–polyfluorene–PBLG triblock copolymers and showed that changes in copolymer morphology could be correlated with the solvent induced α-helical-to-coil transitions of the PBLG blocks.13 Likewise, others have shown that PBLG can be readily adopted into a variety of block copolymers that are capable of undergoing self-assembly to form micellar or vesicular aggregates.12,14,15
Regioregular poly(3-hexylthiophene) (P3HT), a π-CP well known for both its excellent electronic properties and synthetic versatility, has also been incorporated into block copolymers as a means to influence self-assembly.16,17 Following seminal reports by McCullough describing the synthesis and characterization of P3HT–polyacrylate and P3HT–polystyrene block copolymers,18 a host of novel and functional P3HT-containing block copolymers have since been reported.5 For example, Botiz and Darling19 disclosed a block copolymer comprised of P3HT and the base-labile poly(L-lactide). The latter material was etched away following self-assembly which afforded fibrillar domains of P3HT surrounded by vacancies that were subsequently filled with electron accepting fullerenes.
Seeking to integrate the attractive properties of the aforementioned materials and to expand upon emerging classes of CP/polypeptide copolymers,13 we describe herein the synthesis and self-assembly characteristics of P3HT–block–PBLG. To the best of our knowledge this represents the first example of a polythiophene–polypeptide diblock copolymer.§
As summarized in Scheme 1, ethynyl functionalized P3HT (P3HT–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) (1) was prepared from 2,5-dibromo-3-hexylthiophene and isopropylmagnesium chloridevia a Ni-catalyzed Grignard metathesis (GRIM) polymerization according to literature procedures.17 After allowing the reaction to proceed for 10 min at room temperature (rt), ethynylmagnesium bromide was added which simultaneously installed an ethynyl end-group and quenched the polymerization reaction. Using this method, a variety of P3HT–C
CH) (1) was prepared from 2,5-dibromo-3-hexylthiophene and isopropylmagnesium chloridevia a Ni-catalyzed Grignard metathesis (GRIM) polymerization according to literature procedures.17 After allowing the reaction to proceed for 10 min at room temperature (rt), ethynylmagnesium bromide was added which simultaneously installed an ethynyl end-group and quenched the polymerization reaction. Using this method, a variety of P3HT–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH homopolymers with different number average molecular weights (Mns) were synthesized by adjusting the initial monomer to catalyst ratio (see Table 1). Following precipitation from methanol, the desired materials were isolated in 60–80% yields by filtration. Analysis of the isolated polymers by size exclusion chromatography (SEC) showed narrow molecular weight distributions characteristic of GRIM polymerizations, and the incorporation of the ethynyl end-group was confirmed by 1H NMR spectroscopy (diagnostic signal at δ = 3.52 ppm, CDCl3) and FTIR spectroscopy (υC
CH homopolymers with different number average molecular weights (Mns) were synthesized by adjusting the initial monomer to catalyst ratio (see Table 1). Following precipitation from methanol, the desired materials were isolated in 60–80% yields by filtration. Analysis of the isolated polymers by size exclusion chromatography (SEC) showed narrow molecular weight distributions characteristic of GRIM polymerizations, and the incorporation of the ethynyl end-group was confirmed by 1H NMR spectroscopy (diagnostic signal at δ = 3.52 ppm, CDCl3) and FTIR spectroscopy (υC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C = 2098 cm−1, KBr).¶
C = 2098 cm−1, KBr).¶
 | ||
Scheme 1 Synthesis of P3HT–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (1) and PBLG–N3 (2) and their respective block copolymer P3HT–block–PBLG (3). CH (1) and PBLG–N3 (2) and their respective block copolymer P3HT–block–PBLG (3). | ||
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (1), PBLG–N3 (2), and the corresponding block copolymers P3HT–block–PBLG (3)
CH (1), PBLG–N3 (2), and the corresponding block copolymers P3HT–block–PBLG (3)
| Sample | [M]0/[Ni]0a | M n (kDa)b | PDIb | Isolated yield (%) |
|---|---|---|---|---|
