Multicompartment micelles from blends of terpolymers†‡
Jean-François
Gohy
*a,
Nathalie
Lefèvre
a,
Cécile
D'Haese
a,
Stephanie
Hoeppener
b,
Ulrich S.
Schubert
b,
Georges
Kostov
c and
Bruno
Améduri
c
aInstitute of Condensed Matter and Nanosciences (IMCN), Bio- and Soft Matter (BSMA), Université catholique de Louvain (UCL, Place Pasteur 1, 1348, Louvain-la-Neuve, Belgium. E-mail: jean-francois.gohy@uclouvain.be; Fax: +3210479269; Tel: +3210479269
bLaboratory of Organic and Macromolecular Chemistry, Friedrich-Schiller-University Jena, Humboldtstrasse 10, 07743, Jena, Germany
cIngénierie et Architecture Macromoléculaire, Institut Charles Gerhardt, Ecole Nationale Supérieure de Chimie de Montpellier (UMR 5253-CNRS), 8, rue de l'Ecole Normale, 34296, Montpellier Cedex 1, France
First published on 8th September 2010
Abstract
Poly(VDF-ter-HFP-ter-TFMA) terpolymers (in which VDF, HFP and TFMA stand for vinylidene fluoride, hexafluoropropylene, and α-trifluoromethacrylic acid, respectively) were synthesized by iodine transfer radical terpolymerization. These copolymers were further blended with a poly(S-b-2VP-b-EO) triblock terpolymer (in which S, 2VP and EO stand for styrene, 2-vinylpyridine and ethylene oxide, respectively) in N,N-dimethylformamide to lead to micelles containing a core formed of poly(VDF-ter-HFP) segments and TFMA/2VP hydrogen-bonded complexes, and a corona of PS and PEO chains. Depending on the content of VDF in the used poly(VDF-ter-HFP-ter-TFMA) terpolymers, microphase-separated fluorinated nanodomains can be visualized inside the core.
Introduction
The ability of block copolymers to self-assemble in selective solvents has allowed the formation of various well-defined nanostructures with tunable sizes and morphologies.1 Among those structures, multicompartment micelles have raised special interest since they can be considered to some extent as mimics of complex natural systems such as proteins and cell organelles.2 Distinct micellar compartments are generally generated by self-assembling block copolymers containing non-soluble hydrocarbon and fluorocarbon segments.2In this respect, fluoropolymers have been introduced in triblock terpolymers containing a water-soluble block and a hydrophobic hydrocarbon block in addition to the fluorocarbon block. This approach has been previously validated by several authors, including Laschewsky and co-workers for linear3 and comb-like4triblock copolymers and Lodge and co-workers for mikto-arm star copolymers.5 Since those works, many other reports have appeared in the literature on that topic. For example, we have recently proposed the use of metallo-supramolecular tetrablock quaterpolymers6 and of triblock terpoly(2-oxazoline)s7 as precursors of multicompartment micelles.
However, the synthetic routes towards fluoro-containing triblock terpolymers may be cumbersome. An alternative strategy to introduce fluoro-containing segments in block copolymer micelles consists in using block copolymer complexation between an hydrogenated triblock copolymer and a fluoro-containing (co)polymer. Indeed, it has been previously demonstrated that micellization can be triggered by introducing additional non-covalent interactions in an initially soluble block copolymer.8 The non-covalent complexes resulting from these interactions should be insoluble to induce micellization. Such insoluble complexes can be generated by mixing, in a non-selective solvent for all the individual blocks, two block copolymers, containing mutually interacting segments. This mixing process therefore may lead to insoluble non-covalent complexes, which further aggregate into micellar cores stabilized by the uncomplexed blocks. Such a strategy has been successfully implemented in both aqueous and non-aqueous solvents to create interesting stimuli-responsive systems, mainly using ionic interactions and hydrogen-bonding.8,9
In this contribution, we propose to prepare multicompartment micelles by blending a poly(S-b-2VP-b-EO) triblock with poly(VDF-ter-HFP-ter-TFMA) terpolymers in N,N-dimethylformamide (DMF). Hydrogen-bonding interaction should occur between the 2VP and TFMA units eventually leading to insoluble complexes that will further aggregate into micellar cores. Those micellar cores will be stabilized by polystyrene and poly(ethylene oxide) segments. Moreover, the VDF and HFP segments could microphase separate from the 2VP/TFMA complexes in the core, leading to multicompartment micelles.
Experimental
Materials
Vinylidene fluoride (VDF, bp = −82 °C), hexafluoropropylene (HFP, bp = −28 °C) were kindly offered by Solvay S.A. (Tavaux, France and Brussels, Belgium), while α-trifluoromethacrylic acid (TFMA) was donated by Tosoh F-Tech Company (Shunan, Japan). 1,6-Diodoperfluorohexane was purchased at Ugarit (Pierre-Benite, France). It was worked up with sodium thiosulfate and then distilled prior to use. Na2S2O8 (purity 99%), acetonitrile, dimethylformamide (DMF), tetrahydrofuran (THF), methanol, methylethylketone and dimethylacetamide (DMAc) of analytical grade were purchased from Aldrich Chimie (Saint Quentin-Fallavier, France). A poly(styrene)-block-poly(2-vinylpyridine)-block-poly(ethylene oxide) (PS13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-b-P2VP9000-b-PEO16
000-b-P2VP9000-b-PEO16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, the numbers in subscript represent the number averaged molar mass of each block) triblock terpolymer was purchased from Polymer Source Inc. (Montréal, Québec, Canada) and had a narrow polydispersity index of 1.1.
500, the numbers in subscript represent the number averaged molar mass of each block) triblock terpolymer was purchased from Polymer Source Inc. (Montréal, Québec, Canada) and had a narrow polydispersity index of 1.1.
Synthesis of poly(VDF-ter-HFP-ter-TFMA) terpolymers
Iodine transfer copolymerizations of VDF, HFP and TFMA were carried out in the presence of 1,6-diiodoperfluorohexane as chain transfer agent and initiated by Na2S2O8 at 80 °C. Example of P3 (Table 1): a 160 mL Hastelloy (HC-276) autoclave, equipped with inlet and outlet valves, a manometer and a rupture disk, was degassed and pressurized with 30 bar of nitrogen to check eventual leaks. Then, a 20 mmHg vacuum was operated for 30 min. Under vacuum, were transferred into the autoclave 0.230 g (0.001 mol) of Na2S2O8, 3.0 g (0.0067 mol) of 1,6-diiodoperfluorohexane (IC6F12I), 4.8 g (0.034 mol) of TFMA and 80.0 g of deionized water. Then, by double weighing, 6 g (0.40 mol) of HFP and then 10.0 g (0.10 mol) of VDF were introduced in the mixture. Afterwards, the autoclave was progressively heated to 80 °C, by carrying out plateau at 50, 60, 70 °C for 2 minutes. A small exotherm of ca. 5 °C was observed and then a sharp drop of pressure to 40 bars. After 6 h reaction, the autoclave was placed in an ice bath for about 60 minutes and 4 g of unreacted VDF and HFP were progressively released (yield 85%). After opening the autoclave, about 100 g of latex emulsion were obtained. The water was removed by lyophilization to lead to a white powder. Unreacted TFMA was eliminated by precipitation from methanol (yield = 65–70%). The sample was characterized by 19F and 1H NMR spectroscopies and SEC analysis. The poly(VDF-ter-HFP-ter-TFMA) terpolymer was soluble in polar solvents, such as acetone, DMF, dimethyl acetamide, DMSO, and methyl ethyl ketone. Average molar mass assessed by SEC = 3700 g mol−1 and by 19F NMR = 5900 g mol−1.| Sample | M n/g mol−1 | VDF (mol%) | HFP (mol%) | TFMA (mol%) | PDI |
|---|---|---|---|---|---|
| P1 | 5400 | 80 | 8 | 12 | 1.4 |
| P2 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
45 | 6 | 49 | 1.8 |
| P3 | 5900 | 46 | 1 | 53 | 1.5 |
Techniques