| a Initial monomer (2,5-dibromo-3-hexylthiophene) to catalyst (Ni(dppp)Cl2) (dppp = 1,3-bis(diphenylphosphino)propane) ratio. b M n and polydispersity index (PDI) were determined by SEC (THF as the eluent, UV-vis detection at 450 nm). It has been shown that molecular weight determinations of P3HT using SEC against polystyrene standards are overestimated.23 c M n and PDI were determined by SEC (DMF as the eluent, refractive index detection). d The relatively broad PDIs measured for 3c and 3d may be due to the presence of small amounts of unreacted homopolymers and/or the formation of triblock copolymers. | ||||
| 1a | 75/1 | 12.1 | 1.10 | 80 |
| 1b | 45/1 | 7.24 | 1.14 | 71 |
| 1c | 25/1 | 3.79 | 1.26 | 60 |
| 1d | 54/1 | 8.47 | 1.26 | 75 |
| 2a c | — | 11.7 | 1.07 | 77 |
| 2b c | — | 9.08 | 1.27 | 76 |
| 2c c | — | 4.45 | 1.13 | 65 |
| 3a (1a + 2a) | — | 21.5 | 1.22 | 72 |
| 3b (1b + 2b) | — | 15.9 | 1.32 | 67 |
| 3c (1c + 2c)d | — | 9.67 | 1.60 | 70 |
| 3d (1d + 2c)d | — | 12.7 | 1.64 | 60 |
Separately, azide end-functionalized PBLG (2) of varying Mns and narrow molecular weight distributions were prepared via the polymerization of γ-benzyl-L-glutamate NCA using 1-azido-3-aminopropane as the initiator.12a The ROPs were carried out in DMF at rt for 40 h under nitrogen, after which the resulting viscous solutions were poured into excess diethyl ether. The precipitated polymers were then collected viafiltration as white solids in good overall yields (60–70%) and characterized by SEC, as well as 1H NMR and FTIR spectroscopy, the latter of which revealed a signal diagnostic of azides (υN3 = 2100 cm−1).
With the end functionalized homopolymers P3HT–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH and PBLG–N3 in hand, subsequent efforts shifted toward linking these materials together. Using modified literature procedures,20copper(I) bromide and N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA) were employed as the catalyst/ligand and the cycloaddition reactions were performed in THF at 55 °C. Although relatively long reaction times were required (3 d), a slight excess (1.2 equiv) of PBLG-N3 was found to drive the conversion of P3HT–C
CH and PBLG–N3 in hand, subsequent efforts shifted toward linking these materials together. Using modified literature procedures,20copper(I) bromide and N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA) were employed as the catalyst/ligand and the cycloaddition reactions were performed in THF at 55 °C. Although relatively long reaction times were required (3 d), a slight excess (1.2 equiv) of PBLG-N3 was found to drive the conversion of P3HT–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH to the desired P3HT–block–PBLG (3) product. After the P3HT–C
CH to the desired P3HT–block–PBLG (3) product. After the P3HT–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH was consumed, as monitored by removing aliquots and analyzing by SEC, the resulting copolymers were isolated viafiltration after precipitation from methanol. Thorough washing of the precipitated solids with methanol and DMF effectively removed residual PBLG homopolymer and afforded 3 in 60–72% isolated yields. 1H NMR spectroscopy of the isolated products revealed signals attributable to both coupling partners along with the disappearance of the signal assigned to the alkynyl moiety (δ = 3.52 ppm, CDCl3). FTIR spectroscopy also supported successful coupling of the homopolymers, as evidenced by the disappearance of the diagnostic υC
CH was consumed, as monitored by removing aliquots and analyzing by SEC, the resulting copolymers were isolated viafiltration after precipitation from methanol. Thorough washing of the precipitated solids with methanol and DMF effectively removed residual PBLG homopolymer and afforded 3 in 60–72% isolated yields. 1H NMR spectroscopy of the isolated products revealed signals attributable to both coupling partners along with the disappearance of the signal assigned to the alkynyl moiety (δ = 3.52 ppm, CDCl3). FTIR spectroscopy also supported successful coupling of the homopolymers, as evidenced by the disappearance of the diagnostic υC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH and υN3 signals.‡ Moreover, the SEC traces of the P3HT–block–PBLG copolymers were monomodal with narrow molecular weight distributions that correlated well with their expected molecular weights (Fig. 1A and Table 1).