The composition and structure of the terpolymers obtained by iodine transfer terpolymerization were determined by 19F and 1H NMR spectroscopies. The NMR spectra were recorded on Bruker AC 250 or 400 (250 or 400 MHz) instruments, using deuterated acetone, dimethylsulfoxide (DMSO) or DMF as solvents and tetramethylsilane (TMS) (or CFCl3) as references for 1H (or 19F) nuclei. Coupling constants and chemical shifts are given in Hz and ppm, respectively. The experimental conditions for 1H (or 19F) NMR spectra were the following: flip angle 90° (or 30°), acquisition time 4.5 s (or 0.7 s), pulse delay 2 s (or 5 s), number of scans 16 (or 64), and a pulse width of 5 µs for 19F NMR. Size Exclusion Chromatography (SEC) analyses were performed with a Spectra-Physics apparatus equipped with two PLgel 5 µm Mixed-C columns from Polymer Laboratories and a Spectra Physics SP8430 Refractive Index (RI) detector. Dimethylformamide (DMF) containing 1.5 wt% LiCl was chosen as the eluent at T = 70 °C. Polystyrene (PS) standards were used for calibration.Preparation of the micelles
Micelles were prepared by mixing known amounts of poly(S-b-2VP-b-EO) with poly(VDF-ter-HFP-ter-TFMA) terpolymers. The amount of each polymer and the volume of DMF have been adjusted to obtain a concentration of 5 g L−1 after mixing. A TFMA/2VP 1/1 mol/mol stoichiometry was used for all samples. The micellar solutions were obtained by first dissolving one partner into DMF, followed by the addition of the second partner as a powder. The solutions were stirred for 15 minutes before measurements. Identical micelles were obtained whether poly(S-b-2VP-b-EO) is first dissolved and poly(VDF-ter-HFP-ter-TFMA) terpolymer was added as a powder, or the reverse.Dynamic light scattering
DLS measurements were performed on either a Malvern CGS-3 apparatus equipped with a He–Ne laser with a wavelength of 632.8 nm or an automated DynaPro™ Plate Reader™ from Wyatt Technology Corporation for measurements performed at different temperatures. Size distribution histograms were obtained by the CONTIN method. The polydispersity index (PDI) of the micelles was estimated from the Γ2/Γ12 ratio in which Γ1 and Γ2 represent the first and second cumulant, respectively.Transmission electron microscopy
Transmission electron microscopy (TEM) was performed on a LEO 922 microscope, operating at 200 kV accelerating voltage in bright field mode. The images were formed by unscattered electrons only. Samples for TEM experiments were prepared by casting a drop of the solution of micelles on a carbon-coated TEM grid. The radius (RTEM) of the micelles was calculated by averaging measurements performed on a minimum of 100 objects taken from different TEM pictures.Results and discussion
Synthesis of poly(VDF-ter-HFP-ter-TFMA) terpolymers
An interesting way to insert carboxylic acid functions in fluoropolymers arises from the copolymerization of VDF with comonomers containing acid groups. Actually, acrylic acid (AA) is too reactive to lead to satisfactory poly(VDF-co-AA) copolymers,10 but TFMA has already demonstrated an appropriate reactivity with VDF.11,12 In addition, the conventional free radical terpolymerization of TFMA with VDF and HFP was successfully carried out in organic solvent11 while TFMA radical homopolymerization failed.13,14Iodine transfer polymerization (ITP)15 has been chosen in this contribution since it is a suitable technique to control the polymerization of fluorinated olefins.16 The ITP terpolymerization of VDF with HFP and TFMA was carried out in aqueous solution, initiated by Na2S2O8 at 80 °C, in the presence of 1,6-diiodoperfluorohexane as the chain transfer agent (Scheme 1). Interestingly enough that reaction was carried out without any organic solvent. The accordingly obtained polymer chains were essentially composed of several oligo(VDF-co-HFP) blocks separated by one TFMA unit due to the non-propagation of TFMA.13,14 At the end of the copolymerization, the emulsion was freeze-dried to remove water. These products were precipitated from methanol and characterized by 1H and 19F NMR, and SEC. 19F NMR allowed us to determine the characteristic end-groups of the copolymers, which have been assigned to –CH2CF2I and –CF2CH2I, respectively (see ESI‡ for details). The presence of such end-groups can be exploited to incorporate a range of chemical functionalities,15 such as alcohol, carboxylic acid, azido, ester, allylic functions, or to synthesize block polymers.17,18 Analysis of the 19F NMR spectra also allowed to assess the composition of the terpolymers (see Table 1 and ESI‡ for further details). The terpolymerization of VDF, HFP and TFMA was also confirmed by 1H NMR (see ESI‡) while SEC allowed the determination of PDIs. These are close to 1.7–1.9 (Table 1) and indicate the pseudo-control of the radical polymerization. Also, the molecular weight of the terpolymers can be tuned by varying the concentration of the chain transfer agent. As expected, the increase of the TFMA concentration in the feed ratio favored an increase of the TFMA monomer in the copolymers. All the details concerning the characterization of the accordingly obtained copolymers are available in the ESI.‡
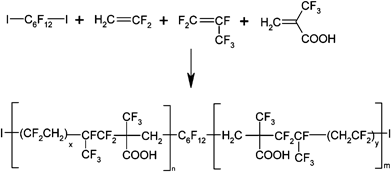 | ||
| Scheme 1 Iodine transfer terpolymerization of trifluoromethacrylic acid (TFMA) with vinylidene fluoride (VDF) and hexafluoropropylene (HFP) performed in the presence of 1,6-diiodoperfluorohexane. | ||
A series of three poly(VDF-ter-HFP-ter-TFMA) terpolymers that essentially differ from the content of incorporated TFMA has been further considered for complexation with poly(S-b-2VP-b-EO). Their molecular characteristic features are summarized in Table 1.
Micelles from poly(VDF-ter-HFP-ter-TFMA)/poly(S-b-2VP-b-EO) blends
The use of hydrogen bonds to trigger complexation and further aggregation of polymer blocks has recently emerged as a powerful strategy towards the formation of micellar structures in organic solvents.8 This methodology was previously used to control micellar structures of mixtures of poly(styrene-block-4-vinylpyridine) and poly(acrylic acid) (PAA) in various organic solvents.7 Moreover, it was possible to create micelles containing two different types of coronal chains by mixing a poly(S-b-2VP-b-EO) triblock terpolymer with either PAA or PAA-containing graft copolymers in DMF.19 In this case, a micellar core formed by 2VP/AA complexes was surrounded by a corona of mixed PEO and PS chains. In some cases, it was possible to induce segregation between the PS and PEO chains.19In this contribution, we would like to extent this strategy for the incorporation of fluorinated compartments into the micellar core. To this aim, a poly(S-b-2VP-b-EO) triblock has been complexed with poly(VDF-ter-HFP-ter-TFMA) terpolymers. The complexation has been achieved in DMF, a non-selective solvent for both poly(S-b-2VP-b-EO) and poly(VDF-ter-HFP-ter-TFMA) terpolymers. In this respect, DLS measurements on those terpolymers dissolved separately in DMF at a concentration of 5 g L−1 showed a very weak scattered intensity that revealed, after analysis of the DLS data, the presence of unimers. No signal indicating the formation of dense micellar structures was detected. The situation changed dramatically for the mixtures of poly(S-b-2VP-b-EO) with poly(VDF-ter-HFP-ter-TFMA) terpolymers. Indeed, all those solutions macroscopically displayed the characteristic bluish color of micellar solutions. Since the individual components do not show any aggregation, this indicates that complexation between 2VP and TFMA units results in insoluble complexes that further aggregate into micellar cores. This observation is in line with our previous results on poly(S-b-2VP-b-EO)/PAA mixtures in DMF.19 The accordingly obtained solutions were further analyzed by DLS and the formation of micellar structures was confirmed. The DLS data are summarized in Table 2.