CH and υN3 signals.‡ Moreover, the SEC traces of the P3HT–block–PBLG copolymers were monomodal with narrow molecular weight distributions that correlated well with their expected molecular weights (Fig. 1A and Table 1).
 | ||
Fig. 1 (A) Representative SEC traces of P3HT–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (1b) and the corresponding block copolymer P3HT–block–PBLG (3b). (B) DSC thermograms of P3HT homopolymer (Mn = 8.17 kDa) (top), P3HT–block–PBLG (3d) (middle), and PBLG–N3homopolymer (2c) (bottom) (heating rate: 10 °C min−1; atmosphere: N2). CH (1b) and the corresponding block copolymer P3HT–block–PBLG (3b). (B) DSC thermograms of P3HT homopolymer (Mn = 8.17 kDa) (top), P3HT–block–PBLG (3d) (middle), and PBLG–N3homopolymer (2c) (bottom) (heating rate: 10 °C min−1; atmosphere: N2). | ||
Finally, efforts were directed toward studying the self-assembling characteristics of P3HT–block–PBLG in solution and in the solid state. Slow addition of DMF (via syringe pump) to a stirred THF solution of 3c ([3c]0 = 1.0 mg mL−1) induced a color change from bright orange to purple (λmax: 442 → 484 nm), consistent with the aggregation of P3HT chains.21 Following evaporation of the solvent, analysis of the resulting dried aggregates by transmission electron microscopy (TEM) revealed the formation of spherical particles having an average diameter of 287 ± 52 nm (see Fig. 2A). Combined with the UV-vis data, these results suggested to us that aggregates containing a P3HT core and a PBLG shell were formed in solution.
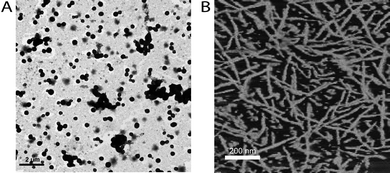 | ||
| Fig. 2 (A) TEM image of nanoparticles (stained with 1 wt% aqueous solution of phosphotungstic acid) formed from 3c. (B) Tapping mode AFM phase image of 3c spin cast from CHCl3 onto a Si wafer. | ||
In the solid state, analysis of P3HT–block–PBLG using differential scanning calorimetry (DSC) revealed that the material was capable of undergoing phase separation, as glass transition temperatures (Tg) assignable to both P3HT and PBLG phases were observed (Fig. 1B). Moreover, FTIR analysis of a film casted from a CHCl3 solution of 3c revealed that the PBLG segment of the copolymer adopted a typical, α-helical, rod-like structure,‡ as evidenced by the amide I absorption energies observed in the 1650–1660 cm−1 region.||12e No indication of β-sheet or random coil secondary structures were observed. The solid state morphology adopted by 3c was also investigated by tapping mode atomic force microscopy (AFM). As shown in Fig. 2B, films of 3c spin casted onto silicon wafers exhibited a nanofibrillar morphology consisting of a series of fibrous structures with persistent lengths > 200 nm. For comparison, thin films of 3a on silicon wafers were also examined by AFM and found to adopt more entangled, wormlike morphologies, suggesting to us that molecular weight influences the self-assembly or phase separation characteristics of P3HT–block–PBLG in the solid-state.‡
In conclusion, a series of polythiophene–polypeptide rod–rod diblock copolymers were synthesized via coupling of ethynyl-terminated P3HT with azide-terminated PBLG. The copolymers were obtained in high yields and were free of homopolymer impurities after a simple purification procedure. Detailed analyses of the copolymers revealed their abilities to self-assemble into spherical nanostructures in solution and into fibrillar morphologies in the solid state. Furthermore, the electronic properties inherent to P3HT and the α-helical secondary structure inherent to PBLG were preserved, as determined by UV-vis and IR spectroscopy, demonstrating that the desirable properties of both homopolymers were successfully integrated into a single block copolymer material. We believe that the straightforward synthetic approach described herein will facilitate the systematic study and applications of P3HT–block–PBLG, particularly in OPVs and related applications.