| Sample | DLS | R TEM /nm | |
|---|---|---|---|
| R h/nm | PDI | ||
| P1/poly(S-b-2VP-b-EO) | 52.6 | 0.23 | 31 |
| P2/poly(S-b-2VP-b-EO) | 39.5 | 0.14 | 22 |
| P3/poly(S-b-2VP-b-EO) | 28.7 | 0.07 | 13 |
R h values reported in Table 2 were determined by extrapolation of the apparent Rh measured at 5, 2, 1 and 0.5 g L−1 to zero concentration. The Rh values were observed to increase as a function of the VDF + HFP content in the poly(VDF-ter-HFP-ter-TFMA) terpolymers (compare P1/poly(S-b-2VP-b-EO) to P2/poly(S-b-2VP-b-EO) and P3/poly(S-b-2VP-b-EO)) and by the molecular weight of the poly(VDF-ter-HFP-ter-TFMA) terpolymers for the same VDF content (compare P2/poly(S-b-2VP-b-EO) to P3/poly(S-b-2VP-b-EO)). The PDI of these micelles were rather broad (see values in Table 2), except for the P3/poly(S-b-2VP-b-EO) sample. This was confirmed by the CONTIN histograms that show rather broad, size distributions. The experimental intensity correlation function measured by DLS as well as the CONTIN histogram obtained after analysis of the DLS data are shown in Fig. 1 for the P1/poly(S-b-2VP-b-EO) mixture and in the ESI‡ for the other mixtures investigated in this paper.
 | ||
| Fig. 1 Experimental intensity correlation function (top) and CONTIN size distribution histogram (down) obtained for the P1/poly(S-b-2VP-b-EO) mixture in DMF at 1 g L−1. | ||
To get more details on the morphology of the micelles, transmission electron microscopy (TEM) experiments were performed. These results confirmed the formation of polydisperse spherical micelles for all the investigated poly(S-b-2VP-b-EO)/poly(VDF-ter-HFP-ter-TFMA) terpolymers mixtures. The average radii of the micelles (RTEM) measured by statistical analysis of the TEM pictures are reported in Table 2. RTEM is following the same trend as Rh, i.e. it increases with the amount of VDF + HFP in the micelles and with the molecular weight of the poly(VDF-ter-HFP-ter-TFMA) terpolymers for a same copolymer composition. RTEM is systematically smaller than Rh since TEM measurements have been performed on dried micelles. Micellar cores are thus essentially imaged by TEM. Interestingly enough, dark dots have been imaged for the micelles formed by the P1/poly(S-b-2VP-b-EO) blend containing the higher amount of VDF + HFP (see Fig. 2). Since fluorinated domains are well-known to give an increased electronic contrast in TEM experiments, those dots have been attributed to VDF + HFP microdomains microphase separated from the 2VP/TFMA hydrogen-bonded complexes in the micellar core. Multicompartment micelles are thus formed in this case. The existence of fluorinated nanodomains in those micelles has been confirmed by the selective encapsulation of a fluorophilic dye (see ESI‡).
 | ||
| Fig. 2 TEM image (left) of micelles formed by the P1/poly(S-b-2VP-b-EO) mixture. Focus on a multicompartmentalized core (right, scale bar is 40 nm). Fluorinated domains are represented in dark grey. | ||
Finally, the temperature dependence of the DLS data was investigated. Those experiments were realized in parallel at a concentration of 1 g L−1 in the temperature range from 20 °C to 60 °C. The measured apparent hydrodynamic radius (Rh,app) has been reported as a function of temperature, as shown in Fig. 3.
 | ||
| Fig. 3 Evolution of Rh,app as a function of temperature for the P2/poly(S-b-2VP-b-EO) (squares), poly(VDF-ter-HFP-ter-TFMA) terpolymers P3/poly(S-b-2VP-b-EO) (triangles) and P1/poly(S-b-2VP-b-EO) (dots) mixtures. | ||
From these data, it is clear that the micelles formed at room temperature reorganize into smaller structures at ca. 50 °C. It is remarkable that all the three investigated poly(S-b-2VP-b-EO)/poly(VDF-ter-HFP-ter-TFMA) terpolymers complexes seem to lead to the same structure at and above 50 °C (see Fig. 3). To gain more information, more accurate DLS measurements were realized at 50 °C with the Malvern CGS-3 equipment. The data are shown in Fig. 4. A sharp decrease in scattered intensity was noted in agreement with the formation of smaller, less dense and/or more solvated objects at 50 °C.