The material presented is based upon work supported as part of the program “Understanding Charge Separation and Transfer at Interfaces in Energy Materials (EFRC:CST),” an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Basic Energy Sciences under Award Number DE-SC0001091.
Notes and references
- S. Gunes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324–1338 CrossRef; G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- N. C. Greenham, S. C. Moratti, D. D. C. Bradley, R. H. Friend and A. B. Holmes, Nature, 1993, 365, 628–630 CrossRef; A. C. Grimsdale, K. Leok Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897–1091 CrossRef CAS.
- H. Sirringhaus, Adv. Mater., 2005, 17, 2411–2425 CrossRef CAS; S. R. Forrest, Nature, 2004, 428, 911–918 CrossRef CAS.
- R. A. Segalman, B. McCulloch, S. Kirmayer and J. J. Urban, Macromolecules, 2009, 42, 9205–9216 CrossRef CAS; I. Botiz and S. B. Darling, Mater. Today, 2010, 13, 42–51 CrossRef CAS.
- M. C. Iovu, C. R. Craley, M. Jeffries-EL, A. B. Krankowski, R. Zhang, T. Kowalewski and R. D. McCullough, Macromolecules, 2007, 40, 4733 CrossRef CAS; M. C. Iovu, M. Jeffries-EL, E. E. Sheina, J. R. Cooper and R. D. McCullough, Polymer, 2005, 46, 8582 CrossRef CAS; D.-A. Dai, W.-C. Yen, Y.-H. Lee, C.-C. Ho and W.-F. Su, J. Am. Chem. Soc., 2007, 129, 11036 CrossRef CAS; T. Higashihara and M. Ueda, React. Funct. Polym., 2009, 69, 457 CrossRef CAS; M. G. Alemseghed, S. Gowrisanker, J. Servello and M. C. Stefan, Macromol. Chem. Phys., 2009, 210, 2007 CrossRef CAS; S.-J. Park, S.-G. Kang, M. Fryd, J. G. Saven and S.-J. Park, J. Am. Chem. Soc., 2010, 132, 9931 CrossRef CAS; Z. Li, R. J. Ono, Z.-Q. Wu and C. W. Bielawski, Chem. Commun., 2011 10.1039/c0cc02166k.
- U. Scherf, A. Gutacker and N. Koenen, Acc. Chem. Res., 2008, 41, 1086–1097 CrossRef CAS; U. Scherf, S. Adamczyk, A. Gutacker and N. Koenen, Macromol. Rapid Commun., 2009, 30, 1059–1065 CrossRef CAS.
- J. Hollinger, A. A. Jahnke, N. Coombs and D. S. Seferos, J. Am. Chem. Soc., 132, pp. 8546–8547 Search PubMed.
- T. J. Deming, Adv. Drug Delivery Rev., 2002, 54, 1145–1155 CrossRef CAS.
- B. D. Olsen and R. A. Segalman, Mater. Sci. Eng., R, 2008, 62, 37–66 CrossRef.
- Y.-b. Lim, K.-S. Moon and M. Lee, J. Mater. Chem., 2008, 18, 2909–2918 RSC.
- T. J. Deming, Nature, 1997, 390, 386–389 CrossRef CAS.
- C. Schatz, S. Louguet, J. F. Le Meins and S. Lecommandoux, Angew. Chem., Int. Ed., 2009, 48, 2572–2575 CrossRef CAS; K. T. Kim, C. Park, G. W. M. Vandermeulen, D. A. Rider, C. Kim, M. A. Winnik and I. Manners, Angew. Chem., Int. Ed., 2005, 44, 7964–7968 CrossRef CAS; H. R. Marsden, J.-W. Handgraaf, F. Nudelman, N. A. J. M. Sommerdijk and J. Kros, J. Am. Chem. Soc., 2010, 132, 2370–2377 CrossRef CAS; X. Kong and S. A. Jenekhe, Macromolecules, 2004, 37, 8180–8183 CrossRef CAS; A. Sanchez-Ferrer and R. Mezzenga, Macromolecules, 2010, 43, 1093–1100 CrossRef.
- L. Rubatat, X. Kong, S. A. Jenekhe, J. Ruokolainen, M. Hojeij and R. Mezzenga, Macromolecules, 2008, 41, 1846–1852 CrossRef CAS; X. Kong and S. A. Jenekhe, Macromolecules, 2004, 37, 8180–8183 CrossRef CAS.
- K. T. Kim, G. W. M. Vandermeulen, M. A. Winnik and I. Manners, Macromolecules, 2005, 38, 4958–4961 CrossRef CAS.
- H. Tian, C. Deng, H. Lin, J. Sun, M. Deng, X. Chen and X. Jing, Biomaterials, 2005, 26, 4209–4217 CrossRef CAS.
- R. Miyakoshi, A. Yokoyama and T. Yokozawa, J. Am. Chem. Soc., 2005, 127, 17542–17547 CrossRef CAS; E. E. Sheina, J. Liu, M. C Iovu, D. W. Laird and R. D. McCullough, Macromolecules, 2004, 37, 3526–3528 CrossRef CAS.
- M. Jeffries-EL, G. Sauve and R. D. McCullough, Macromolecules, 2005, 38, 10346–10352 CrossRef CAS.
- J. Liu, E. Sheina, T. Kowalewski and R. D. McCullough, Angew. Chem., Int. Ed., 2002, 41, 329–332 CrossRef CAS.
- I. Botiz and S. B. Darling, Macromolecules, 2009, 42, 8211–8217 CrossRef CAS.
- Y. Tao, B. McCulloch, S. Kim and R. A. Segalman, Soft Matter, 2009, 5, 4219–4230 RSC; J. A. Opsteen and J. C. M. van Hest, Chem. Commun., 2005,(1), 57–59 RSC; B. S. Sumerlin and A. P. Vogt, Macromolecules, 2010, 43, 1–13 CrossRef CAS.
- U. Scherf, A. Gutacker and N. Koenen, Acc. Chem. Res., 2008, 41, 1086–1097 CrossRef CAS.
- H.-A. Klok, A. Rosler, G. Gotz, E. Mena-Osteritz and P. Bauerle, Org. Biomol. Chem., 2004, 2, 3541–3544 RSC.
- S. Holdcroft, J. Polym. Sci., Part B: Polym. Phys., 1991, 29, 1585–1588 CrossRef CAS; J. Liu, R. S. Loewe and R. D. McCullough, Macromolecules, 1999, 32, 5777–5785 CrossRef CAS.
Footnotes |
| † This paper is part of a Polymer Chemistry issue highlighting the work of emerging investigators in the polymer chemistry field. Guest Editors: Rachel O'Reilly and Andrew Dove. |
| ‡ Electronic supplementary information (ESI) available: Detailed experimental procedures as well as microscopy, chromatography, and spectroscopy data. See DOI: 10.1039/c0py00299b |
| § Oligothiophene–oligopeptide conjugates composed of discrete tetra(3-hexylthiophene) and GlyAlaGlyAlaGly pentapeptide sequences made via solid phase peptide synthesis have been reported.22 |
¶ Consistent with MALDI-TOF studies,17 end-group analysis of P3HT–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH by 1H NMR spectroscopy revealed that >90% of polymer chains were mono-functionalized. CH by 1H NMR spectroscopy revealed that >90% of polymer chains were mono-functionalized. |
| || The FTIR spectrum of PBLG-N3 homopolymer displayed similar characteristics in the amide I stretching regime.‡ |
| This journal is © The Royal Society of Chemistry 2011 |