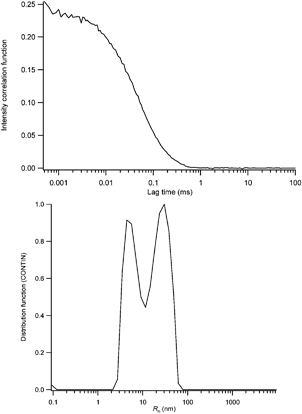 | ||
| Fig. 4 Experimental intensity correlation function (up) and CONTIN size distribution histogram (down) obtained for the P1/poly(S-b-2VP-b-EO) mixture in DMF at 50 °C. | ||
The average Rh of the objects was measured to be 13 nm in agreement with the data obtained in Fig. 3. Moreover, the CONTIN size distribution histogram reveals the presence of two populations (see Fig. 4, down). The first one with a characteristic size of a few nm can be attributed to either poly(S-b-2VP-b-EO) or poly(VDF-ter-HFP-ter-TFMA) unimers while the second one is reminiscent of the initial poly(S-b-2VP-b-EO)/poly(VDF-ter-HFP-ter-TFMA) complexes. It is indeed well-known that hydrogen-bonded complexes can be dissociated by heating.8 In this respect the results obtained in Fig. 3 and 4 can be explained by a dissociation of the micelles containing the poly(S-b-2VP-b-EO)/poly(VDF-ter-HFP-ter-TFMA) complexes in the core into poly(S-b-2VP-b-EO) and poly(VDF-ter-HFP-ter-TFMA) terpolymers solvated non-interacting chains (or unimers) at and above 50 °C. Indeed, it was not possible to image any structured objects above 50 °C. It is also noteworthy that the temperature effect is reversible since the poly(S-b-2VP-b-EO)/poly(VDF-ter-HFP-ter-TFMA) terpolymers complexes immediately do form upon cooling to room temperature. Moreover, the Rh of the re-formed micellar objects was found to be similar to the initial micelles, indicating the formation of (quasi)-equilibrium micelles in the present study.
Conclusions
Iodine transfer terpolymerization of α-trifluoromethacrylic acid (TFMA) with vinylidene fluoride (VDF) and hexafluoropropylene (HFP) led to terpolymers in high yields in aqueous medium in the absence of any surfactants and in the presence of 1,6-diodoperfluorohexane as the chain transfer agent. In a second step, the accordingly obtained poly(VDF-ter-HFP-ter-TFMA) terpolymers have been mixed with a poly(S-b-2VP-b-EO) triblock terpolymer in DMF. Formation of hydrogen-bonded complexes between the TFMA and 2VP units has been observed. These complexes are insoluble and further aggregate into micelles consisting of P2VP/poly(VDF-ter-HFP-ter-TFMA) terpolymers cores surrounded by a mixture of PS and PEO chains. The size of those micellar aggregates depends on the content of fluorinated moieties, especially on VDF + HFP units, in the copolymer and on the molecular weight of the poly(VDF-ter-HFP-ter-TFMA) terpolymers for a same copolymer composition. For the copolymer containing the higher amount of VDF + HFP units, multicompartmentalized cores have been visualized in agreement with the formation of VDF + HFP nanodomains in the micellar core. Finally, those micellar structures exhibit a reversible thermo-responsive behavior since they dis-assemble upon heating above 50 °C and re-assemble upon cooling.Acknowledgements
The authors thank the Solvay S.A. (Tavaux, France and Brussels, Belgium) and Tosoh F-Tech Company (Shunan, Japan, Dr Yoshida and Kawada) for providing with VDF, HFP and TFMA comonomers, respectively. BA also thanks CNRS and Région Languedoc Roussillon (ARPE programme). JFG and NL thank BELSPO for financial support in the frame of the network IAP 6/27. NL thanks FRIA for financial support.Notes and references
- J.-F. Gohy, Adv. Polym. Sci., 2005, 190, 65 CAS.
- A. Laschewsky, Curr. Opin. Colloid Interface Sci., 2003, 8, 274 CrossRef CAS.
- See for example: (a) J.-F. Lutz and A. Laschewsky, Macromol. Chem. Phys., 2005, 206, 813 CrossRef CAS; (b) S. Kubowicz, J.-F. Baussard, J.-F. Lutz, A. F. Thünemann, H. von Berlepsch and A. Laschewsky, Angew. Chem., Int. Ed., 2005, 44, 5262 CrossRef CAS; (c) A. F. Thünemann, S. Kubowicz, H. von Berlepsch and H. Möhwald, Langmuir, 2006, 22, 2506 CrossRef; (d) H. von Berlepsch, C. Böttcher, K. Skrabania and A. Laschewsky, Chem. Commun., 2009, 2290 RSC.
- (a) A. Kotzev, A. Laschewsky, P. Adriaensens and J. Gelan, Macromolecules, 2002, 35, 1091 CrossRef CAS; (b) A. Kotzev, A. Laschewsky and R. Rakotoaly, Macromol. Chem. Phys., 2001, 202, 3257 CrossRef CAS.
- See for example: (a) Z. Li, E. Kesselman, Y. Talmon, M. A. Hillmyer and T. P. Lodge, Science, 2004, 306, 98 CrossRef CAS; (b) T. P. Lodge, M. A. Hillmyer, Z. Zhou and Y. Talmon, Macromolecules, 2004, 37, 6680 CrossRef CAS; (c) Z. Li, M. A. Hillmyer and T. P. Lodge, Macromolecules, 2006, 39, 765 CrossRef CAS; (d) Z. Li, M. A. Hillmyer and T. P. Lodge, Nano Lett., 2006, 6, 1245 CrossRef CAS; (e) Z. Li, M. A. Hillmyer and T. P. Lodge, Langmuir, 2006, 22, 9409 CrossRef CAS; (f) T. P. Lodge, A. Rasdal, Z. Li and M. A. Hillmyer, J. Am. Chem. Soc., 2005, 127, 17608 CrossRef CAS.
- J.-F. Gohy, C. Ott, S. Hoeppener and U. S. Schubert, Chem. Commun., 2009, 6038 RSC.
- K. Kempe, R. Hoogenboom, S. Hoeppener, C.-A. Fustin, J.-F. Gohy and U. S. Schubert, Chem. Commun., 2010, 46, 6455 RSC.
- See for example: N. Lefèvre, C.-A. Fustin, S. K. Varshney and J.-F. Gohy, Polymer, 2007, 48, 2306 Search PubMed.
- For a recent review on that topic, see: N. Lefèvre, C.-A. Fustin and J.-F. Gohy, Macromol. Rapid Commun., 2009, 30, 1871 Search PubMed.
- V. Firetto, O. Scialdone, G. Silvestri, A. Spinella and A. Galia, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 109 CrossRef CAS.
- R. Souzy, B. Ameduri and B. Boutevin, Macromol. Chem. Phys., 2004, 205, 476 CrossRef CAS.
-
B. Ameduri and C. Boyer (assigned to Tosoh F-Tech Inc., Japan), JP 2008/214420, 20
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 070
070![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 301, 2008.
301, 2008. - H. Ito, B. Giese and R. Engelbrecht, Macromolecules, 1984, 17, 2204 CrossRef CAS.
- K. T. McElroy, S. T. Purrington, C. L. Bumgardner and J. P. Burgess, J. Fluorine Chem., 1999, 95, 117 CrossRef CAS.
- G. David, C. Boyer, J. Tonnar, B. Ameduri, P. Lacroix-Desmazes and B. Boutevin, Chem. Rev., 2006, 106, 3936 CrossRef CAS.
- C. Boyer and B. Ameduri, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4710 CrossRef CAS.
- M. Lansalot, C. Farcet, B. Charleux, J.-P. Vairon and R. Pirri, Macromolecules, 1999, 32, 7354 CrossRef CAS.
- D. Valade, C. Boyer, B. Ameduri and B. Boutevin, Macromolecules, 2006, 39, 8639 CrossRef CAS.
- J.-F. Gohy, E. Khousakoun, N. Willet, S. K. Varshney and R. Jérôme, Macromol. Rapid Commun., 2004, 25, 1536 CrossRef CAS.
Footnotes |
| † This paper is part of a Polymer Chemistry issue highlighting the work of emerging investigators in the polymer chemistry field. Guest Editors: Rachel O'Reilly and Andrew Dove. |
| ‡ Electronic supplementary information (ESI) available: Detailed characterization of the poly(VDF-ter-HFP-ter-TFMA) terpolymers and additional DLS data. See DOI: 10.1039/c0py00217h |
| This journal is © The Royal Society of Chemistry 2011 |
